Note: Descriptions are shown in the official language in which they were submitted.
2~9~ 9~
48275-2
RE~DIAq~ION MET~ODS FOR TOXIC MA1~3RI~l;S
The present invention relates to remediation of toxic
waste materials in solid form or in solid and liquid form.
More specifically, the present invention relates to a
process for treating toxic waste materials with nutrients to
remediate the contaminated material and efficiently reduce
the toxic conditions to innocuous levels.
Conventional disposal methods for solid toxic wastes
are landfills and include incineration, the application of
engineered bacteria, land farming or simply removal to
another area. The latter method does not resolve the
problem but merely delays a problem that has to be handled
at a later time.
One method of treating waste water utilizing an
aerating process is disclosed in Canadian Patent 1,182,227
to Hume, issued February 5, 1985. In this process, effluent
is treated such that substantially no sludge is formed.
Toxic effluent is first skimmed to remove floating oil and
other solids. The effluent is then vigorously mixed and
aerated so the waste material present in the effluent is
maintained in suspension such that minimum flocculation
occurs. The mixing and aeration continues for a sufficient
period of time to permit remediation of a substantial
portion of the waste material. The mixed and aerated
effluent is then passed through an oxidation basin or basins
for reduction of substantially all the remaining portion of
the waste material.
The present invention relates to the treatment of waste
materials in solid and/or liquid form using native or
indigenous microorganisms. No engineered microorganisms are
required. The remediation occurs rapidly, for some
2 ~
-- 2
materials in a matter of hours, other materials require a
matter of days. Previous reduction systems can require
months or years. In the process, toxic waste materials,
which may be in solid form, liquid form or in solid and
liquid form, are contact mixed with nutrients in water and
then exposed to the action of air to ensure o~ygen is always
present. The combination of air and the nutrients in
contact with the indigenous microorganisms in the toxic
wastes provide a bioactive structure which rapidly
remediates to specified levels.
The type of toxic wastes to be treated include soils
that have become toxic through contact with contaminants
such as PCBs, oils, creosotes and other organic or petroleum
based products. It is first necessary to excavate the soils
which are contaminated. Water and nutrients are added to
form a homogeneous mass which is then mixed and at the same
time exposed to the action of air. After mixing and air
entrainment, the waste materials are stockpiled or laid out
so that curing occurs with no toxic leachate.
Where heavy contamination is present, a rinse cycle is
employed to remediate contaminated water, nutrients, and in
some cases surfactants, which are added to the toxic wastes.
The contaminated rinse liquids are remediated in a recycle
treatment tan~, a final treatment tank or tanks.
The solid waste materials are preferably screened to
avoid oversized materials from damaging the process
equipment. Crushing, grinding or shredding may be
necessary, and oversized materials that cannot easily be
processed are sprayed with liquid nutrient and water and
stacked or piled up for curing.
In the case where contaminated liquid in substantial
volume is together with solid toxic waste material, two
systems are arranged side-by-side. One system is primarily
for liquids, which may be liquids from a solids contact
-- 3 --
mixing phaseO The liquid remediation system is similar to
that disclosed in Canadian Patent 1,182,227 and both liquids
and solids can be recycled between the two systems~ In this
way metals may be removed from the waste materials. The
heavy molecules of the nutrients assist in the process of
precipitating the metals for mechanical removal from the
liquid treatment system.
The present invention provides a process for treating
toxic waste materials contaminated with organic substances
comprising the steps of: contact mixing solid waste
materials with protein nutrients in water, air entraining
the solid waste materials and protein nutrients during
mixing to form a bioactive structure, exposing the bioactive
structure to cure in air until the toxic waste materials are
remediated to a predetermined innocuous level.
Definitions of terms used throughout the specification
and claims are as follows:
Biological degradation, or biodegradation is the
molecular degradation of an organic substance resulting from
the complex action of living organisms, and in the present
application indigenous microorganisms. Toxic materials are
biodegraded to innocuous metabolites by these indigenous
microorganisms. Biodegradation and/or detoxification is
referred to as remediation or treatment throughout the
present text. The remediation process is a combination of
enzyme catalyzed reactions which proceed to or proceed with
biodegradation to resultant, less toxic metabolites.
Nutrients include hydrolized protein nutrients composed
of naturally occurring amino acids. The nutrients also
include enzymes which are complex proteins ubiquitous in
natllre and have additional chemical bonds that stabilize
fixed macromolecular geometries that are involved in enzyme
reaction.
2 ~ 9 4
-- 4 --
Surfactants, surface active agents, may be used with
some waste materials to reduce interfacial tension between
liquids and ~etween liquids and solids. The surfactants are
selected for their detergency, emulsifying and dispersing
qualities. ~11 surfactants must be compatible with the
nutrients to ensure the remediation process is not reduced.
Bioactive structure includes a cellular bubbled
configuration of air, nutrients, active indiqenous
microorganisms all in contact with the pollutants to enhance
remediation. This structure is created within the solids
mass or as a stable surface floating froth on the liquid.
Curing means the disposition of the bioactive
structured solids in a manner to allow further remediation
(biodegradation - detoxification) at acceptable ambient or
artificially controlled temperatures.
In another embodiment, the present invention provides a
process for treating toxic recycled rinse liquids from the
treatment of solid waste materials with protein nutrients in
water, comprising the steps of contact mixing and air
entraining with protein nutrients in water to form a stable
surface bioactive structure to remediate the rinse liquids
to a predetermined innocuous level for authorized disposal.
In drawings which illustrate embodiments of the
invention,
Figure 1 is a flow sheet showing a process according to
one embodiment of the present invention for treating toxic
solid waste materials~
Figure 2 is a flow sheet showing a process according to
another embodiment of the present invention for treating
toxic rinse liquids from the treatment of toxic solid waste
materials in a recycle treatment tank.
2~59~9~
-- 5
Figure 3 is a flow sheet showing a process according to
a further embodiment of the present invention ~or treating
the rinse liquids in a final treatment tank.
Waste material sites must first be examined to
determine the treatability of the material. Laboratory
analysis and pilot tests are required to select an effective
and economic treatment configuration. Samples of the waste
material are treated with different nutrients in various
concentrations and set out to cure. Tests are taken at
curing times, for example, zero time up to seven days, and
the results tabulated. From these results, variation in the
nutrient content to be added, times, etc., are determined.
Furthermore, the reduction in toxicity is determined based
on the authoritative requirements for a particular toxic
material.
Many toxic materials on waste sites include a
homogeneous mass of liquid and solids. Soils that have been
soaked with oil products or oil related products such as
PCBs, creosote, etc., are first excavated and then if
necessary crushed or ground to increase the active surface
area of the contaminated particles~
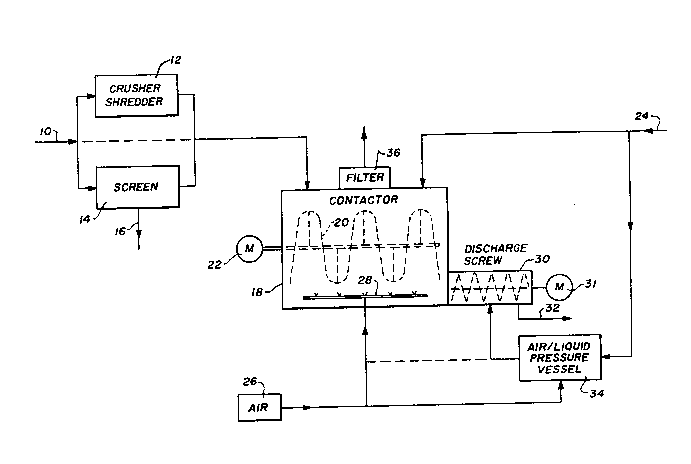
As shown in Figure 1, waste material 10 is crushed,
ground or shredded in a suitable apparatus 12. The material
10 may be screened in a screen 14 to remove oversized
materials 16. These oversized materials 16 may be
remediated by wetting with protein nutrients in water and
leaving to cure on site. In some cases crushing and
screening is not required, in which case the material is fed
directly to a contactor container 18 which has a mixer,
preferably a ribbon mixer 20 therein driven by a motor 22.
Whereas a ribbon mixer 20 is shown, paddle mixers, auger
mixers or other types of known contact mixers may be used.
In one case an auger screw may be provided to move the
material through the container 18 and out at a discharge.
Protein nutrients in water 24 are added to the contactor
2 ~ 9 ~
-- 6 --
container 18, and at the same time a compressed air source
26 provides compressed air through an air entrainer 28 at
the bottom of the contactor container 18.
Sufficient liquid is added to form a homogeneous mass
of solid waste material and the air entrainment causes the
material to form bubbles of air within the mass. The time
of contact mixing can vary depending upon the particular
material being treated and the type of mixer used.
A bioactive structure is formed in the mixing stage,
the air bubbles provide about a 20 to 50% increase in volume
for solids.
After the mixing step, the bioactive structure is
removed from the container 18. In some cases the container
may have a discharge chute or a discharge screw powered by
motor 31 removes the bioactive structure at exit 32 where
the treated material is stockpiled or laid out for further
curing in air. The discharge screw 30 in one embodiment has
added to it protein nutrients in water which ha~e been
entrained with air by air entrainment system 34. In some
instances air entrained protein nutrients in water are also
added to the entrainer 28 as shown in the dotted line in
Figure 1. The contactor container 18 has an air filter 36
at the top to prevent possible contaminated air escaping.
After the bioactive structure has been laid out in air,
it is left to cure. Temperatures preferably in the range of
50 to 90F permit remediation of the structure down to
innocuous levels of toxicity generally in about 4 to 21 days
although the curing time is dependent on a number of
variables, primarily ambient temperature, and varies from
site to site. The treatment appears to proceed via an
enzyme-catalyzed reaction leading to biodegradation to less
toxic metabolites.
2 ~
-- 7 --
The basic protein nutrients are hydrolized protein
materials and, in some cases, include surfactants depending
on the ease or otherwise of wetting the contaminated waste
material satisfactorily. Indigenous microorgani~ms pre~ent
in the toxic materials are utilized. Good contact between
the waste material and the protein nutrients and water
solution is necessary for the effectiveness of the process.
The selection of the protein nutrient, the concentration in
a water solution and the option, selection and concentration
of the surfactant is also determined by tests on the waste
material prior to the process. The protein nutrient is
selected from a number of protein nutrient sources including
powdered cow's milk, soya bean oil, soya bean meal, fish
oil, fish meal, rendering plant byproducts, slaughterhouse
byproducts, brewery residues and brewery bottoms. The
selection of protein nutrient is made after initial tests in
a laboratory and on the site.
Gravel, sand, silt and clay may all be treated by this
process. In the past, silt and clay have been difficult to
treat, but they may be processed according to the present
invention.
In some cases where heavy contamination is apparent,
excess water is added to the solid waste material and a
rinse cycle takes place where the contaminated rinse liquid
is treated.
As shown in Figure 2 a recycle treatment tank 40 is
provided for an input of contaminated rinse liquid 52. The
recycle treatment tank 40 has an aerator 44 fed from the air
supply 26 and a discharge screw 46 driven by motor 48.
Protein nutrients and water 24 are added to the recycle
treatment tank 40. The liquid from the recycle treatment
tank 40 is recirculated through line 50 to the contactor
container 18 and then further recycled back through line 52
into the recycle treatment tank 40. Solids that build up in
the recycle treatment tank 40 pass through the discharge
2~
-- 8
screw 46 and are recirculated back to the contactor
container 18 through line 54. In another embodiment the
solids from the discharge screw 46 leaving the recycle
treatment tank 40, instead of being recycled, go to disposal
and may be laid out or stockpiled for further curing. After
treatment specification liquid from the recycle treatment
tank 40 passes through outlet 56 to an authorized disposal.
Any heavy metals that are released during the processes,
drop into the metal removal container 58 for removal from
the recycle treatment tank 40.
Any liquid such as oil that settles above the solids in
the contactor container 18, is removed at outlet 38 and may
be remediated in the recycle treatment tank 40.
Recirculation of liquid between the contactor 18 and
the treatment tank 40 and the addition of the protein
nutrients in water creates a stable surface bioactive
structure.
In another embodiment the recycled contaminated rinse
liquids 52 from the contactor container 18 or from the
recycle treatment tank 40 are fed to a final treatment tank
60 as shown in Figure 3, through line 62. Liquid such as
oil removed from the contactor container ~8 from outlet 38
may also be fed to the final treatment tank through line 62.
The final treatment tank 60 has an aerator 44 fed from the
air supply 26. Protein nutrients and water 24 are added to
the final treatment tank 60 and air is entrained into the
liquids in the final treatment tank 60. Oil on the top of
the liquid in the final treatment tank 60 is bioactively
structured. Metals in the oil products chelate and drop to
the bottom of the final treatment tank 60 where they are
collected in the metal removal 58. After treatment the
remediated liquid passes through outlet 64 to authorized
disposal.
2 ~ 3 k
The application of the process can all occur at a site.
Commercially available equipment may be utilized with little
or no modification needed. The protein nutrients and their
concentration in water, the surfactants and their
concentration, and the crushing, grinding or shredding
requirements are all determined for a specific site.
Example 1. At one waste site the pollutant in soil was
gasoline/diesel fuels and the soil was a silty clay with
fine sand. The analysis of the pollutant was modified EPA
8015 for Total Fuel Hydrocarbons, and EPA 802~ for benzene,
toluene, xylene and ethyl benzene. Contamination levels
often exceeded 5,000 ppm, total fuel hydrocarbons and the
requirement was to reduce the overall concentration to less
than 100 ppm and eliminate the strong odour associated with
thi~ contamination. EPA methods to determine ~ontaminants
are standards adopted by laboratories (see Brown and
Caldwell Laboratories Technical Fact Sheet No. 2).
A batch mixing method was employed according to the
arrangement shown in Figure 1. Over 200 cubic yards of soil
were treated per day. The temperature was ambient varying
from 50 to 90F, and after mixing, which occurred in less
than five minutes per batch, curing proceeded for four days
with resnltant contaminant levels consistently reduced to
less than 100 ppm. ~ir monitoring during the process failed
to detect any release of contaminant volatiles during the
mixing or curing of the treated materials. This example
treated only solid toxic materials.
Example 2. In this example the waste materials
included polychlorinated biphenyls (PCBs) Aroclor 1254. The
analysis was EPA 8080. The waste material was very fine
greenish silty sediment and very dusty with the dry density
of flour. The site was a dried cooling tower blow-down pond
located at a natural gas compressor station. The process
was the same as disclosed in Example 1. The material was
treated and analysis conducted before treatment and at 1, 3,
2 ~
-- 10 --
7 and 14 days of curing time. The PCB contamination ranged
from 183 to 77 ppm in the original material and the PC~
contamination after 14 days ranged from 2.5 to 1.3 ppm with
the isomer shifting to the chromatographic signature of
Aroclor 1260.
Example 3. A test was conducted to investigate the
effectiveness of the process to detoxify coal tar deposits.
The material was coal tar saturated soil sediments. The
pollutants were polynuclear aromatic hydrocarbons (PAH)
namely, naphthalene, anthracene, acenaphthene, plenanthrene,
methylated naphthalenes, etc. The analysis was EPA 8240 and
EPA 8270 (8 various MAH's and 17 various PAH's).
The method of treatment was the same as that disclosed
in Example l. The material was treated and analysis
conducted before treatment and at 1, 4, 7, and 14 days of
curing time. The total volatiles were reduced from 8,900
ppb on day 1 to 35 ppb on day 14. The total base/neutrals
were reduced from 5,927 ppm on day 1 to 839 ppm on day 14.
The results shows that two, three, four and five-ring
compounds were de~raded 96 percent, 88 percent, 55 percent
and 44 percent respectively, in a period of 14 days.
Example 4. A spill disposal pit containing heavy fuel
oils (Bunker C) was tested to treat both solid and liquids
and the system used was that disclosed in Figure 2. The
material was fine sand with clay lenses and the analysis was
polynuclear aromatic hydrocarbons (PAH), total organic
carbons (TOC) and total petroleum hydrocarbons (TPH). A
full scale pilot test was run for four days. Both solids
and liquids were treated. Analysis was conducted
immediately at the start of the treatment and then at one
and four days of solids curing and liquid treatment. The
results were as follows.
2 ~
TABLE
Sample Analysis PAH TOC TP~ (ppm)
Day 0 before 5502,500 20,000
Day 0 after ND 1,500 7,500
Day 1 ND 1.5 13.0
Day 4 ND 1.0 9.8
ND indicates Not Detectable
Rinse water had polynuclear aromatic hydrocarbons
concentrations reduced from 2,388 ppb on day 1 to 39 ppb on
day 4 and was subsequently discharged through an industrial
sewage system.