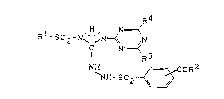Note: Descriptions are shown in the official language in which they were submitted.
2~ ~ 0 ~ 3 ~
The invention relates to new substituted arylsulphonyl-
aminoguanidinotriazinas, thei.r preparation, and their use
as herbicides.
It has already been di~closed that certain sulphonyl-
aminoguanidinoazines such asl for example, N'-(4,6-
dimethyl-pyrimidin-2-yl)-N''-(4-methyl-phenylsulphonyl-
amino)-N'''-(2-chloro-phenyl-sulphonyl)-guanidineandN'-
(4,6-dimethyl-pyrimidin-2-yl)-N''-(2-methoxycarbonyl-
phenylsulphonylamino)-N'''-(2-methoxycarbonyl-phenyl-
sulphonyl)-guanidine, have herbicidal properties (cf.
EP-A 121,082 and EP-A 302,378). However, the herbicidal
action of these known compounds is not -~atisfactory in
all respects.
Other ~ubstituted arylsulphonylaminoguanidinoazine~ are
the sub~ect of an earlier, but not prior-published patent
application (cf. DE-P (German Patent Specification)
3,927,770 of 23.08.1989).
Novel sub~tituted arylsulphonylaminoguanidinotriazines of
the general formula (I)
Le A 28 200 - 1 -
2 0 ~
R4
H N==~
C N- ~ 3
¦ R 2 (I)
NH~==~_CO~
`NH-5O
in which
Rl represent~ in each case optionally substituted aryl,
aralkyl or heteroaryl,
S R2 repre~ents hydroxyl, amino or in each ca~e option-
ally substituted alkoxy or alkylamino,
R3 represents halogen, alkyl, halogenoal~yl, alkoxy-
alkyl, alkoxy, halogenoalkoxy, alkylthio, halo-
genoalkylthio, alkylamino or dialkylamino and
0 R4 represents halogen, alkyl, cycloalkyl, alkoxy,
alkylthio, alkylamino or dialkylamino,
have now baen found, N~-(4,6-dimethoxy-s-triazin-2-yl)-
N''-(2-methoxycarbonyl-phenylsulphonylamino)-N'''-(2-
bromophenylsulphonyl)-guanidine, N'-(4,6-dLmethoxy-s-
triazin-2-yl)-N''-(2-methoxycarbonyl-phenyl~ulphonyl-
amino)-N'''-(2-phenyl-phenylsulphonyl)-guanidine, N'-(4-
methoxy-6-methyl-3-triazin-2-yl)-N''-(2-methoxycarbonyl-
phenylsulphonylamino)-N'''-(2-phenyl-phenylsulphonyl)-
guanidine, N'-(4,6-dimethoxy-s-triazin-2-yl)-N''-(2-
Le A 28 20Q - ~ -
2 ~ } c~ ~
methoxycarbonyl-phenylsulphonyl.~mino)-N'''-(2-methylthio-
phenylsulphonyl)-guanidine and N-(4,6-dimethoxy-3-tri-
azin-2-yl)-N~'-(2-methoxycarborlyl-phenylsulphonylamino)-
N'''-(2-isopropoxycarbonyl-phenylsulphonyl)-guanidine
being excepted (cf. DE-P (Genman Patent Specification)
3,927,770 or EP Application No. 90115373.4/EP-A-
0,414,067).
The general formula (I) represents the individual
possible tautomers of the formulae (IA), (IB) and (IC)
R4
Rl-5O2-N~ ,NH ~ N
C N ~ 3 (I)
NH ~ oR2
~NH-5O2 ~
N ~ (IB)
Rl-5O2-NH~ ~N ~ ~ N
NH ~ oR2
`NH-SO
Le A 28 200 - 3 -
2a~
R -502-NH~ ,NH--~, ~N
ll R3 ( IC )
N~ ~=~C oR2
NH- 52~_~>
and mixtures of these tautomers.
The new substituted arylsulphonylaminoguanidinotriazines
of the general formula (I) are obtained when
(a) sulphonyl compounds of the general formula (II)
H N==~
R1-SO2-N~-,N ~ ~ N (II)
R5
in which
R' R3 and R4 have the abovementioned meanings and
Rs represents one of the leaving groups mentioned
below
R6-So2-N-oR7 or -Q-Re,
where
Le A 28 200 - 4 -
2 ~ 8
R~ has the meaning mentioned above for Rl, but need
not be identical to Rl in each individual case,
R7 represents alkyl, alkenyl or aralkyl,
R~ represents alkyl, aralkyl or aryl and
Q represents oxygen or sulphur,
are reacted with sulphonylhydrazides of the qeneral
formula (III)
~ oR2 (III)
H2N-NH- S02~
in which
R2 has the abovementioned meaning,
if appropriate in the presence of a diluent, or when
(b) aminoguanidinotriazines of the general formula (IV)
1 ~H, Nc=~
R -5O2-N~ ;N ~\ /N
I R3 (IV)
NH
~NH 2
in which
Le A 28 200 - 5 -
R1 R3 and R4 have the abovementioned meanings,
are reacted with sulphonyl halides of the general
formula (v)
oR2
X-502~ (V)
in which
R2 has the abovementioned meaning and
X represents halogen,
or with sulphobenzoic anhydride of the formula (VI)
CO '
S2 ~ (VI)
if appropriate in the presence of a diluent and if
appropriate ln the presence of an acid acceptor, or
when
(c) substituted arylsulphonylaminoguanidinotriazines of
~he general formula tI), in which R2 represents
Le A 28 200 - 6 -
2 ~ o ~ ~
optionally substituted alkoxy and Rl, R3 and Ri have
the abovementioned meanings,
are reacted with aqueous alkali metal hydroxide
solutions or with ammonia or with optionally sub-
S stituted alkylamines, if appropriate in the presence
of a diluent.
The novel substituted arylsulphonylaminoguanidinotri-
azines of the general formula (I) are distinguished by
powerful herbicidal activity.
The invention preferably relates to compounds of the
formula (I) in which
R10
R1 repre3ents the radical ~ where
R9
R9 and R10 are identical or different and represent
hydrogen, fluorine, chlorine, bromine, iodine,
cyano, nitro, C1-C6-alkyl (which is optionally
~ubstituted by fluorine, chlorine, bromine,
cyano, carboxyl, C1-C4-alkoxycarbonyl, Cl-C4-
alkylamino-carbonyl, di-(C1-C4-alkyl)-amino-
carbonyl, hydroxyl, Cl-C4-alkoxy, formyloxy,
C1-C4-alkyl-carbonyloxy, C1-C4-alkoxycarbonyl-
oxy, Cl-C4-alkylaminocarbonyloxy, Cl-C4-alkyl-
thio, C1-C4-alkylsulphinyl, Cl-C4-alkyl-
~ulphonyl, di-(C1-C~-alkyl)aminosulphonyl,
Le A 2~ 200 - 7 -
2 ~
C3-C6-cycloalkyl or phenyl), or represent
C2-C6-alkenyl (which is optionally substituted
by fluorine, chlorine, bromine, cyano, C~-C4-
alkoxycarbonyl, carboxyl or phenyl), or
S represent C2-C6-alkinyl (which is optionally
substituted by fluorine, chlorine, bromine,
cyano, C~-C4-alkoxy-carbonyl, carboxyl or
phenyl), or represent C~-C4-alkoxy (which is
optionally substituted by fluorine, chlorine,
bromine, cyano, carboxyl, C~-C4-alkoxy-
carbonyl, C~-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-
alkylsulphinyl or C~-C4-alkylsulphonyl), or
represent C~-C4-alkylthio (which is optionally
substituted by fluorine, chlorine, bromine,
cyano, carboxyl, C~-C4-alkoxy-carbonyl, Cl-C4-
alkylthio, C,-C4-alkylsulphinyl or C~-C4-
alkylsulphonyl), or represent C3-C6-alkenyloxy
(which i3 optionally substituted by fluorine,
chlorine, bromine, cyano or C~-C4-alkoxy-
carbonyl), or repre~ent C2-C6-alkenylthio
(which is optionally ~ubstituted by fluorine,
chlorine, bromine, cyano, nitro, C~-C3-
alkylthio or C,-C4-alkoxycarbonyl), C3-C6-
alkinyloxy, C3-C6-alkinylthio or the radical
-S(O)p-Rl1 where
p represents the numbers l or 2 and
Rll represents Cl-C~-alkyl (which is optionally
~ubatituted by fluorine, chlorine,
Le A ?8 200 - 8 -
bromine, cyano or Cl-C4-alkoxy-carbonyl),
C3-C6-alkenyl, C3-C6-alkinyl, Cl-C4-alkoxy,
Cl-C4-alkoxy-Cl-C4-alkylamino, C~ C4-alkyl-
amino, di-(Cl-C4-alkyl)-amino or the
S radical -NHORl2 where
Rl2 represents C1-C12-alkyl (which is
optionally substituted by fluorine,
chlorine, cyano, C1-C4-alkoxy, C1-C4-
alkylthio, Cl-C4-alkylsulphinyl,
Cl-C4-alkylsulphonyl, Cl-C4-alkyl-
carbonyl, Cl-C4-alko~ycarbonyl, Cl-C4-
alkylamino-carbonyl or di-(Cl-C4-
alkyl)-amino-carbonyl), or repre-
sents C3-C6-alkenyl (which is
1~ optionally substituted by fluorine,
chlorine or bromine), or represents
C3-C6-alkinyl, C3-C6-cycloalkyl, C3-C6-
cycloalkyl-Cl-C2-alkyl,phenyl-Cl-C2-
alkyl (which is optionally
~ubstituted by fluorine, chlorine,
nitro, cyano, C1-C4-alkyl, Cl-C4-
alkoxy or Cl-C4-alkoxy-carbonyl), or
represents benzylhydryl, or repre-
sents phenyl (which is optionally
substituted by fluorine chlorine,
nitro,cyano, C1-C4-alkyl, trifluoro-
methyl, Cl-C4-alkoxy, Cl-C2-fluoro-
alkoxy, Cl-C4-alkylthio, trifluoro-
methylthio or C1-C4-alkoxy-carbonyl),
Le A 28 200 - 9 -
2 ~ v ~
Ra and R10 furthermore represent phenyl or phenoxy,
or represent amino, C,-C4-alkylcarbonylamino,
Cl-C4-alkoxy-carbonylamino, Cl-C4-alkylamino-
carbonyl-amino, di-~C1-C4-alkyl)-amino-carbon-
ylamino, or represent the radical -Co-R13
where
Rl3 represents Cl-C6-alkyl, Cl-C6-alkoxy, C3-C6-
cycloalkoxy, C3-C6-alkenyloxy, C1-C4-alkyl-
thio, C1-C4-alkylamino, C1-C~-alkoxyamino,
C1-C4-alkoxy-C1-C4-alkyl-amino or di-(C1-C4-
alkyl)-amino (which are optionally sub-
stituted by fluorine and/or chlorine),
R9 and R10furthermore repre4ent C1-C4-alkylsulphonyl-
oxy, di-(C1-C4-alkyl)-~minosulphonylamino,
thiazolyloxy, or represent the radical
-CHzN-~14 where
R14 represents C1-C6-alkyl which is optionally
substituted by fluorine, chlorine, cyano,
carboxyl, Cl-C4-alkoxy, Cl-C4-alkylthio,
Cl-C4-alkylsulphinyl or Cl-C4-alkylsul-
phonyl, or represents benzyl which is
optionally substituted by fluorine or
chlorine, or represents C3-C6-alkenyl or
C3-C6-alkinyl, each of which is optionally
substituted by fluorine or chlorine, or
represents phenyl which is optionally ~ub-
stituted by fluorine, chlorine, bromine,
Cl-C~-alkyl, Cl-C~-alkoxy, trifluoromethyl,
Le A 28 200 - 10 -
2 ~
trifluoromethoxy or trifluoromethylthio,
or represents C~-C6-alkoxy, C3-C6-alkenoxy,
C3-C6-alkinoxy or benzyloxy, each of which
is optionally substituted by fluorine
S and/or chlorine, or represents amino,
Cl-C4-alkylaminoI di-(Cl-C4-alkyl)-amino,
phenylamino, Cl-C4- alkyl-carbonyl-amino,
Cl-C4-alkoxy-carbonylamino, Cl-C4-alkyl-
sulphonylamino, or represents
phenylsulphonylamino which is optionally
substituted by fluorine, chlorine, bromine
or methyl,
furthermore
R17
R1 represents the radical -CH ~ where
R15 R16
Rl5 represents hydrogen or C1-C4-alkyl,
R15 and R17 are identical or different and represent
hydrogen, fluorine, chlorine, bromine, nitro,
cyano, C1-C4-alkyl (which i8 optionally sub-
stituted by fluorine andtor chlorine), C1-C4-
alkoxy (which is optionally substituted by
fluorine and/or chlorine), carboxyl, Cl~C4-
alkoxy-carbonyl, C1-C~-alkylqulphonyl or di-
tCl-C~-alkyl)-aminosulphonyl; furthermore
Le A 28 200 - 11 -
2~ o'~
R18~Rlg
Rl represents the radical where
R1~ and Rl3 are identical or different and represent
hydrogen, fluorine, c:hlorine, bromine, nitro,
cyano, Cl-C4-alkyl (which is optionally sub-
stituted by fluorine and/or chlorine) or C1-C4-
alkoxy (which i~ optionally substituted by
fluorine and/or chlorine); furthermore
R20
Rl represents the radical ~ where
R21
R20and R21 are identical or different and represent
hydrogen, fluorine, chlorine, bromine, nitro,
cyano, Cl-C4-alkyl (which is optionally sub-
stituted by fluorine and/or chlorine~, C1-C4-
alkoxy (which is optionally substituted by
fluorine and/or chlorine), or represent C1-C4-
alkylthio, Cl-C4-alkylsulphinyl or Cl-C4-alkyl-
sulphonyl (which are optionally sub6tituted by
fluorine and/or chlorine), and also represent di-
(C1-C~-alkyl)-aminocarbonyl or C1-C4-alkoxy-
carbonyl; furthermore
R1 represents the radical R22 ~ 23 where
Le A 28 200 - 12 -
2~o~8
R22 and R23 are identical or different and reprsent
hydrogen, fluorine, chlorine, bromine, Cl-C4-alkyl
(which is optionally sub~tituted by fluorine
and/or bromine), Cl-C4-alkoxy (which is optionally
sub~tituted by fluorine and/or chlorine), or
represent C1-C"-alkylthi.o, C1-C4-alkylsulphinyl or
C1-C4-alkylsulphonyl ~which are optionally
substituted by fluorine and~or chlorine), or
represent di-(C1-C4-alkyl)-aminosulphonyl;
furthermore
R24
R1 represents the radical ~ 2s where
R24 and R25 are identical or different and represent
hydrogen, fluorine, chlorine, bromine, cyano,
nitro, C1-C4-alkyl (which is optionally substi-
tuted by fluorine and/or chlorine), C1-C4-alkoxy
(which is optionally substituted by fluorine
and/or chlorine), C~-C4-alkylthio, C1-C4-
alkylsulphinyl or C1-C4-alkylsulphonyl (which i5
optionally substituted by fluorine and/or
chlorine), di-(C,-C4-alkyl)-amino-sulphonyl or
C~-C4-alkoxycarbonyl, and
Al represents oxygen, sulphur or the group N-Zl,
where
zl represents hydrogen, Cl-C4-alkyl (which is
Le A 28 200 - 13 -
optionally substituted by fluorine,
chlorine, bromine or cyano), C3-C6-cyclo-
alkyl, benzyl, phenyl (which is optionally
substituted by fluorine, chlorine, bromine
or nitro), Cl--C4-al~ylcarbonyl, Cl-C4-
alkoxy-carbonyl or di-(Cl-C4-alkyl)-amino-
carbonyl; furthermore
R26
Rl represents the radical ~ ~_N where
R25 represents hydrogen, Cl-Cs-alkyl or halogen,
R27 represents hydrogen or Cl-C5-alkyl and
yl represents sulphur or the group N-R2a where
R2B represents hydrogen or Cl-C5-alkyl,
furthermore R31
Rl represents the radical ~ ~ 30 where
N
l29
R2~ represent3 hydrogen, Cl-C4-alkyl, phenyl,
naphthyl or (iso)quinolinyl,
R30 represents hydrogen, halogen, cyano, nitro,
Cl-C4-alkyl (which i~ optionally sub3tituted by
Le A 28 200 - 14 -
fluorine and/or chlo:rine), Cl-C4-alkoxy (which
is optionally substituted by fluorine and/or
chlorine) or Cl-C4-alkoxycarbonyl and
R31 represents hydrogen, halogen or C1-C4-alkyl
furthermore
R1 represents the radical ~ 2 where
R32 -R33
R32 represents Cl-C3-alkyl and
R33 represents Cl-C4-alkyl,
furthermore ~
R1 represents the radical
furthermore
R2 represents hydroxyl, amino, or represents Cl-C4-
alkoxy or C1-C4-alkylamino, each of which is option-
ally substituted by fluorine, chlorine, methoxy or
ethoxy,
R3 represents fluorine, chlorine, bromine, Cl-C4-alkyl,
Cl-C~-halogenoalkyl, Cl-C2-alkoxy-Cl-C2-alkyl, Cl-C4-
alkoxy, Cl-C~-halogenoalkoxy, Cl-C4-alkylthio,
Le A 28 200 - 15
Cl-C4-halogenoalkylthio, Cl-C4-alkylamino,
dimethylamino or diethylamino, and
R4 represents f luorine, chlorine, bromine, C,-C4-alkyl,
cyclopropyl, Cl-C4-alkoxy" Cl-C4-alkylthio, C1-C4-
alkylamino, dimethylamino or diethylamino,
N ' - ( 4, 6-dimethoxy-s-triazin-2-yl ) -N ' ' - ( 2-methoxycarbonyl-
phenylsulphonylamino ) -N ~ ~ ~ - ( 2 -bromo-phenylsulphonyl ) -
guanidine, N ' - ( 4, 6-dimethoxy-s-triazin-2-yl ) -N ' ' - ( 2-
methoxycarbonyl-phenyl~ulphonylamino ) -N ' ~ ~ - ( 2 -phenyl-
phenylsulphonyl ) -guanidine, N ~ - ( 4-methoxy-6-methyl-s-
triazin-2-yl ) -N ' ~ - ( 2-me~hoxycarbonyl-phenylsulphonyl-
amino ) -N ~ ~ ' - ( 2-phenyl-phenylsulphonyl ) -guanidine, N ' -
( 4, 6-dimethoxy~s-triazin-2-yl ) -N ' ' - ( 2-methoxycarbonyl-
phenylsulphonylamino ) -N ~ ' ' - ( 2-methylthio-
phenylsulphonyl ) -guanidine and N- ( 4, 6-dimethoxy-s-tri-
azin-2 -yl ) -N ' ' ' - ( 2-methoxycarbonyl--phenylsulphonylamino ) -
N ' ' ~ - ( 2-isopropoxycarbonyl phenylsulphonyl ) -guanidine
being excepted (cf. DE-P (German Patent Specification)
3,927,770) .
In particular, the invention relates to compounds of the
formula ( I ) in which R1 o
R1 represents the radical ~ where
R
R~ represents fluorine, chlorine, bromine,
methyl, trifluoromethyl, methoxy,
difluoromethoxy, trifluoromethoxy,
Le A_28 200 - 16 -
2 ~ t, 8
Cl-C3-alkylthio, Cl-C3-alkylsulphinyl~ Cl-C3-
alkylsulphonyl, dimethylaminosulphonyl,
diethylaminosulphonyl, N-methoxy-N-
methylaminosulphonyl, phenyl, phenoxy, methoxy-
S carbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, chloroethoxycarbonyl,
methoxyethoxycarbonyl, n-, i- or s-butoxy-
carbonyl, n-, i- or s-pentoxycarbonyl, and
Rl represents hydrogen, fluorine or chlorine;
furthermore
R17
Rl represents the radical ~l ~ where
RlS 6
Rl5 represents hydrogen,
Rlfi represents fluorine, chlorine, bromine, methyl,
methoxy, ethoxy, difluoromethoxy, trifluoro-
methoxy, methoxycarbonyl, ethoxycarbonyl,
methylsulphonyl or dimethylamino~ulphonyl, and
Rl7 represents hydrogen, fluorine or chlorine;
furthermore
R1 repre~ent~ the radical ~~ 1l where
R represents methyl or ethyl, or
Le A 28 200 - 17 -
~ ~ J~ 0
Ro-C ~ ~
R represents the radical N~N where
c~3
R represents methyl or ethyl,
R2 represents hydroxyl, amino, methoxy, ethoxy, prop-
oxy, isopropoxy, 2-chloro-ethoxy, 2-methoxy-ethoxy,
2-ethoxy-ethoxy, methylamino, ethylamino, propyl-
amino or isopropylamino,
R3 represent~ chlorine, methyl, trifluoromethyl,
methoxy, ethoxy, methylthio, e~hylthio~ methylamino,
ethylamino, dimethylamino or diethylamino, and
R4 represents methyl, ethyl, cyclopropyl, methoxy,
ethoxy, propoxy, isopropoxy, methylthio, ethylthio,
methylamino, ethylamino, dimethylamino or diethyl-
amino,
N'-(4,6-dimethoxy-3-triazin-2-yl)-N''-(2-methoxycarbonyl-
phenylsulphonylamino)-N'''-(2-bromo-phenylsulphonyl)-
guanidine, N'-(4,6-dimethoxy-s-triazin-2-yl)-N''-(2-
methoxycarbonyl-phenylsulphonylamino)-N~ (2-phenyl-
phenyl ulphonyl)-guanidine, N'-(4-methoxy-6-methyl-s-
triazin-2-yl)-N''-(2-methoxycarbonyl-phenylsulphonyl-
amino)-N'''-(2-phenyl-phenylsulphonyl)-guanidine, N'-
(4,6-dimethoxy-s-triazin-2 yl)-N''-(2-methoxycarbonyl-
Le A ?8 200 - 18 -
2 ~
phenylsulphonylamino ) -N ~ 2 -methylthio-
phenylsulphonyl)-guanidine and N-(4,6-dimethoxy-s-tri-
azin-2-yl)-N''-(2-methoxycarboslyl-phenylsulphonylamino)-
N'''-(2-isopropoxycarbonyl-phenylsulphonyl)-guanidine
S being excepted (cf. DE P (German Patent Specification)
3,q27,770) .
Very particularly preferxed compounds of the fonnula (I)
are those in which thP -COR2 group is in the ortho-
position to the SO2 group, and R1, R2, R3 and R4 have the
meanings mentioned above as being particularly preferred,
the previously mentioned compounds being excepted.
If, for example, N'-(4,6-dimethoxy-s-triazin-2-yl)-N''-
methoxy-N'',N'''-bis-(2-fluoro-phenylsulphonyl)-guanidine
and 2-methoxycarbonyl-benzenesulphonylhydrazide are used
as starting substances, the course of the reaction in
process (a) according to the invention can be outlined by
the following equation:
Le A 28 200 - 19 -
5 ~
6~ N=< H2N-NH-502
<~S02-N-OCH3
F OCH3
~< N=c<
---- ~ ~502-N~ ,NH--<~ ~N
-Q--502-NHocH3 NH~ OCH3
F NH- S02~)
COOCH3
If, for example, N'-(4,6-dimethoxy-s-triazin-2-yl)-N~'-
amino-N'''-(2-difluoromethoxy-phenylsulphonyl)-guanidine
and 2-ethoxycarbonylbenzenesulphonyl chloride are used as
starting substances, the course of the reaction in
process (b) according to the invention can be outlined by
the following equation:
HF2 ~ CH3 COOC2H5
NH-NH2
Le A 28 200 20 -
g ~ 8
~ OCHF
-HCl ~ 5O2-N~ ,NH--~ ~N
¦ OCH~
NH~ r==~
NH-5O
Cot)c2H5
If, for example, N'-(4-methoxy-6-methyl-s-triazin-2-yl)-
N''-(2-methoxycarbonyl-phenylsulphonylamino)-N'''~(2-
methoxycarbonyl-phenylsulphonyl)-guanidine and ammonia
are used as starting Rub~tances, the course of the
S reaction in proce~s (c) according to the invention can be
outlined by the following equation:
Le A 28 200 - 21 -
COOC~3 OCH3
r - ~ N ~ ~ NH3
S02 - N~ ,NH ~ ~N >
C N ~ - HOCH3
C~
NH
NH-502 ~ ~
COOCH3
COOCH3 OC~3
N
C N ~
¦ CH3
N~
r--~
NH-50
CONH2
Formula ~II) provides a general definition of the sul-
phonyl compounds to be used as starting substances in
proce~s (a) according to ~he invention. In formula (II),
Rl, R3 and R4 preferably, or in particular, have those
meanings which have already been mentioned above in
connection with the description of the compounds of the
formula (I) according to the invention as being pre-
ferred, or particularly pref~rred, for Rl, R3 and R4, and
R5 preferably represents one of the leaving groups
mentioned below
R6 -SO -N-oR7
2 1 or -Q-R , where
Le A 2~ 200 - 22 -
2 ~
R6 has the meaning mentioned above ~s being
preferred for Rl, but need not be identical to
R1 in each individual case,
R7 represents C1-C4-alkyl, C3-C4-alkenyl or benzyl,
R8 represents Cl-C4-alky:L, benzyl or phenyl and
Q represents oxygen or sulphur.
In particular, R5 repre~ents the group
R6 - S02 - N- oR7 ~ where
R6 has the meaning given above as being particu-
larly preferred for R1, but need not be identi
cal to R1 in each individual case, and
R7 represents methyl,
or R5 represents the group
_Q_R8 where
R3 rspresents methyl or phenyl and
Q represents oxygen or sulphur.
The starting substances of ~he formula (II) are known
and/or can be prepared by processes known per se (cf.
Le A 28 200 - 23 -
2 ~ ~ ~(3~ ~
EP-A 121,082, EP-A 172,957, EP-A 173,321, EP-A 173,956,
EP A 224,078, EP-A 5,986 and E$'-A 24,215).
Formula (III) provides a yeneral definition of the
sulphonylhydrazides further to be used as starting
substances in the process according to the invention. In
formula ~III), R2 preferably, or in particular, has the
meaning which has already been mentioned above in connec-
tion with the description of the compounds of the formula
(I) according to the invention as being preferred, or
particularly preferred, for R2.
The following may be mention~d as examples of the com~
pounds of the formula (III):
2-methoxycarbonyl-,4-methoxycarbonyl-,2 ethoxycarbonyl-
and 4-ethoxycarbonyl-benzenesulphonylhydrazide. ~
The starting substances of the formula (III) are known
and/or can be prepared by processes known per se (cf.
Org. Synth. 40 (1960), 93 - 95; EP-A 302,378; Eqypt. J.
Pharm. Sci 22 (1981), 207-221 - cited in Chem. Abstracts
100, 191704y).
Process (a) according to the invention for the
preparation of the new compounds of the formula (I) is
preferably carried out using diluents. Preferred diluents
which are suitable for this are water and/or polar
organic solvents ~uch as methanol, ethanol, isopropanol,
butanol, isobutanol, sec-butanol, tert-butanol, glycol
dimethyl ether, diglycol dimethyl ether, tetrahydrofuran,
Le A 28 200 - 24 -
2~3~g5~
dioxane, methyl acetate, ethyl acetate, acetonitrile,
propionitrile, dimethylformamide, dimethylacetamide,
N-methylpyrrolidone, dimethyl sulphoxide and tetramethyl.-
ene sulphone.
When carrying out process (a) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
temperatures between 0C and 150CI preferably at temper-
atures between 10 DC and lOO~C.
For carrying out process (a) according to the invention,
between 1 and 5 moles, preferably between 1 and 3 moles,
of sulphonylhydrazide of the formula (III) are generally
employed per mole of sulphonyl compound of the formula
~II).
In general, the reactants are combined at room tempera-
ture or with ice-cooling, and the reaction mi~ture is
stirred until the reaction i8 complete, if necessary at
elevated temperature. After cooling, the products of the
formula (I) are generally obtained in crystalline form
and can be isolated by filtration with suction.
Formula (IV) provides a general definition of the amino-
guanidinotriazines to be used as starting substances in
proces~ (b) according to the invention for the prepar-
ation of compounds of the formula (I).
In formula (IV), Rl, R3 and R~ preferably, or in
Le A 28 200 - 25 -
particular, have those meanings which have already been
mentioned above in connection w:ith the description of the
compounds of the formula (I) according to the invention
as being preferred, or particularly preferred, fo~ R1, R3
and R4.
The ~tarting 6ubstances of the formula (IV) are known
and/or can be prepared by processes known per se (cf.
EP-A 224,078, US-P (US Patent Specification) 4,725,303,
EP-A 343,462).
The aminoguanidinotriazines of the formula (IV) are
obtained when sulphonyl compounds of the general formula
(II) - above - are reacted with hydrazine or a
hydrazine/water adduct ("hydrazine hydrate") analogously
to process (a) according to the invention, if appropriate
in the presence of a diluent such as, for example,
methylene chloride, methanol or ethanol, and if
appropriate in the presence of a desiccant such as, for
example, sodium sulphate, at temperatures between -20C
and +80C, preferably between 0C and 50C.
Formula (V) provides a general definition of the sul-
phonyl halides furthermore to b~ used as starting
subs~ances in process (b) according to the invention.
In formula (V), R2 preferably, or in particular, has the
meaning which has already been mentioned above in connec-
tion with the description of the compounds of the formula(I) according to the invention as being preferred, or
Le A 28 200 - 26 -
2~ 3~
particularly preferred, for R2, and X preferably repre-
sents fluorine, chlorine or bromine, in particular
chlorine.
Examples of the starting substances of the formula (V)
which may be mentioned are:
2-methoxycarbonyl- and 2-ethoxycarbonyl- and also 4-
methoxycarbonyl- and 4-ethoxycarbonyl-benzenesulphonyl
chloride.
The starting substances of the formula (V) - as well as
the starting compound of the formula (VI), are known
and/or can be prepared by processes known per se (cf.
EP-A 173,320 and EP-A 173,321).
Process (b) according to the invention for the prepar-
ation of the new compounds of the formula (I) is prefer-
ably carried out using diluents. Suitable diluents forthis purpo e are virtually all inert organic solvents.
These preferably include aliphatic and aromatic, option-
ally halogenated hydrocarbons such as pentane, hexane,
heptane, cyclohexane, petroleum ether, benzine, ligroin,
benzene, toluene, xylene, me~hylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, chloroben7ene
and o-dichlorobenzene, ethers such a~ d~ethyl ether and
dibutyl ether, glycol dimethyl ether and diglycol
dimethyl ether, tetrahydrofuran and dioxane, ketones such
a~ acetone, methyl ethyl ketone, methyl isopropyl ketone
and methyl isobutyl ketone, esters ~uch as methyl acetate
and ethyl acetate, nitriles such as, for example,
Le A 28 200 - 27 -
2~4~ J~
acetonitrile and propionitrile, amides such as, for
example, dimethylformamide, dimethylacetamide and
N-methyl-pyrrolidone, and also dimethyl sulphoxide,
tetramethylene sulphone and hexamethylphosphoric
triamide.
Acid acceptors which can be employed in process (b)
according to the invention are all acid-binding agents
which can customarily be used for reactions of this type.
~he following are preferably suitable: alkali metal
hydroxides such as, for example, sodium hydroxide and
potassium hydroxide, alkaline earth metal hydroxides such
as, for example, calcium hydroxidel alkali metal carbon-
ates and alkali metal alcoholates such as sodium carbon-
ate, potassium carbonate, sodium tert-butylate and
potassium tert-butylate, furthermore aliphatic, aromatic
or heterocyclic amines, for example triethylamine,
trimethylamine, dimethylaniline, dimethylbenzylamine,
pyridine, 1,5-diazabicyclo-[4,3,0]-non-5-ene (DBN),
1,8-diazabicyclo-[5,4,0]-undec-7-ene (DBU) and 1,4-diaza-
bicyclo-[2,2,2]-octane (DA~CO).
When carrying out process (b) according to the invention,
the reaction temperature~ can be varied within a substan-
tial range. In general, the process is carried out at
temperatures between -100C and +100C, preferably at
temperatures between -70~C and +70C.
Process (b) according to the invention i~ generally
carried out under atmospheric pressure. However, the
Le A 28 200 - 28 -
~ iL7' ~
process can also be carried out under increased or
reduced pressure.
For carrying out process (b) according to the invention,
the starting substances required in each case are gener-
ally employed in approximately equimolar amounts. How-
ever, it is also possible to use one of the two
components employed in each case in a larger excess. The
reactions are generally carried out in a suitable dilu-
ent, if appropriate in the presence of an acid acceptor,
and the reaction mixture is stirred for several hours at
the particular temperature required. Work-up in process
(b) according to the invention i8 carried out in each
case by customary methods.
With the proviso that, in formula (I), R2 represents
optionally substituted alkoxy, this formula provides a
general definition of the substituted arylsulphonylamino-
guanidinotriazines to be used as starting substances in
process (c) according to the invention. In this case, Rl,
R3 and R4 preferably, or in particular, have those mean-
ings which have already been mentioned above in connec-
tion with the description of the compounds of the formula
(I) according to the invention as being preferred, or
particularly preferred, for R~, R3 and R4, and R2 prefer-
ably represents C1-C4-alkoxy which is optionally
substituted by fluorine, chlorine, methoxy or ethoxy, and
in particular represents methoxy, ethoxy, propoxy,
i~opropoxy, 2-chloro-ethoxy, 2-methoxy-ethoxy or 2-
ethoxy-ethoxy.
Le A ?8 200 - 29 -
2 ~
Tha compounds of the formula (I) to be employed as
starting substances in process (c) according to the
invention are new compounds according to the invention;
they can be prepared by processes ~a) or (b) according to
the invention.
Aqueous alkali metal hydroxide solutions which can be
employed in process (c) according to the invention are
solutions of alkali metal hydroxides, such as, for
example, of lithium hydroxide, sodium hydroxide or
pota~sium hydroxide, in water. It i~ preferred to employ
aqueou~ ~odium hydroxide solution in process (c) accord-
ing to the invention.
In process (c) according to the invention, ammonia can be
employed as a gas or in solution, preferably as an
lS aqueous solution.
Optionally substituted alkylamines which can be employed
in process (c) according to the invention are preferably
alkylamines which have 1 to 4 carbon atoms and which are
optionally substituted by fluorine, chlorine, methoxy or
ethoxy, in particular methylamine, ethylamine, propyl-
amine and isopropylamine.
If appropriate, process (c) according to the invention is
carried out u~ing diluents. ~esides water and alcohols
such as, for example, methanol, ethanol and isopropanol,
suitable diluents are virtually all inert organic
Le A 28 2~0 - 30 -
2 ~
solvents. These preferably include aliphatic and
aromatic, optionally halogenated hydrocarbons such as
pentane, hexane, hep~ane, cyclohexane, petroleum ether,
benzine, ligroin, benzene, to:Luene, xylene, methylene
chloride, ethylene chloride, chloroform, carbon
tetrachloride, chlorobenzene and o-dichlorobenzene,
ethers such as diethyl ether and dibutyl ether, glycol
dimethyl ether and diglycol dimethyl ether,
tetrahydrofuran and dioxane, ketones such as acetone,
methyl ethyl ketone, methyl isopropyl ketone and methyl
isobutyl ketone, esters such as methyl acetate and ethyl
acetate, nitriles such as, for example, acetonitrile and
propionitrile, amides such as, for example,
dimethylformamide, dimethylacetamide and N-
methylpyrrolidone, and also dimethyl sulphoxide,tetramethylene sulphone and hexamethylpho~phoric tri-
amide.
When carrying out process (c) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out attemperatures between 0C and 120C, preferably at tempe-
ratures between 20C and 100C.
Process (c) according to the invention is generally
carried out under atmospheric pressure. However, it is
also possible to carry out the process under increased or
reduced pressure.
To carry out process (c) according to the invention,
Le A 28 200 - 31 -
2 ~ 8
between 1 and 10 moles, preferably between 1 and 5 moles,
of al~ali metal hydroxide, ammonia or amine are generally
employed per mole of starting compound of the
formula (I).
In general, the starting compo~lnd of the formula (I) is
introduced first, at room temperature, if appropriate in
a diluent, and the aqueous alkali metal hydroxide solu-
tion, the ammonia or the amine is then metered in, if
appropriate at increased temperature. The reac~ion
mixture is stirred until the reaction is complete and
worked up by customary methods (cf. the Preparation
Examples).
The active compounds according to the invention can be
used as defolian~s, desiccants, agents for destroying
broad-leaved plants and, especially, as weed-killers. By
weeds, in the broadest sense, there are to be understood
all plants which grow in locations where they are unde-
sired. Whether the substances according to the invention
act as total or selective herbicides depends essentially
on the amount used.
The active compounds according to the invention can be
used, for example, in connection with the following
plants:
Dicotyledon weeds o~ the genera: Sinapis, Lepidium,
Galium, Stellaria, Matricaria, Anthemis, Galinsoga,
Chenopodium, Urtica, Senecio, Amaranthus, PoEtulaca,
Le A 28 200 - 32 -
Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania,
Ambrosia, Cirsium, Carduus, Sonchus, Solanum, Rorippa,
Rotala, Lind~rn:ia, Lamium, V~eronica, Abutilon, Emex,
Datura, Viola, Galeopsis, Papaver, Centaurea, Trifolium,
Ranunculus and Taraxacum.
Dicotyledon cultures of the qenera: Gossypium, Glycine,
Beta, Daucus, Phaseolus, Pisum, Solanum, Linum, Ipomoea,
Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
Lactuca, Cucumis and Cucurbita.
~onocotyledon weeds of the qenera: Echinochloa, Setaria~
Panicum, ~igitaria, Phleum, Poa, Festuca, Eleusine,
Brachiaria, Lolium, Bromus, Avena, Cyperus, Sorghum,
Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria,
Eleocharis, Scirpus, Paspalum, Ischaemum, Sphenoclea,
Dactyloctenium, Agrostis, Alopecurus and Apera.
~onocotyledon cultures of_ the genera: Ory~a, Zea,
Triticum, Hordeum, Avena, Secale, Sorghum, Panicum,
Saccharum, Anana~, A~paragus and Allium.
However, the use of the active compounds according to the
invention is in no way restricted to these genera, but
also extends in the ~ame manner to other plants.
The compounds are suitable, depending on the concentra-
tion, for the total combating of weeds, for example on
industrial terrain and rail tracks, and on paths and
squares with or without tree plantings. Equally, the
Le A 28 200 - 33 -
2 ~
compounds can be employed for combating weeds in peren-
nial cultures, for example afforestations, decorative
tree plantings, orchards, vineyards, citrus groves, nut
orchards, banana plantations, coffee plantations, tea
S plantations, rubber plantations, oil palm plantations,
cocoa plantations, soft fruit plantings and hopfields,
in lawns, turf and pasture land, and for the selective
combating of weeds in annual cultures.
The compounds of the formula (I) according to the inven-
tion are particularly suitable for selectively combating
dicotyledon weeds in monocotyledon cultures, both by the
pre-emerg~nce and the post-emergence method.
The active compounds can be converted into the customary
formulations, such as solutions, emulsions, wettable
powders, suspensions, powders, dusting agents, pastes,
soluble powders, granules, suspension-emulsion concen-
trates, natural and synthetic materials impregnated with
active compound, and very fine capsules in polymeric
substances.
These formulations are produced in a known manner, for
example by mixing the active compounds with extenders,
that is liquid solvents and/or solid carriers, optionally
with the use of surface-active agents, that is emulsify-
ing agents and/or dispersing agent~ and/or foam-forming
agents.
In the case of the use of water as an extender, organic
Le A 28 200 - 34 -
2 ~
solvents can, for example, also be used as auxiliary
solvents. As liquid solvents, there are ~uitable in the
main: aroma~ics, such as xyl.ene, ~oluene, or alkyl-
naphthalenes, chlorinated aromatics and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloro-
ethylenes or methylene chloride, aliphatic hydrocarbons,
such a~ cyclohexane or paraffins, for example petroleum
fractions~ mineral and vegetable oils, alcohols, such as
butanol or glycol as well 2S their ethers and esters,
ketones, ~uch as acetone, methyl ethyl ketone, methyl
isobutyl ketone or cyclohexanone, strongly polar sol-
vents, such as dimethylformamide and dimethyl sulphoxide,
as well as water.
As solid carriers there are suitable: for example ammon-
ium salts and ground natural minerals, such as kaolins,
clays, talc, chalk, quartz, attapulgite, montmorillonite
or diatomaceous earth, and ground synthetic minerals,
~uch as highly disperse silica, alumina and silicates; as
solid carriers for granules there are suitable: for
example crushed and fractionated natural rocks such as
calcite, marble, pumice, sepiolite and dolomite, as well
as synthetic granule~ of inorganic and organic meals, and
granules of organic material such as sawdust, coconut
shells, maize cobs and tobacco stalks; as emulsifying
and/or foam-forming agents there are suitable: for
example non-ionic and anionic emulsifiers, such as
polyoxyethylene iatty acid esters, polyoxyethylene fatty
alcohol ethers, for example alkylaryl polyglycol ethers,
alkylsulphonates, alkyl sulphates, arylsulphonates as
Le A 28 200 - 35 -
2 ~ S 8
well as albumen hydrolysis products; as dispersing agents
the following are suitable: for example lignin-sulphite
waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and natural and
synthetic polymers in the form of powders, granules or
latexes, such as gum arabic, polyvinyl alcohol and
polyvinyl acetate, as well as natural phospholipids, such
as cephalins and lecithins, and synthetic phospholipids,
can be used in the formulations. Further additives can be
mineral and vegetable oils.
It is possible to use colorants such as inorganic pig-
ments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dye-
stuffs, and trace nutrients such as salts of iron,manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1 and 95
p~r cent by weight of active compound, preferably between
0.5 and 90~.
For combating weeds, the active compounds according to
the invention, as such or in the form of their formula-
tions, can also be used as mixtures with known herbi-
cides, finished formulations or tank mixes being pos-
8 ible.
Suitable herbicides for the mixtures are known
Le A 28 200 - 36 -
2 ~
herbicides, such as, for example, 1-amino-6-ethylthio-3-
(2,2-dimethylpropyl)-1,3,5-triazine-2,4(lH,3H)-dione
(AMETHYDIONE) or N-(2-benzothiazolyl)-N,N'-dimethyl-urea
(METABENZTHIAZURON) for combating weeds in cereals; 4-
amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one
(METAMITRON) for combating weeds in sugar beet, and 4-
amino-6-(1,1-dimethylethyl)-3-methylthio-1,2,4-triazin-
5(4H)-one (METRIBUZIN) for combating weeds in soya beans;
furthermore also 2,4-dichlorophenoxyacetic acid (2,4-D);
4-(2,4-dichlorophenoxy)-bu~yric acid (2,4-DB); 2,4-
dichlorophenoxypropionic acid (2,4-DP); 3-isopropyl-
2,1,3-benzothiadiazin-4-one-2,2-dioxide (BENTAZONE);
methyl5-(2,4-dichlorophenoxy)-2-nitrobenzoate(BIFENOX);
3,5-dibromo~4-hydroxy-benzonitrile (BROMOXYNIL); 2-
chloro-N{[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino]-
carbonyl}bsnzenesulphonamide (CHLORSULFURON); N,N-di-
methyl-N'-(3-chloro-4-methylphenyl)-urea (CHLORTOLURON);
2-[4-(2,4-dichlorophenoxy)-phenoxy]-propionic acid, its
methyl ester or its ethyl ester (DICLOFOP); 4-amino-6-t-
butyl-3-ethylthio-1,2,4-triazin-5(4H)-one (ETHIOZIN); 2-
{4-[(6-chloro-2-benzoxazolyl)-oxy]-phenoxy}-propanoic
acid, its methyl eqter or its ethyl ester (FENOXAPROP);
[(4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)-oxy]-acetic
acid or its l-methylheptyl e~ter (FLUROXYPYR); methyl 2-
[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-lH-
imidazol-2-yl]-4(5)-methylbenzoate (IMAZAMETHABENZ);
3,5-diiodo-4-hydroxyben20nitrile (IOXYNIL); N,N-
dimethyl-N'-(4-isopropylphenyl)-urea (ISOPROTURON);
(2-methyl-4-chlorophenoxy)-acetic acid (MCPA);
(4-chloro-2-methylphenoxy)-propionic acid (MCPP);
Le ~ 28 200 - 37 -
20~a~8
N-methyl-2-(1,3-benzothiazol-2-yloxy)-acetanilide (MEFEN-
ACET); 2-{[~((4-methoxy-6-methyl1,3,5-triazin-2-yl)-
amino)-carbonyl]-amino]-sulphonyl}-benzoic acid or its
methyl ester (METSULFURON); N-(1-ethylpropyl)-3,4-di-
S methyl-2,6-dinitroaniline (PENDIMETHALIN); 4-ethylamino-
2-t-butylamino-6-methylthio-s-triazine (TERBUTRYNE);
methyl 3-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-
amino]-carbonyl~-amino]-sulphonyl]-thiophene-2-
carboxylate (THIAMETURON); 5-2,3,3-trichloroallyl N,N-
diisopropylthiolcarbamate. Surpri~ingly, some mixturesalso show synergistic action.
A mixture with other known active compounds, such as
fungicides, insecticide~, acaricides, nematicides, bird
repellants, plant nutrients and agents which improve soil
structure, is also possible.
The active compounds can be used as such, in the form of
their formulations or in the use forms prepared therefrom
by further dilution, such as ready-to-use solutions,
suspensions, emulsions, powders, pastes and granules.
They are used in the customary manner, for example by
watering, spraying, atomizing or ~cattering.
The active compounds according to the invention can be
applied either before or after emergence of the plants.
They can al~o be incorporated into the soil before
sowing.
Le A 28 200 - 38 -
2 ~ 8
The amount of active compound used can vary within a
substantial range. It depends lessentially on the nature
of the desired effect. In general, the amounts used are
between 0.01 and 10 kg of active compound per hectare of
soil surface, preferably between 0.05 and 5 kg per ha.
The preparation and use of the active compounds according
to the invention can be seen from the following examples.
Preparation ExamPles:
Example 1
COOC2H5 CH~
SO2-NH~ ~N-~ ~ N
¦ OCH3
NH
NH-5O
C OOC 2H 5
(Process (b))
A mixture of 7.5 g (18.3 mmol) of N'-(4-methoxy-6-methyl-
s-triazin-2-yl)-N''-amino-N'''-(2-ethoxycarbonylphenyl-
sulphonyl~-guanidine, 2.28 g (20.3 mmol) of diazabicyclo-
[2,2,2]-oct~ne (DABCO) and 100 ml of methylene chloride
i cooled to -70C, and a solution of 4.53 g (18.3 mmol)
of 2-ethoxycarbonyl-benzenesulphonyl chloride in 20 ml of
methylene chloride is added dropwise with stirring to
Le A 28 200 - 39 -
this mixture. Afker the cooling bath has been removed,
the reaction mixture is stirred for a further lS hours
(at the end at 20C). It is then diluted with 100 ml of
methylene chloride and washed twice with approx. 200 ml
of water each time. The organic phasP i5 dried with
magnesium sulphate and filterecl. The solvent is removed
from the filtrate by distillation under a waterpump
vacuum, the residue is brought to crystallisation with
ethyl acetate, and the crystalline product is isolated by
filtration with suction.
6.3 g of a first fraction of N'-(4-methoxy-6-methyl-s-
tria~in-2-yl)-N''-(2-ethoxycarbonyl-phenylsulphonyl-
amino)-N'''-(2-ethoxycarbonyl-phenylsulphonyl)-guanidine
are obtained. The mother liquor is concentrated, and the
residue which remains here is recrystallised using
ethanol, during which process a second product fraction
is obtained (1.55 g). Total yield: 7.85 g (69 % of
theory); melting point: 165C.
Other examples of the compounds which can be prepared
analogously to Example 1 and following the general
description of the preparation process according to the
invention are those of the formula (I) listed in Table 1
below.
R4
C N ~ (I~
NH r~ COR2
~NH-SO
Le A 28 200 - 40 -
~ !
~ I
O I
:C ~ ~ ~ U
O O O O O O
I
T S tr)
P I O O ~ O O U
_~
H
N
U
I ~ U U ~ U ~
I O O O O O O
O l O O O O O O
S ~ ~, I U o ~ U tJ
~ o 8 1 _ ~ N N N t\~
O Pl I I
U~ I
O
C~ I
O
U
I u~ S
~ ~ ~ ~ ~r T
O N ~ N t )
I O O ~ ~ ~.1 0
~ S~ ~ S~ b ~b ~b ~
..
~ ~ I
I
. I
E~l X z ¦ N ~
Le A 28 200 - 41 -
c~ l
o
~ - l
~ c l ~o ~ ~ - -
~ o l
C4 1
ll
T T 3 T
O O O O O
I
T :C ~t'J T T
ll
ll
U'~
(`~ r~.
a ~N ~ N ~
ll
,0 1 .
b b b b
~ ~ I
~ ~.1
E~ ~ Z I ~ ~ _ _
Le A 28 200 - 42 -
c~ l
o
D`-- I
~ o l
~ ~ l
T T T, S T
U U U ~ U
O O O O O
t~
T
T U T 5 U
D; I U O U ~ O
I
U') U~
S ~q T T
S t~
U U U ~ U_
o I o 8 8 $ o
.,, ~ U U U ~ U
~ N I l I
O U I -- _ N N
P~ I
1 t'~
' ` u
~o~ U~b~ b~
~ ~ I
~ ~ I
Li ~' Z ~
Le A 28 200 - 43 -
2 ~
- l
u l
o
~
~ o ~
~ ~ I
D I S ~ ~' T
U U U U U
O O O O O
U o O
I
U~
~r~ X
r ~ T ~
U U U
3 , u u u u u
O U I N ~ N N ~
I T r
g
I
_ ~1 1
_I I
0~ 1
~ ~ I
E-~ X ~ -- N
Le A ?8 200 - 44 -
2 ~
U I
o I
.,, ~,
~, ~ I~ o ~ ~ ;~,
a) o I ~ ~ ~
X ~,
P: , S :C T
U ~.) U ~ 1 ~
O O O O O O
~ ~7
"~ I T trl ~ ~ T T
:C S T
O ~ U O O
~ U'~ U-
X ~
N ;: N S :C N
U U U
O l O O O O O O
r~ I O O O O O O
Ul O I
O U I N N N N N N
N N
OI -- _
~ T
I N N
~ oZN oN
~ b b ~b ~b ~
~ ~ ,
~ ~ I
. I
~ X o
E~ ~ Z ~ ~ u~
N t~ N N N N
Le A 28_200 - 45 -
c~ l
o
~ - l
~ N
a) o I
I
~' I U o oU T
I
.. - U I S U IU
ll
ll
It)
T ~ ~N r
~ I O O O O O
o I O O O O O
~ U
U~ O ¦ N N N N N
O U I ~ ~ -- _ _
~ I
I
ll
rl I S 2 2 N
S
O o rl ~ o ~,
UbUbU~ U~
_~ ~ I
~ ~-1
0 X O I ~ O ~ N t~
E-l ~ Z I N
Le A 28 200 - 46 -
g 5 $
U I
~ _ t
3 ~ t ~ 0
~ ~ t
t
tt ,."
g O O O O
r S T T
~ I ~J O O O O
t u~
T ~q T ~1 T
~ ::C N T
t o o o o o
.~ t
~ ~. , _ _ _ _ ~
,, ~ I , , , , I
UJ O I N ~
O U I ~
' t
t u ~ T
t 1 8 ~ N ~ N I ~L I
tu~ ~, `10 13 `O `O ~
Q~
E~ ~Z
Le A 28200 - 47
_ 1 2 ~
U
o
~,,
~ O I
, , T T T
O O O O O O
1'') ~ ~ ~ T T
T T
~ O O
I
ll
U') 11~ U')
T ~ t'-~
N T ~ T T
O O O O O O
O l O O O O O O
~ I _ _ _ _ ~ _
Lq O I N N N N N N
O ~
I
L~
O I T
._~ I N
~ I t" ~" t" f'l ~
~I b b b b ~ ~
~,
~ ~ I
L ~
E- ~C O I ~ o _ N
Le A 28 200 - 48 -
~ I
o
c l
O O
~ ~ l ~ o o o ~ ~ ~
~ o~ l
1 2 ~ 5 T ~ T
I ~,1 U C. U ~ U ~`
O O O O O O C
t') ~'I
I T
T T U U C~
~ I t.. l U ~_) U O O O
I
I
~ r
N U U N U
O N -- O
t'') N ~ ) N
C ~ ~ U C T
~ U U N t~ U U
T T -- N
'r T
U U ~.1 0 t ~ t ) U
~ I O O O O O O O
I O O O U O O O
U U U I U U U
o O I ~ N N N N N N
~ I I
I C S 2
U t.l U N t~ N
I O O O O ~ U
O O O O O O O
~o~ buo~
~ ~ ,
,
~ ~ I
I
E~ X Z ~ O` o
Le A 28 200 - 49 -
~,, 2 ~
~ I
.,, ~ I
., " , C o t~ C
, o I
~ ~ I
I
o o o
r7 j I T T t'~
O O O O O t_l
T
_I ~ N
O _ ~
N N t7~ N
~r T C T C
N N _ T N
O I O O
~rl ~ ¦ ~ ~ _ A _ _
U~ O I
O C~ I N N N N N N
ll
I
o ~ ~N N N
~ b~bbb
~ ~, .
I
. I
E-l 1~1 Z I N ~
Le A 28 200 - 50 -
2~6~8
~ I
o
..,
u O -- N ~ ~r
I:i, I
I
~ T S og I T
I
T X
ll
N N
~')N N
~ C ~ ~ ~ T
~N U O O U
ll
ll
~ ~ b b b b b
, ~ I
~ ~-,
E-~ w Z ~ ~ ~ o _ N ~
Le A 28 2~0 - 51 -
^, 2~GO~
o~
o~ o o
lll
O O O ~ ~ r
I
~ T ~
N W W
o I N ~ ~ N N N
O ~ X
Y ~b C~ W o~b ~
l ~,
hl W Z
~ o ~ o o
Le A 28 200 - 52 -
~ I 2~
~_ I
C
,, ~ I
C I ~, ~, U. o
Q) O I ~ ~ ~ ¢~
ll
T
O O O O O O
D I S S :S: S S :r:
E
O I ~ ~ ~ ~ S
1~ C I T = T T ~J (`J
3 N I O O O
O U I
0 ~4 1 I N N N ~ N
~ I
C~ I ~ ~
C I _ t.~ S o N
¦ T U t~ Q S r
~ T ~ ~] U ~
~ ~ 1 b b ~b b uo o b
~ ~ ,
~ ~ .
E~
o ~ ~ ~ ~
Le A 28 ? - 53 -
2 ~
u l
o
cr-- I
C I ~ `D ~
` ~ 0 ~o 0
O I
~ ~ l
-- T _ T T
I
~ ~
T -- T T -- --
O t~
I
C I O O O O O ~
O l O O O O O O
J ~ I _ _ _
U~ O I N N N N N ~
O U I -- _ _ _ _ ~
~ I I . .
C C C C
C I ~ S S ~
0~ o~ 0~ 0~ o~ 0
u ~ W ~ 1~
_~ C) I
C) Q~ ~
~ ~ l
r~ x o l ~o ~ ~ ~ o
E~ ~ ~ ' t~ ) 0
Le A 28 200 - 54 -
_ 1 2 0 ~ 8
U I
o
,, ~ I
O ~ O`
o I
~ D. I
I
~ I S S T S S T
U U U U U ~1
O O O O O O
~1 t'l
T T
u SU U O O
I
I
U
N
U) C C SU
~ ~ S
S t`J t~ S t~
C: I U U ~ U ~ ~
O l O O O O O O
~-1 1 0 0 0 0 0 0
~N I U U ~ I U U U
U~ O l
O t~ 1 t~ N
I
S S 5
U ~ ~ U
O O O O
S :C
3 I N ~ S --~ ~ X
u u ~ u T
o I o 1 8 1 o 1 8 lu 8 lu
~ I U~ ~ ~ ~ ~ ,~ U~
o ~,' ~J l W ~J W W
~ ~ ,
~ ~ '
.
E~ ~ Z ~
0 0
Le A 28 200 - 55 -
2 ~ , 8
~, I
o
~_ I
~ , 0 ~J
_I-rl I
o ~
,
T
~ I o U U
I
T ~ ~ .~
~, I O ~ U O O U
U ~ C
N tq T t~) tll ~
:C S N :~: S ~')
tJ U U U U U -
O O O O O O
o I O O O O O O
.,~ I U U U U U U
~ N ¦ ~ '` -- -- -- _
o I N N N N N N
¦ U ; 5U 2U UN o U
o ~ o 1 8 I g I g I
1 ~ U~OU~13U~OU~
_, a) I
~ ~ I
. I
la X 0 1 0 ~ O _ N t'l
E~ ~3 Z ~ ~ ~ o~ o~
Le A 28 200 - 56 -
2 ~
~ I
o
~ ~ l 0 o ~o o
--~-.1 1 O c~ ~ CO r~
~ o l -- --
ll
u u ~ u ~ u
o o o o o o
I
~ ~ T S U U U U
C ~ _
T T N
.~ ~ U oUO Uo O Uo
~ N ¦ U U U U ~_I U
O U I N N N N N ~
P~ I ~ _ _ _ _ _ _
N _ _ _
-- ~ U U
O I U UN UN U C C
N N N 3 T
I o 0~ J~ ~J~ u~ ~)~
~ b~ww
~ ~ I
~ ~-1
E~ ~ zo
Le A_28 200 - 57 -
2 ~
- l
u l
O I
ll
T T 3~ T T
O O C~ O O O
O O O
N t.l
C X ~
tr~ T -. T S
O I O O o o o-
.~1 ~ I , , , , , ,
O U I ~ _ N N N N
1:4 1 1 --
ll
C C C C C C
I S r C T ~-
u= ~ Ub ~b ~b ~ ~ ~
~ ~ ,
h ~1 Z I O O O O O
Le A 28 200 - 58 -
()) ;3 ~
_
o
-- I
o a~
~ 0 t~
,, .," _
~ o
~ I I ~ T T T T
O O O t~
t~ t'''l t'~'1
T ~ ~ t'~
~,~ T U
~C I O U O O U t_)
I
t'q
N N _ ~
U
r~ ~ N
T . T
~ ~ U U U~
T
T T T 1
O O O O O O
O l O O O O O O
~ N
~ ~1 1 1 1 1 1 1 1
o 8, _ _ ~ N N ~
.
S I C C C C C C
O ¦ ~r T ~ C T
1 o o o O O O
b ub ub ~b ~b ~b
~I x z, o o o o
Le A 28 200 - 59 -
2 ~ 5 8
o
~ _ ~ _ 0 N 0
.-1.~1 1
I
T ~ 5 T
T S - T T
ll
N _ C.l N t l
-- U N t' ) N
~ U U S U
S -- N N --
U U U ~ T 5
I t.) U U ~ U t_~
~ N
o I N N N N N N
~ I I
T
C C C C N N
_/ ~ ~
~01 ~ o ~
Le A 28 200 ~60 -
2 ~
~~!
~ ~; 0
S N N S S
PC I N N S S N N
lll
p, U N ~ , ~ U
~b au ~ ~b ~b ~`b U`b
z ~ N N ~ N
Le A 28 200 - 61 -
ul ~a6~85~
o l
'~I ~ I N -- q~ N IJ- O
~11 0 1
T ~ ~ r~ ~ ~1
N ::: S T S
U U
O O t~
ll
U ~J U U U U
I
ll
~r) T ~ T ~) T
N U U
U U U U ~
o ~ N N N N N N
I
O I 11~ 11)
~ N 5 N N N
J X b b b b ~ b
~ ~ I
E-~X ~ ~ N N N N
Le A 28 200 -62 -
5 ~
o
~_,
o ~ r~
~ ~ t ` ~ ~ ~ 0 0
~ o I _
t
u~
S~ ~ N ~ ~' .
U U ~ t~ U
O O O O
U~
S ~ S :~: N N
I
I
t
u~
S N ::C N S N
~J ~ ~ ~ t~ u
o o o o o o
l o o, o o o o
o N N N N N N
.~ I U ~ S
0 1 ~ ~ O O 1
a~ b ~b ~ b ~
, ~ t
_, I
E~ ~ O I
Le A 28 200 - 63 -
2~$~g
c~ l
o
c
N `~
O I
æ ~ I
I N 2 r
U U ~ U ~ ~
2 2
O O O O ~
~ O
t" N N
C ~: 2 2
U ~
O O O
N N N N N N
I
N N
~-~ Sbb~b~b~
Le A 28 200 - 64
2~ 3~j~
o
-- I
!
I
U ~ ~ T ~, I
ll
N ~
C :C" N
N
N UN ~.~ U o o
o I N N N N N N
N N N N
N
8I Q u u T U T U
N
aI u ~ t.l u
~ Ub ~b b b ~b ~
~~ ,
I
,
0X o, ~ .
E~~ Z I
Le A 28200 - 65 -
2 ~ g
~ l
_~ ~1 1 _ _ _ _ _ _
~ o l
x ~ l
I
S ~
~ l o o ou o uo uo
T 2
~: I O O O O O O
ll
(~
C S ~ T
~' T t~J T
U ~ ~
C: I O O O O O O
I O O O O O O
~e~ I _ ~ _ _ _ _
o ¦ ~ ~`J N ~ N t~
P,l I
I
N N N
U U U U U U
N N N
~ ~b ub 8b Bb 8o '~J
~ ~ I
E~ ~ Z ~
Le A 28 200 - 66 -
2 ~
._,
o
~,,
._1 ~ I ~ N c~ t`l
1: 1 ~ ul ~ N --
O) O l
T T T T ,'
U t.) U t_) U
O O O O O O
I
T T T ~
U ~J U U ~ U
~: I O O O O O O
t
r
-- U N
U O
N N 1'')
2 T
U U U~ ~ U
N N t~
~ ~ N ~r~ T
O I oU oUO UO o o UO
r~ I U U U U ~J U
~N I _ _ _ _ _ _
o O ¦ N t~l N N N N
I I
I
N N
lrJ ~'1
I U S
S S C C C C
~ I U U -- -- -- --
O I ~ ~ T
O O OU O U O
.~ I u~ u~ u~ u~ uJ~ u~
UO ~ W
_~ ~ I
Q~ ~4 I
E~ ~Z ~
Le A 28200 - 67 -
~) l
.rl ~ I
C I ~r ~9 o ~ 1~ ~O
'~ I
~ o ~
x ~ l
S ' S ' ~ T
U t U t~ U ~
O O O O O O
t
I
S S S
I U ~, ~, U U ~,
o o o o o o
~ ~ _ U
U o _ ~ o
N ~ t~ N N
C ~ ~ U
(`J N S --
~ T S
U ~ U
I O O O O O O
o O ~,,) ~ _
U~ O I
O C t I I`J N N N N N
~4 1 _ _ _ _ _ _
C 1: N N N N
C I _ _~ C S T ~'
O
.~ I 5 5 N N N N
S ~ U
~ ~b ~b ~b ~b ~b ~b
~ ~ I
a~ ~ I
I
h x o I O ~ N t~
_~ _ _ _ _ _
Le A 28 200 - 68 -
2 ~
I
~ ~ l o
~ o l ~
ll
S 1 T
., S S T
t.~ O O O O
C C S
~ S ~r _
3 1 o u
~n o I
o y I N N N N N
~: ~ S N ~ S N S N r N
3 ~ U u~
u= ~ b b b Ub ~
~ ~ !
~ ~,
~ ~ .
~ X o , ~ ~ ~ ~ o
E~ ~3 Z ~ `D ~D
Le ~ 28_200 - 69 -
_, 2 ~
~u
.,, ~,
2 S T T
~ I c~ T ~ ~ ~ U
ll
N ~'I N
~ j O o o U
O U I N N N t~ N N
lll
N N N N N
T S ~ -- T
J~ U U U ~ U
N U 3 U ~ ~JN
b ub ~b Ugb UOb U~
-'I I
_ E
EX X I -- N
Le A 28 200 - 70 -
a O ?''
O
~: I
O ~ ~ -- O
~ O O`
o U
,~, 1 3~') t~ X S ~ T
C T
U U
O C~
N
t I ~ C C C
b ~ b b b
,
~ ~ I
~ ~ .,
1:~ ~ O I 1~ 0 O~ O ~ N
Le A 28 200 - 71
~ou ~ 3 5 ~
O o N a~ 'r
O O O O O
~y ¦ U N N N N
u ~. ~. u
I
I
..
o
N
o ! o o ~ N
~ N I ~ U
U~ O I I I I ~ ,
o ~ I N N N N N
lll
O
I N
O
_~ ~) I
Q &o 1I Q 0 CO
Le A 28_200 - 72 -
~ ? j~ ~ ~j g
o
C ~ _ N
I
U U U U U U
~ U~
t.l U t~ U C.~
u~
o I U U ~ ~ N ~r '
.rl I O O O O O O
~a o I
O U I N ~ (~ N
O I 11~ T
:~: 2
O O ~ Z
a ' 8 1 _ _ IL I oN
U P: I `O 1=/ ~=/ b o `Ib
_ a~ I
_I
a ~
~ ~ I
~ x o I 0 o~ o _
E~ ~ Z 0 0 ~ ~ o~
Le A 28 200 - 73 -
I
I
o
~ l
c
.~ J~ 1 0 ~ ~n ~o O ~
0 t~ ~ 0
O I
T r ~ T S
,~ I U U U ~ U
O O O O
I
T S :C
~q ~ T U Uo U U U
t~'t f') ~')
S I 2
U ~ U U
O U O O
N N N N
S T T T
S
~ ~ S
U U U U ~ U
O O O O O O
O ~ O O O O O O
U U U U U U
U~ C~ N N N t~i
O C~
I
~ ~ U U
I _ -- N C N
T T U ~ U
I U U ~
S tr~ ~ T
~ I Z Z ~ U U U
I O i N 1 8 1 , , o
~J w
I
_, a~ I
~ ~ l
~ ~ l
E~ X z I ~ O` ` ~ ~ _
Le A 28 200 - 74
2 ~
u l
o
~ - l
~ co o ~
x ~ l
~ U U 5 ~r T
U t~ O U ~I
N ~
~- T ,N N
~ N ~ .) O
O I N N N N N N N
P~ I I
~x ~ b ~b b
, ~,
x Z t '' g N N
LQ A 28 2nO - 75 -
5 8
U,
O
o
~ ~ I
~ I X T 'r ~ T
o o o o
U O U
U~ O I N N N N ~
O y ~ _ _ _ _ _
I
ll
U~
V b b ~ s ~
~ ~ o
E-~ X ~Z; I o O O _ ~
Le A_28 200 - 76 -
2 ~
O I
o
~ O I
x p~ l
T
O O O O O
I
~r ~
O O O
N
U
O O O U U
-1 ~: I I I I I I
o ¦ N N N N N
I
t~
u ~ ~ e b b
X O I --~ N N N N
Le A 2 8 ? Q
206~3a8
I
~ I
~ ~ I
I
.,, I
O ~
I
I
~ ~ r~
~ I U T T T U U
O O O O O O
t~ C T
T U U U U
~2 1 U U O O O O
i
r TN S T N T
O O O O o oU
O lO O O O O O
~rl I U U U U U ~)
U~ OI N N N N N N
O CJI -- -- --_ _ _
~ I I
I
I
O ~ ~" T ~
1 5 ~ U
U N U N U N
I O I O I O I O I O I O
~ U~ ~ U~ ~
11 I l l 11 l
V' ~ V ~J ~ ~J
_~ ~ I
_~ I
~ ~ i
,q ~ o ~ ~
~ X O I ~ N N N
i~ i~l Z ' N N N N N N
Le A 28200 - 78 -
2 ~
o
I
,~ I r~
o i - ,
O O O O O O
(r~ tq trJ ~ 5 5
X ~ r T ~ ~
S ~ S ~ U
o I N t~ t~l N t~l
ll
ll
C ~ ~
~ b ~ ~ o ~,
_~ ~ I
_I I
E~ x O I ~) N ~ ~ N
Le A 28 200 - 79 -
2 ~ 8
o
~ ~ l
~ o l
x ~ l
T ~C T T T
~ I O O O O O O
I
I
T 5 T U
U O ~ O U O
U U U U
N N
I I
I
t~
_
I C C 5 S C C
I X -- -- ~ X
~b ~b ~b Ub ~b ~
~ ~,
~2 ~ I ` -- N tr~
E1 ~ Z I ~ ~ ~ N N
Le A 28 200 - 80 -
-
~,
o
-~ J (`1 0 0
J c: ~r ~r) O N
Q) O ,~
~:: D.
~:
~; ~ $ :r: o
C~
r~
U~
(~7
C: O O O '~
O ~ O
J ~
o , N N
O I N `~ -- N
~ _ _
O N IN I N
,~ O O O
~ O O O
~ 3
U ~
~ ~-
a ~ ~ ~ 0
~ X o ~ ~ ~ ~
E~ ~ z N N N N
Le A Z8 20û - 81 -
s ~
o
c
N 0 0 N t~
J C ~ t~ O ~ (~`
r~ ~1 ~ _~
tl~ t~
~ O.
~r X ~: X X X
O O O O O
X X X X
N
N t~
~ _ ,n C
:1: C I X
~ tr C~ J
O O O o o , X 1l~
J O O O C~ O
~; C~
'0 ~
O I N N N N N
_ ~
N N N N N
t') tr~ tr~ t~ tr~
O C
.,~ O U C~
J _ z z z Z
N N N N N
o ~ ~~3 ~3 ~3 b ~3
., .~
tD O.
_~ ~ tJ~ o _~ t~ t~
X O ~ ~ ~ ~ ~
~ ~ Z N N N N N
Le A 28 200 - 82 -
2 ~
-
t~
G)--
C 11~ ~0 N 00 N
J C: t~ tr) ~D U) ~'
_~
O t~
:~: a.
tr~ tr~ tr~ tr~ tr
X
~r c~ U
K O O O O O
t'l t" tr~ tr~ t`l
tr
t~
tr~
~ t~ N
C~ O
t`J N ~'~
~ C :~ c
C~ O t` t~ t~
~J ~J :~ -- X
X
C ~ ~ t~ U U
O O O O O O
O O O O O
t~
r~ O ~ ~ ~ _ _
U~ ~
O I N N N N N
N N
C tr~ trl t~ ~ t`~ ~r t~ X
O I~
.~ z z ' ~ ` '
:~ N N N N N
C O I O I O I O I O
u ~ ~3 ~3 ~3 ~ 3 ~3
tl~ ~.
X o ~ ~ ~ ~ ~
N N ~ N
Le A 28 200 - 83 -
2 ~
t~
Dl `-
C O U~ O t_
J C t`
~ .,. ~ ~ ~ .
tD o
:~ t~,
t~ X
~; o o o o
t`~
O L~ t~ t
-- In C
t~ ~ ~ ~ t~
t ~ t~ t~ ~C
:r: X 11~ :C
t t ~ t ~ ~
o o o o o
,.tl o o o o
J ~ t~ t~ t~ t~
rl O l ^
o I t\l t~ t~ t~
D~
t~ tr~ ~ t'~
C t~ ~r t~ '1 1 t~
o I
"~ '~ t~lo '~ , '`
~3 ~3 ~3 ~3
., tD
t E'
t~ o -~
ro X O
7~ t~ t~ t~ t~J
Le A28 200 - 84 -
2 ~
t~
t
~, J O ~ t~ ~
J ~: O` t~ In O
_ ,~ ~ ~ ,_,
tU O
.
tr~ tr) tr) t~
t_~ t~ t~U
~; o o otn
t~ tr3
t~ $ :r: t~
'r; ~ o 't~ t~
t~
u t~ t~
O o o
t J
tr :I: tr
~ J ~ t'~
t~ t,~ t~ t~
o o o o 8
J ~ t_~ t_) U t_)
~r~
Ul t~
O I N t\l t~ t~l
t~
trJ~
C t~ X t~ tT) ~r
o I t ~ r t~
.,- t~ O ~ t~ O
\ ~ Z ~ t~
t~l t~ t~ ~
C O I O I O I C~ I
c ~ ~3 tn ~3 ~ ~3 ~3
,, tu
tU D.
_~
tti ~ t~
1~ X O U~
1~1 7~ t`J t~ t~ t`J
Le A 28 200 - 85 -
2~$~
t,
D)-- D
J 0 N D,
J C r~ ~ L ~r 0
~ ~ O ~ ~
o
D, ~a
X
~; U~ O O O
t'~ ~ X X N X
u~
~ 3
o o o o o o
"~, o o o o o
J ~ t~
.,~ O _
~ I I I I
O ' N N N N N
~ _ _ _ ~_ _
C U~
.,~
J 1 J t'~ N
1~1 t~
u ~ ~3 1~3 1 3 ~3 ~
a) D,
D (~ ~ t' 0 ~ O _-
1~ X O Il~ u U~ `D ~
E-~ [~ ~Z N N N N N
Le A 28 200 - 86 -
~"
C r~ t1` ~ oD O
C O O O O N
~ .t ~ ~1 ~ ~ ~
tV O
t~.
:I:t'l rr) r'l r
O r ) O O t~
1~
r~ r~ r~
r'~ ~ r: t`~ :C X
~; r~ r ) r r~ U
,_ N ~4
U -- r~
t~
r~ U~ rX
~) tJ ~'1 -- (
r X X S X S
O O O O O O
O O O O O
J 11:; r~ r~ r_) r_) ~
.,~ o _ _ _ _ _.
U1 ~ I I
O I I N N N N N
,.
N ~
-- N U U
~: t`l -- N N
o r~ r~ r~
,~ -- r~ N N
J
r~ Z r~ r~
:~ O t\l O O
-~ I ~ O I O I O I O
u ~ ~ ~3 ~3 ~3 ~3
tV E~'
,R ~ t~ r~
,a x o `D ~ `O ~ `D
E~ k~ Z N N N N t J
Le A 28 200 - 87 -
2 ~ 5'
Dl ~ ~ O 11~ 0 ~D
J ~:: ~ _l O O LO
.1 .
tD O
S o,
tr~tr)t'l
~ $ :c
C
~ O tn U~ tJ~ O
t~
$ 1:
a; o t~
C $
~ U> t~
X 3: t'l ~C ~ J
(" ~`~ $ t J
C ~J t ~ t~
O O O O O O
-'t~ O O O O O
~) ~ t~ ~ ~) t) C~
,. O _
I I ~ ~ ~
O I N t~ N N t`1
tL _ _, _ _ _
~ t'~
~,~ 3: 'r tr~ I
J t~ t~l
O ~ 0~
o ~ J
t
tD 1:1,
_. e
.0 ~ ~ ~ t~` O _~
X O
~d ;Z N t`~ N ~`J N
Le A28 200 - 88 -
2 ~
-
t~
c
J I` t~ ~r O
a7 ~ _I tJ`
~ .~ ~
tD O
7,
tl7 U7 ~U> t~7
X N N :~
O O O O
r7 t~7 t77
t'~
~; C,~ C~ t~ O
t~7
t O
t~
t `~ (~7 X ( J
~ t J
C U ~
~-~t J o o O O
~; t_l t~ t,~ C~
.,~ o _ _ _ _
~7 ~
t7 1 N N N N
Il, ~
C t~7 X t~7 3 ~C
O ~ t_) N
.,~ t~7 ¦ ~.) O L7 0 ~ t~7
J X N ~ S 2
t~ ~ æ z ~,~ t~
::~ O ~1 N N O ,---
C O I O I O I o~ I
c ~3 ~3 ~3 ~3
t
tu
L7 ~ t J tl~7
X O ~' t' ~ t`
1~ ; N t J N N
Le A 28 200 - 89
-
~) ` -
t~ t' ~ `D t~
.J C t'` N oD O tS~
tl) O
:~: Q.
u~ In
r~ t~ t`~
t`~ t~
~r u
O O O O O
Ln U'>
t'l trl ~ t~
t" t-~ U ~" N :I:
u~ In In U~
~ X ~J
C:
O O O O O O
O O O O O
~r1 0 I ^ ~ _ _ _
~n ~ I I
O I N t~ N N t~
t~ t'~ t" Lr~
tt) tr~ tr) t,~ t_) C.)
c ~3 ~ ~ 3
~
. tD
a~ ~.
_I E
~ CD ~
O
~ X o ~ ~ t~
t~ t~ 7 t~J t~ ~J t~ tJ
Le A 28 200 - 90 -
ru l ~
r~l - u~ t~
~ c o o o o
~1
a~ o
~ Ln
:c ~ x x
N 3: ~`1 N
t~
O O O O
~
(~
~ ~ O
C
In t~ u~
t~
O O O O O
~(`1 O O O O
J ~:; t~
-~ O l ^ -- _ _
O I ~ N (\~ t`J
tl~ _ _ _ _
:~
.,,
~ X 3:
.~ ub ub U~ U~X~
..
I D.
_ k
, ~ ~ . t`3
I X O o~ CD ~ 0
;~
Le A 28 200 - 91 -
.
2~$~
.
t~
,,
C o~ o
t~ U~ o o
. ,, ~ ..
~ o
X Q.
t,~ tr) ~ t~
X ~ X
~:r u ~ t~ t~ t ~
O O O O O
tl~ t~ ~ t~) t~l
to; tX t~ t~
t3
t~ O t~
t_~ ~) t~
_ _ ~ J
~ X
U t~ t~ t~ t~
Q O O O O O
~'J I o o o o o
; I t~ t ~ t ~ t~ ~
-~ o ~
ID t.l
O I t~J N t` J t~
tl. _
.- ~ xU~
t~ t~ tr~ ~ t~
(~ U~
O- I O I g I O I O-
c 1~3~3 ~3 ~3 ~3
I
¦ G.
Ll ~ ~ 1~
~C X t~ a:~ 0 0 0 ~1
~d
Le A 28 200 - 92 -
20~58
t~ I
t~, I
c
._~ J O N ~ O N
C O ~ ~ t'` ~O
,~ ~ .1 ~ _~
tl) O
Q.
t~J tT~ t~) t~ t~)
X 3: :C
U U U U U
~; o tn o o o
t'l trJ
t" t`l ~ X ~"
t~) ~ ~ u t.,~
D~ U u O O U
t,
I
O
(`~ C C C
X ~ _I
U U~ ~1 ~ .
t~
C I U U U ~ U
O l O O O O O
O O O O O
J ~ t.) U U U U
. _~ o _ ~
I I I ~ I
O I N N N N N
tL.
c ~In
O
~, U t~ t~ X
J ~ ~ N 3:
U--U,- U U U
O ,-' tr~ O O O
c o~ o I o I o
D.~ ~3 ~ ~3 ~3 ~ 3
., t
to o
,, E
.a ttl --~ N tt~ ~
~ x o t~ tJ~ tJ~ o~ tr
E~ ~ N N t`~ t`~ t~
Le A 28 200 - 93 -
20~$58
c
.,~ J O ~ ~o O
'J C er ts~ o~ t~
.
O O
t~
U C~ ~ U
O O O O
~r) t~
r~ ~: t~
~'1 5: C~ U :C
~; C~ O O C~
~ X X X
:I: U ~'~ V t~ U
~l~ X ::C X ~ X X
C I U U---- C ~ C )~ _) U-~
O l O O O O
-~] l O O O O
J~ I U U U U
~-l O l ^
10 ~ I I I
O ~ I N t~ t`J N
~ _ ~_ _ _
t~ u~ In
.,, X ~ X t"
r~ X
u U ~) u
~C I g I O I O
~ ~ c~3 u~3 c~ ~3 c~3
~ to
t~ o
,
R 111 ~ 1/~ ~ t~ t~
1~ X O t~` tl` t1` tJ`
rJ ~ t~ r~
Le A 28 20Q - ~4 -
5 ~
~,
o
~ ~ ~ 0 ~ ~ ~
~ ~ o~ o 0 ~ 0
~ .,. ~ ~
~ o
X o.
1:) t~ ~ t~ t~
U U C~
o o o o o
t~ tr~
'c~ ~ g g t~ ~
~`J N t`J
(') ~" t~
U t~ t'~
U~
I~ X
(J ~ t~ t.~ t~
U t~ t~ t~ tJ t~
c c~ rJ o o u o
O O O O O O
~t ~ O O O O O
J ~ O t~ t~ t~ L~
~-~ O l ^
Ul U I
O I ¦ N N N N N
.~1 X t~l ~ t~ 1:
J t~ t~
o O o O
c~ ~3 ~3 ~3 ~ 3
U ~
Q~ O.
O --I t~J t~
t~ x o ~ o o o o
W :~; N t`~ t~ ~ t~
Le A 28 200 - 95 -
2 ~ 0 ~ s~
-
o
r~ ~ S10 0 N O
J C 0 u ~ S!` SJ` S~
_ ,1 ,1 _~ ~
Sll O
:~: S~.
~ ~ SY~
$ :r: X s~ N
U U U U U
~r; o o o o o
:r:
(') U
~ O U U ~ U
~: S~ C
S~` O` S~
~ ~ q' I SJ
U U U U U
O O O O O O
O O O O O
u ~ u u u
,~ O _ _ _ _ _
O I ~ N ~ N N
~ _ _ _ _ _
S~l N
t~
~rl u u
U U U Z Z;
3 O O O N S~
C: O I O I O I O I O
c ~ ~3 ~3 ~3 ~3 ~3
.- a)
~ o
_, E
u~
x o o o D o o
ld ~ s~
Le A 28 200 - 96 -
2~6~8
~,
c
,~ J U~
C a:~ co O`
.
~) O ~ oD
O. ~O
LO
N ~ :1: $
O :C U C~
O ~.) O ~') O
O $
O
~
rl :I: X :~:
t~
O ~')
C ~ ~
X ~ ~ C :I:
C
O I O C~ O :~: O
~ l O O O ~ O
J 1~ O t~
o I _ ~) -- O
~ _ I O
O ~ N I N ~,) N
_ ~
~_ I
N
~ t" t')
In U~
.,~ X ~: O O O
J ~ ~`~ O O O
~3 ~3
C) o.
. o o ~ ,
~ X o o
E~ td % ~ ~
Le A 28 200 - 97 -
2 ~ 5 Y
~,
~,~
c ~ o o~
J C: C~` O O Cl` O
_ ,- ~ ,~
~ O
:~: o,
~ ;~
X
t~
O O O O O
X ~ X X
~ ~ ~ ~ C~
a~; o o o o L~
t`~
r
--~ ~n C u~
C
a` U !
~ J
C ~
O O O O O O
~" I O O O O O
^
Ul ~.) I I I I
O ' I N N N N N
CL, j -- _ _ _ _
X 5: X ~ X
C
O O O O O O
._~ O O O O O
J
., ~ ~ ~
c ~ L-~' I=,U' L-i~ L-~U' L-~
l ~
_I D.
rO ~ In ~D t~ 0
I X O ~ _I ~ ~ ,~
Le A ?8200 - 98 -
2 ~ 5 ~
r~
D)--
t
-~ ~ N tr1 ~ ~r
~ ~ ~ 0
_ .,,
t~ o
~: u.
t~) t~ c~
.~ C~ CJ ~1
~; O O O O
tr~ t~ tr~ t
t_1 U
~; O O O O
N
t~l
N ~r
u
t~ _
C ~: C
t~ C~ tJ` t )
C C) ~ t) L~
o O O O O
O O O O
I C) tA~ ~ C_~
~-~
0 C
O I N N N N
D~ ~_ _ _ _
t~ tr~ ~ t~
c r~ X t~ X t~ ~ t~ 5
O C~
.,., ~ o c~ o t~ o c~ o
ZN N
c O I O I O I O
D ~ ~,3 ~3 ~:3 ~:3
I t"
tl E
.L ~ 0~ O ~ N
¦ X O ~ N N N
;~; t~ t~ t~
LeA 28 200 - 99 -
-
2~8~
-
o
~,~
C O ~ ~D `D
J C co t~` ~ O
~ ~ ~ ~4 ~ .~
t~.
t'~ t7 tr~ t'~
tS S
~: O O O O
X I'~ ~ ~ tr~
t7 C,) t,) t.) ~
~; O O O O
t.~
t~ ~ C
S --~ C
L)" ~,3 (-~ X
C I X ~: X
O I O O O C:
.,,-; O O O O
~; t~ L~ t~ t_~
,1 o
Ul
O I t~3 t~ t`J t~
0
t~l
t7 t7 t7
t7 ~ t7 Y t7 ~r t7
O S t~ S t ~ :~ C,) S
.~ '` ` '`:1 t~l N t`~ t~l
o ~ ~:3 n~3 n~3 n ~3
.. t~
to E
t~ ~ In ~
X O N t`J N t~J
t~) t7 t~) t7
Le A 28 200 - 100 -
8 ~ ~
~ I
o I
~,
C N ~ ~ ~'I ~
C: ~1 0 ~ ~D O
O
X
~:; O O O O O
X
~; O O O O O
(~
-- U) C
C X
O l O O O O O
O O O O O
_l O ~ _ _ _ _
U) ~,)
O ~ N N N N N
i:~ ~
N N N N N
C
O ~
J _ _ _ _ _
Z Z Z Z Z
~1 ~ N
o ~ l b ~3 ~3 ~~:3 ~3
t~
.
E
.4 ~ ~ ~ 0 o~ o _~
1ll X O N N N ~ ~')
Le A 28 200 - 101 -
2 ~
w~ -
.,~ ~ tJ` O U~ X) t5`
J ~: ~ tJ` t~
,~ ~ ..
O
O.
t~ t~ tr~ tr~ tr~ t~)
t~ t_~ tJ t~ t~ t~
O O O O O O
t~ tr~ t`~ tr~ t~
~ X t~
tr~ t_~ tJ t_~ t_~ t~
r~ o o o o o t~
ll
I
U
t`~
t~
~ t~ tJ
C t_~ t_l t ~ t~ ~) t)
O O O O O O O
O O O O O O
J ~ I ~ t~ t~ t~ t~ t~
_~ o _ _ _ _ _ _
O I N N N N N N
t~ _ _ _ _ _ _
l l l l I
N N N N (J
Z ~ ~ ~ ~-t~
N ¦ l l ¦ O
O I t~ t~ O
~, '~ ~ O O O O t~
_~ ~ ~ O O O O t~l
1: l t_~ t_~ N N 1:
O .~ l ~ tl~ ~ t_~
~ ~,~ . 3~ $ X~ $
_~
a) o.
_ E
.o ta (~ t~
ro X O tr) t~l tr~ t l t
!; t1 ~ ') t')
Le A28 2Q0 - 102 -
o
t~.
~r~ ~ O t~
tJ` t~ O O t
t~
tD O
O.
t~ t`l t`l tr~
X
t~ O O O O
t~ t~) t~l tY1 t~
tYl
t
t,.~ ~ t_~ t,~ ~
O O O O O O
-lt`~ O O O O O
~J ~ t,) t~ t~ t~ t~
`~ O I -- ^ _ _ _
tl t ) I
O l N N N N N
N N
t~ 3 3 t ~ N
~ ~ O ~ O ~
~ 3 ~3 ~t
N
_l tD
I O,
¦ t~ ~ t~ tJ` O --~ N
I X O t'~ tl~
Z t~
Le A 28 200 - 103 -
~ ~ 43 ~
I
o I
C N 0 ~D t~
O oD t~ 0 tt`
O
Q,
tr~ tr) tr) t~ t')
~!:; O O O O O
t" trl tr) t'~
~: :c X :r: t~
t'~ t~
~ O O O O
u) In
t~ X t1 ~r: t'J
:C t~ ~: t ~ :C
t:: I U t~ ~ O t_~
O l O O O O O
.,~r~ O O O O O
J ~:; C~ ~ U C.~ C~
-1 0 1
Ul t,) l l l l l
O I N N N N N
N
C~ I
,_ tY~ t~ ~
O , ~ I ~ r ~ I
`,( ~ t J :~ t~ O ~ ~ t~
J ~ t_) I O O t~ 5
Il~ O ~ O ~ ~ O 0 ~1
::~ O I O I ~ ~ O
~3 ~3 ~ ~3
~ tu
,
~ R.
R 111 tr~ ~ 1
,~ x o ~ ~r
; t') t~ t~ t'~
Le A 28 200 - 104 -
2~.3~
~ I
t,
~" _
C I co N t~ 1~~
t~l ~ ~ ~~D t'`
~) c a~ ~ ~ ~ ~
~ .~
tll O
Q.
tr~ t~ trl tr) trl tr
U U U t~ C~U
O O O O O O
t') tY~ t~l t~) ~`'I t`l
t'l I :C X
~ I W U t~ Ut 3 C~
U~
X t') X t'') ~ t~l
t ~ ; t~ ~ t
C I U t~ U t~ U t~
t~ I O O O O ~ O
~-~'J I O O O O O O
I U ~ t~ t) t~ t~
-' O l -- ^ ^ _ _ _
In ~,I I I I I I I I
O I I t\~ N t~ N t~ t~
~, I _ _ _ _ _ ~
:~ '
U t~)
~3 ,~3 It'~, It~, I
O ~It~ ~0 ~o ~3
Og~ r ~r
.,, u~ t~ t~ O u g
J l ll tllt`~ O O t~
e 1 11 ~ u u u
o .~~ ~ U U t')
~- ~; ~ _ _ X
~, tl~
tl) ~.
_ E
0 a~ o ~ t\~ t~
~o X o ~ ~ U~
E~ ~ t'~ t~ t~ tr~ t~
Le A 28 200 - 105 -
C N ~D 1' ~ O
._. ~ tt` O oD t~`
J ~ ~
tU O
tr~ trl ~ tr~ t~
X
O O O O O
t'l t~l t~l t~
r~ ~ t~ O
U~ U>
t`~ I t~
t~
o I o o O O o
O O O O O
J ~; I C~ L) ~ ~ _
O ~ I t\l t~ t~ t~l t~
L~
1~ I(J It~ J l~i~
J~
~ 0 ~ O
C O O
,~ tr~ tr)
J C~ t~ O O
C: t~ t~ O O
O
C C X L~
t` t`
C C
.4 tD
I ~'
L IT~ ~ ¦ ~ IS~ ~ t`` CO
x o I u~ ~ In
~s~ % ~ t~ t~
Le A 28 200 - 106 -
- l
c~
D)--
C N O 11~ ~ t~
.,~ J t' 0 1~ ~ t~)
J ~:
tU O
:~: t~.
tY~ t" t~ ~" tr)
~) t_~ t~
~ O O O O O
t~ tr~ ~'1 t~) t~
~r~ ~ ~ t.
~ O O O O O
I
1: t'~ I:t'~:C
t ~ t ~ ~ t `~
C I t~
t~ l O O O O O
~-'(J I O O O O O
J ~ I t~ t~
-' O l ~
0 t~
O I N N N N N
tl. _ _ _ _ _
t, It,
I:
~ 1 r~3
~`~ t`~ ~ t`~ t'~ ~`J
O ~ O ~ O ~ ~ ~
c ~3 ~3 ~3 ~ ~
.,, C C
o ,,
U ~
.~ tU
tU Cl.
_l
.a ~ tJ` O ~ N trl
X O U) `D
~rl Z t'~ t'~ t~l t~
Le A 28 ? - 107 -
2 ~
~ I
u
~) -
c o ~c 0 ~ 0
` ~I J ~ t_ Cl:l CO
~) C
a) o I
: o.
~r
O O O O O
t~
~'~ t~ $
O O L~ C~
~ x
C (J ~ (J
~: I U
Ol O O O O O
] I O O O O O
'-' O I ^
LO ~ I I
I I ~`J
~ ~ t~
~ ~30~U~3 U1~3 Ub
., i
I ~
I o.
i ,~ . ~ U ~ t 0
I X O ~
Le A 28 200 - 108 -
~ I
C I ~o u~~ `D
.,~ J ~tl` ~71 t`
_ ~ ~ ~
~ r~
~ O
o.
1 t~
o o o o
t~ t')
~ ~o ~)ro o
o I o o o o
._", I o O
J t~
-~ O l -- _ _ _
O I I ~ N ~`J N
t~ to
~ N
C~ U
C
o ~ o
~,~ ~ O ~)
J 8 I g I / '~ o
~3 ~3 ~ 3
U ~
.4 tD
O~ O.
_.
.q ~ ~ o _~
O `D t~ t` t~
t~
LeA 28 200 - 109 -
c~ l
C I ~ tr~ o o
~-~ ~ o ~ ~ ~
~ c ~ ~ ~ ~
x o
t~ tY~ tr~ tl7
L~
o~ o o o o
t~ t~3
t, I t~ :C~ ~ t~
o c~ o c~
x
o o o o o
o o o o
J ~ I ~ t~ ~ t~
~ O ~
O I t`J N N t~
~ _ _ _ _
t\~
_ t~
t~ _
:r: tT~
O ~ t`~
,~ ~ Z
.. ~ O O t.~ O t~l
O I O I O I O
-~ ~3 ~3 ~3 ~3
U ~;
Q)
Q El'
~R ~ ~ t~ ~ U~ `D
1~ X O ~
E~ ~ t'1 t~ t'l t')
Le A 28 200 - 110 -
~085~
,
t~
~,
C tr~ o o
.,~ ~ O O t~ N
~ .
_ ,1
tD O
O.
tr1 t~3 t" tr
X X
t~ [~ U
~; O O O O
t'l t'~ tll t~,
t~ I t~ t~ t~ t~
O O O O
C
O l O O O O
-lt~ l O O O O
J 1~ t_) t~ t~
~,1 0 ~ _ _ _
U) ~.)
O ~ t~ t'l t~J t~
D, _ _ _ _
_ t~
t~ O
t~ t\~
t~ t~ tr~ I
O ~ ~ ~ t~l
.,., t_l t_~ t~
$ 1 8 1 o '~
.c '~
U ~
.4 tD
I E'
r ~ ~ t~ 0 t~ O
t~ X O I ~ tO
Le A 28 200 - 111 -
2 ~
t~
too
t......~ ~ In
t~t~` o
J ~: ~
._1
tu n
x o~
tr~t~ ~ tr~
x
t~
~ o o o o
t~
t~ x 3~ :r
t~ ~ t.. ~
o o t~
I
I
I
t~ C X :1:
o l o o o o
-ltJ I O O O O
I t_) t ~ t~ t~
-l O l ^
tn U ~
O ' I N N N N
N
t')
X
t~ N
X X t~
t_) N X
N t_~ t~ t )
X X X t~ ~
,1 t~ t~ ~ Z
t~ O 0'''1-- t~ I O
c t~ ~ t~,~ o ~J tn~
~ tu
tu o.
-~
R t~ ~ ~ t~ t~
I X O tS) 0 t~ a~
1~ Z t~ t') [" t
Le A 28 200 - 112 -
2 0 ~
-
c
.,. J
J ~:
~ ,.
a~ o
3,
S~
~ U U
~; O O
t~) U ~:
U
ll
O
O O
D~ I u U
~ O l
O l (`~ (`1
L
r
U~ ~ O U r~
.~ Z U--U,
D 0~ 0
U ~;
., a)
a nl
t~
Le A 28 200 - 113 -
9 8 .~ ~
Startinq compounds of the formula (IV!:
Exam~le (IV-ll
COOC2H5 OCH3
N ~
5O2-NH-C=N-~\ ~ N
NH~ CH3
~H2
A mixture of 6.5 g (15.3 mmol) of N'-(4-methoxy-6-methyl~
s-triazin-2-yl)-N''-(2-ethoxycarbonylphenylsulphonyl)-S-
methyl-isothiourea, 0.77 g (15.3 mmol) of hydrazine
hydrate and 150 ml of methylene chloride is stirred for
30 minutes at 20C and then stirred with magnesium
sulphate and silica gel, and subjected to filtration with
suction. The filtrate is concentrated, the residue is
stirred with diethyl ether, and the product which is
obtained in crystalline form is isolated by filtration
with suction.
4.9 g (78 ~ of theory) of N'-(4-methoxy-6-methyl-s-
triazin-2-yl)-N''-amino-N'''-(2-ethoxycarbonyl-
phenylsulphonyl)-guanidine of melting point 141C are
obtained.
Le A 28 200
- 114 -
Use Exam~es:
Example A
Pre-emergence test
Solvent: 5 parts by weight of acetone Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active compound,
l part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added, and the concentrate is diluted with water to
the desired concentration.
Seeds of the test plants are sown in normal soil and,
after 24 hours, watered with the preparation of active
compound. It is expedient here to keep constant the
amount of water per unit area. The active compound
concentration in the preparation is of no importance,
only the amount of active compound applied per unit being
decisive. After three weeks, the degree of damage to the
plants is rated in % damage in comparison with the
development of the untreated control. The figures denote:
0 % = no action (like untreated control)
lO0 % = total destxuction
In this test, a powerful action against weeds combined
with good tolerance by wheat is shown, for example, by
the compounds of the Preparation Examples l, 4, 5, 6
and 7.
Le A 28 200
- 115 -
Example B
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1
part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the
desired concentration.
Test plants which have a height of 5 - 15 cm are sprayed
with the preparation of the active compound in such a way
as to apply the particular amounts of active compound
desired per unit area. The concentration of the spray
liquor is so chosen that the particular amounts of active
compound desired are applied in 1,000 l of water/ha.
After three weeks, the degree of damage to the plants is
rated in % damage in comparison to the development of the
untreated control. The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, a powerful action against weeds combined
with good tolerance by wheat is shown, for example, by
the compounds of the Preparation Examples l, 4, 5, 6
and 7.
Le A 28 200 - 116 -