Note: Descriptions are shown in the official language in which they were submitted.
W 1 ~~~~nA
COMPOST3'ION FOFt CE2~lEldl'IP1C~ OII, TrdEhhs
A'f ~OTJ Z'EMEERATURE
The present invention applies to the field of oil and
lp associated industry services, and, in particular, that of
cementing the annulus of an oil, gas, water, geothermal or
analogous well.
Such cementing operations, as well as their importance
for the life and production of the well, have been known to
the man of the art for several decades and there is no need to
reiterate the principle and details thereof.
It will simply be mentioned, to permit correct
understanding of the text and of the invention, t hat it
involves injecting a cement slurry into the casing of the
well. This cement slurry goes downhale through the casing and
then, under the pumping pressure, returns upwards through the
annulus between the borehole and the casing, up to the surface
of the well. There exist perfectly conventional method) fax'
ensuring that, upon completion of pumping, the in~t~.c~e of the
casing contains an inert fluid, and not the s:l.urry. The cement
slurry is -then allowed to set.
The general object of the operation is to maintain the
casing in the well, to isolate the underground areas traversed
and to consolidate the well itself.
The cement slurry is pumped under pressure, and passes
from the on-surface preparation conditions tambient pressure
and temperature) to the downhole conditions (higher pressure
and temperature), part of the slurry then returning through
the annulus to Conditions close to those of the surface. Under
certain conditions, in arctic areas or in submarine wells in
- 3
the North Sea, for example, the surface conditions can be very
cold and ensuring that the slurry sets correctly poses quite
special problems.
Despite such pressure and temperature cycles, the slurry
must, of course, remain pumpable; it must perfectly displace
the drilling mud (a critical factor in ensuring that the
cementing operation is carried out correctly) , it must not
sustain any fluid loss in the areas it passes through, and it
must set as swiftly as possible, but precisely at the point
intended, i.e. up the height of the annulus, and under no
circumstances, of course, inside the casing. Finally, the
hardened cement must possess excellent mechanical properties
and, in particular, good compressive strength, which must
develop as quickly as possible~after setting.
When the well passes through a gas-containing area, it
is extremely important for the hydrostatic pressure exerted by
the column of fluid in the annulus to remain greater than the
gas pressure in the formation throughout the cementing
operation. The density of the drilling fluids, as well as that
of the cement, are thus adjusted accordingly.
Once the slurry is in place, it will begin to set. This
setting is firstly reflected by the gelatian of the slurry.
Bonds are created between the cement particles, as well. as
between the cement and the wal:Ls of the wel:L. Owing to these
bonds, the column of cement supports itself, which is
accompanied by a reduction in the.hydrostatic pressure exerted
opposite the gas-containing area. During this period, the
viscosity of the cement is not yet sufficient to resist the
migration of gas bubb7.es. The end of this period corresponds
to hydration, which is an exothermal reaction between the
cement and the water reflected by a temperature ri se, the
development of compressive strength and the chemical shrinkage
of the system. During this phase, the pressure opaosite the
gas-containing area decreases even further and space is
created in the form of porosity in the cement matrix, through
which the gas can then migrate.
CA 02062183 2002-12-02
- 3 -
Throughout the setting time, the cement must be capable of
withstanding invasion by the gas, which would have serious
consequences, ranging from poor cementing to a disastrous gas
blowout on the surface.
The mere enumeration of the requisite properties, which are
often mutually conflicting or contradictory, the temperature and
pressure conditions, and the serious or disastrous consequences
arising from failure to observe the properties, gives an
indication of the difficulties involved in developing a slurry
having the properties in question.
The invention provides a cement slurry that possesses all
of the properties enumerated above, and which is thus
particularly suitable for the most difficult wells, notably
those that pass through gas-containing areas, and having low
surface temperature, i.e. the wells that accumulate the greatest
difficulties.
According to one aspect of the present invention there is
provided a cement slurry composition for oil, gas, water,
geothermal and analogous wells, comprising cement, 66 to 150%
alpha plaster by weight of cement, 15 to 25% microsilica by
weight of cement and plaster, and 30 to 50% water by weight of
the cement and plaster. The invention also provides for the use
of such a cement composition in cementing oil, gas, water,
geothermal and analogous wells. The cement slurry compositions
preferably contain, apart from the mixing water:
- alpha plaster,
- ordinary Portland cement (API class A or C),
- microsilica, and, insofar as necessary,
- a retarder and a dispersing agent.
Cementing a well at low temperature (in particular <30°C)
presents very special difficulties. In particular, it is
difficult, in the case of such wells, to obtain reasonable
setting for the cement slurry after several hours and
compressive strength in the order of 3500 kPa after eight hours.
It should be noted that the setting time is the time required
CA 02062183 2002-12-02
- 3a -
for the consistency of the slurry measured using a consistometer
in accordance with API Standard No.lO to reach 100 Bc (8c:
Bearden consistency).
Known accelerating agents, such as calcium chloride, can
provide a solution, but with a slow increase in consistency up
to 100 Bc and, when setting commences, it takes several hours
CA 02062183 2002-12-02
- 4 -
for the cement to harden. During this lapse of time,
unacceptable shrinkage occurs and there is a possibility of gas
migration. In addition, below 15°C, it is very difficult to
obtain acceleration.
It is already known (USP 3,891,454) to use plaster
(semi-hydrate calcium sulphate, CaS041/2H20) in a cement slurry
to reduce the setting time. This involves replacing the cement
by a plaster-cement mixture in a mass ratio ranging from
approximately 50:50 to 75:25. However, the addition of plaster
to a cement slurry can entail serious drawbacks.
In the first place, the setting time depends very much
on the type of plaster. In particular, most plasters are far
too reactive in the presence of cement, and we often observe an
acceleration of setting at low temperature, which is, in the oil
industry, a serious drawback (see above). The over-fast setting
can be compensated for, in certain cases, by a large quantity of
setting retarder, but then setting can take days.
In the second place, plaster/cement systems have
practically zero filtrate control. Finally, plaster is known to
be a thixotropic agent in the case of cement, whereas, according
to the invention, a fluid slurry is sought after.
Microsilica has already been used in a cement slurry
(GB 2,179,933; GB 2,212,150; GB 2,212,489). This is a by-
product derived from the manufacture of ferrosilicons. The
microsilica particles have a mean size of approximately 0.15
microns and a specific surface in the order of 20 mg2/g. Owing
to the difficulty involved in manipulating fine particles in a
raw condition, it is preferred to use microsilica in suspension
in water.
However, at low temperature and in the range of
concentration in which it has to be used, the microsilica
prolongs the cement setting time and delays the development of
compressive strength, thereby further impairing one of the
essential properties of gas migration control.
G'~ a ~~ ~) ~.
-
Unsurmountable drawbacks were thus encountered in prior
art .
The invention provides a system that solves for the
first time the cumulative problems posed by cementing at low
temperature (approximately 0-30°C) and the need for a system
capable of ensuring good gas migration control.
There are known to be two main categories of plaster
alpha and beta type semi-hydrate calcium sulphate, hereinafter
referred to as alpha plaster and beta plaster. Their
difference originates essentially from the preparation
process. Alpha plasters are prepared under controlled steam
pressure, whereas beta plasters are produced using a
continuous fluidized bed process. Alpha plasters have an
apparent density of approximately 1000 kg/m3, with spherical
particles, whereas beta plasters have an apparent density of
approximately 600 kg/m3 and take the form of platelets.
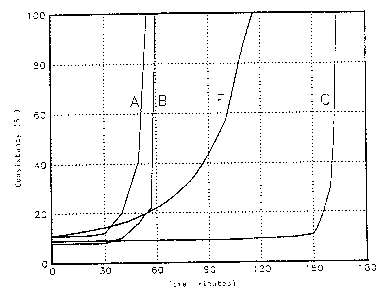
Figure 1 below shows the development of consistency as a
function of time for different types of plaster. The operating
conditions are as follows: 60 o plaster, 90 j cement, API
class A, density 1.89 kgll, with testing at 20°C.
Curves A, B and C correspond to alpha plasters and offer
the highly desirable characteristic of r,ight~-angle set. Two
beta type plasters, U and 1r, led to uncontrol.J.ed flash getting
immediately upon mixing. Curve F Corresponds to a beta plaster
for. wh:Lch it proved possible to obtain a reasonable setting
time through the use of retarder without, hawevez~, obtaining
either a right-angle set or good compressive strength in t he
short term.
A right-angle set correspond to a very fast increase in
consistency at the time of setting, only a few minutes being
taken to increase from 30 Bc to 100 Bc.
It will be remembered that right-angle setting is one of
the parameters that plays a part in gas migration control.
These results show that the use of alpha plaster in the
present invention is of absolutely prime importance.
CA 02062183 2002-12-02
- 6 -
Figure 2 shows the influence of temperature on setting
time, in the case of a system with 60 % alpha plaster, 40 % API
class A cement and a density of 1.89 kg/1. Unlike cement, the
setting time of which is reduced as the temperature increases,
the opposite is observed in the case of a plaster/cement system.
The aforegoing shows the complex nature of the physico-chemical
mechanisms involved in the invention.
Table 1 hereinafter groups together the results obtained
concerning the thickening time (TT) and compressive strength
(CS) after 8 or 48 hours. Use was made of an alpha plaster
available from Lambert Corporation, an ordinary Portland cement
(CPA-HP), slurry density 1.89 kg/1, temperature 20°C, 0.3 % by
weight of dispersing agent (sodium/formaldehyde polynaphtalene
sulphonate or "PNS") and the retarder is sodium or calcium
lignosulphonate.
TABLE 1
Plaster Cement Retarder TT CS at 8 CS at 48
Weight Weight % Weight h:min h h
% % kPa kPa
60 40 0.6 3:50 8015 13405
50 50 0.6 3:35 7700 15750
40 60 0.7 3:17 6160 13335
70 0.8 3:52 1575 12320
25 The larger the quantity of cement, the shorter the plaster
thickening time. However, a retarder such as lignosulphonate
makes it easy to adjust the thickening time, under the test
conditions according to Table 1 above.
Furthermore, it is very clearly observable that compressive
strength after 8 hours is ensured by the setting of the plaster
and that the useful range for the alpha plaster in order to
obtain compressive strength in the order of 3500 kPa at 8 hours
is beyond 30 % by weight of the mixture.
According to the invention, it is essential to incorporate
into the plaster/cement system a considerable
quantity of microsilica, in particular for applications in
which gas migration control is required. The proportion of
microsilica will be preferably between 15 and 25 ~ by weight
approximately of the plaster/cement mixture. '.Chi.s quantity is
required owing to the need to ensure that the system has a
filtrate control value of less than 100 cm3/30min (see Table 2
hereinafter). The use of microsilica in such proportions has,
surprisingly, only a minor effect on the increase in the
rheology of the system, which can easily be reduced through
the use of a conventional dispersing agent of the "PIS" type.
It is further observed that the presence of microsilica makes
the slurry particularly stable, which is reflected by the
complete absence of free water and sedimentation.
During development of the invention, serious
difficulties were experienced in connection with gelation
problems when using certain oil well cements of class G in
particular. This drawback is removed if use is made
exclusively of Portland cement (APT Class A or C).
Furthermore, preference should be given to ordinary Portland
cements.
To obtain sufficient compressive strength within a short
space of time (8 hours), and in the presence of high
microsilica concentrations, it is essentla.l to use in tl:~e
system a sufficient quantity of plaster. On the: other hand, it
can be observed that an excessive quantity i.s pre judicial, to
the long-term mechanical properties of the system.
Consequently, we shall confine ourselves in the present
invention to plaster/cement mixtures having mass ratios
ranging from 40:60 to 60:40, a proportion of 60:40 being
generally the most suitable (see Table 3 hereinafter).
It is observed that the nature of the alpha plaster, as
well as the variety of ordinary Portland cement, only have a
slight effect upon the properties of 'the system (Tables 3 and
4 herei.nafter). Similarly, the right-angle setting property
sought aft er is not affected either by the plaster/cement
_a_
ratio or by the presence of rnicrosilica in the proportions
recommended in the invention.
Mast of the systems studied during development of the
invention have a density of 1.89 kg/1. They contain a 60:40
plaster: cement mixture, to which is added 20 ~ of microsilica
by weight of the mixture. Under these conditions, the quantity
of mixing water is 40 o by weight of the plaster/cement
mixture. It is possible to lower the density of the slurry
without affecting either the stability or the rheology; on the
other hand, filtrate control and compressive strength will be
considerably reduced. To remain within the framework of the
invention, in which the ob ject is to obtain a slurry having
good anti-gas migration properties, the lower limit for
density is 1.80 kg/1, which corresponds to a quantity of
mixing water representing 50 o by weight of the plaster/cement
mixture.
From reading the present description, together with
Tables 1 to 4 and Figures 1, 2, 3, 4, 5, 6 and 7, a man of the
art will appreciate that a representative composition of the
invention is characterized in that it contains essentially
- cement,
- 66 t o 150 ~ of alpha plaster in relation to the weight of
the cement,
- 15 t o 25 ~ by we:Lght of microsilica in relation to the
weight of the cement v plaster mixture,
- 30 to 50 ~ aP water in relation to the weight of the cernent
+ plaster mixtuxe,
- and possibly a retarder and a dispersing agent.
On the other hand, higher densities can easily be
contemplated, but these are often of no practical value in
respect of the operations to which the invention especially
relates.
The plaster/cement/microsilica systems described above
thus possess certain characteristics essential to gas
migration control: stability, low rheology, good filtrate
9 -
control, right-angle set and swift development of cornpressive
strength.
There is no existing API standard test designed to
measure the capability of a cement slurry to retain gas . The
method used for this invention is briefly described below.
A 33 cm high cell (Figure 3) , closed at the upper end,
is filled with cement slurry and placed in a thermostatically
controlled container. The particular shape of the cell has
been developed to avoid experimental artefacts and to force
the gas to migrate inside the cement, and riot at the
cement/wall interface. The bottom of the cell is connected to
a nitrogen line at a pressure of 17.5 bar. During the
experiment, measurements are made of the pressure at the top
of the cell, the temperature of the slurry and the flaw rate
for nitrogen entering the cell.
Throughout the gelation period, see above, the slurry
behaves like a liquid that integrally transmits the gas
pressure. During this period, the pressure measured at the top
of the cell remains constant, as do the gas flow rate and the
temperature. Then tames the exothermal part of hydration. 'This
is reflected by a temperature rise, chemical shrinkage of the
cement and development of campressive strength. The pore
pressure in the cement matrix drops owing to the space created
by chemical shrinkage . According to the system studied, the
gas will penetrate the ini:eriar of the cement to a varying
extent. A system will bay termed resistant to gas invasion if
the pressure at tYxe top of the cell decreases and if the gas
entry rate remains low, or better still, nil.
The physical behaviour of a plaster/cement system is
illustrated in Figure 9. It relates to a system containing
60 ~ by weight of alpha plaster and 40 % of API class A cement
(CPA-HP). The first temperature peak, at approximately 3
hours, corresponds to the setting of the plaster and we can
observe, at the same time, a gas entry peak to f~.11 the pores
created by the chemical shrinkage of the system. At between
s ; () '::!
- 10 ,~, ~ ~ ~ ~ ~, t.) r.3
8 and 20 hours, approximately, we observe a second gas entry
which corresponds to the setting of the cement . Throughout the
experiment, the gas pressure is transmitted to the top of the
cell, there has been gas migration through. the system and
permanent communication between the bottom and the top of the
cell.
If, in the course of a gas migration experiment, there
is permanent communication between the bottom and the top of
the cell, the total quantity of gas entering the cell is equal
to the quantity of chemical shrinkage. In the course of the
experiment illustrated in Figure 4, the total quantity of gas
corresponds to 7 ~ of the volume of the cell, whereas, for a
cement alone, we observe only 4 0 of shrinkage during this
stage. The inner shrinkage of a plaster/cement system is thus
very markedly greater than the shrinkage of cement alone which
favours gas invasion, which was naturally considered to be a
serious drawback by a man of the art.
The effect of the microsilica is indicated in Figure 5,
wherein 20 0 of microsilica per weight of plaster plus cement
have been added to the previous system. At the start of the
test, the pressure drops quickly at the top of the cell, down
to approximately 6 bar. Because of this differential pressure
(11.5 bar over 33 cm), a very slight amount of gas enters
during the setting of the plaster. The pressi.rre at the top of
the cell rises aga~.n to 11.5 bar, and then drops down once
more at approximately 28 hours. ThrougY~out the duration of the
experiment, the pressure measured at the top of the cell
remained below the applied gas pressure. There has thus never
been any gas communication between the bottom and the top of
the cell, despite the differential pressures brought into
play.
We can observe (Figure 6) the same type of behaviour at
5°C with another type of cement (LAFARGE, class A).
The right-angle set in a plaster/cement systern is not,
therefore, sufficient to oppose gas migration. The presence of
11 _
microsilica is thus essential, in synergy with the cement and
the plaster, for the particular applications contemplated.
The results given above bear witness to the remarkable
properties of alpha plaster/ordinary Portland cement/
microsilica systems for gas migration control during a
cementing operation.
Once the system has set, it is also important for the
integrity of the cement column to be maintained throughout the
life of the well (several years). Now, plaster/cement systems
are notorious for presenting longevity problems. Indeed, the
sulphate ions contained in the plaster react chemically with
the aluminate phases (C3A) contained in the cement. The
reaction product (ettringite) has a volume larger than the sum
of the volumes of the initial components, which causes the
system to expand. Excessive expansion is liable to lead to the
formation of rnicrocracks inside the material.
Figure 7 represents an expansion curve .for a
plaster/cement system in which a 2.5 ~ expansion of the sample
is effectively observed after approximately five months. From
the slope of the curve, it can be anticipated that there is a
risk of this expansion continuing. On the contrary, the
expansion of the system containing microsilica is limited to
0.4 ~, which appears no longer to progress beyond ~i days. 'Chi.s
particular phenomenon was not foreseeable.
30
CA 02062183 2002-12-02
C
a N ~D e O
~ ~ ,
01 t0 10 If1
'
~"~
~r
O U
b
O tf1 O u1
O O
rW n M ~ emfl
N O N N ~-4
~
U
v . o
a
a0 N 0t a0
~-1 U1 v-1
a H N vD O
a O 0t
~ o.-m~N~o
., ~ a
~ a
.~ 4., ,.~
v, ;
ar
v
ro ~ ro a e1 a~ N
lwG e~
' ;
~~
iN
a ~ MM.
~
~
>, '~ c~ v' o
~ .,~ .,~ at o
~~
m
c,
~ .
~
ac
N ~O O 0t
O 0f O
a ~ 0 0 0 0 0
0 0
0
~ '
W ~ ' 0000000
~y
.
~
3
?
,. U ~
N G
m
'O y ~
~ .
w t1 10 O v~
a f1 ra
'~
y
O t~1 N N
U M N N
~ a
,
~~ x
H
V1-. ,,~,, .E
V7 O
...~ w u0
t~ o .-v
..r .~ e .r
a~ .r o~
.-X000000
0000000
p
~
r a
C i
~ m
.C M M r1 a~
1~1 to l~
p~ 00000
00
ro _
b ..~ >~
;
Ca
M
N
la
i V iJ
m y
b y
~o v0 c0 o~
o O o N .a~
~ o 0 0 0 .-~ O .~
.-~ .~ O
O L1
C r1
x 3
i T1
x ~d
dl r~
U ~rl
ro m~,
~
ro ~
~
F
0
U i f
.>r
i.
't
. ,.Wr
~ ~
~
a e-I >~ E
ra N N N Lrr
w
~
i
~
.
-
>
L
CA 02062183 2002-12-02
,,.~ r O
4 O
H N
~ ~ r 0~
N ~r . Of v~ .
!! O
~ ~ ~ r
r . ~ u1
r 10 M
W
r
i ri r1 r~
w
N
V
0... ,~ r o ao a ao m
f1 O ri
a ''
-1
y'
'
~
'
.
'~ f
1
f
1
G1 r1
'
'
CO (
a 1 r
~1 t'1 e'1 M
Pf
~
''
o
c
~
o E
a >.
a o ao , vo , ao
n N .r
~ u
'
ts. e wo r
vo.
N
~ E
v
V
:.. ~ ,~ 0 0 0 o in o
o m o
V1 N .1 f"1 v~
N fV1 Il1 O
'
C1 : ,~ f
o'~ 1 U7 Af a N M
a '-f N
N
r1 ~-1
~ pry E
G .s7 . ~ r~ ,-, ~o r,
w x -~ .n u, M
. ~ N
y u~ o o~ r
. M r
a -i .-~ o ~, ci
,, c,
O
M rtf C~
Cy-i
~7 d.~ ; a "? a r ,-~ r
.N o, ~,
3 .u ,
C; .~i ~~., ~ N
~ r1 e1 1't N 1
N N
N G
~ v
~
.
en r o N ao r
! ,.
u~ v m vo umn
o . 0 0 0 0
0 0
' 0 0 0 0 0 0 0
'
V ~ .w(
dP
r1 ~O x a .-~ N ~ ,..i
~ N N
U ~. a
~ ._
.~
N
'~
1
. m
,
b
td N
m
it
~o ~o ~o vo ~o
ao o vo ap
O c
0 0 0 0 0 0 ~ O y
o o
S.1 m y G
3 ?i
~ U ~ ro y m
td O :i ~ m ' y
a ~ ~
as
m y m
> 1
l C
r
~
as m
a
f.,.~ ~ ~ o .~ 0 0 0 o
N o O s
r
~
V
~ ' i cL
~ ~
i
. s~
,. ~ .- ~x
~ ,- d
.-
ri .i ,1 ~ .-i
m m .-~
a 3
o m ~ E
a
E
a. Y, U
ts,
0r 0. W 04 ~ H
W W W W
~ ~
m x x x x H v
~ ~
~C ~ 4 C x > w v~
~ H a
m y ~ ~s. W Gc. tr ~ o. ?~ U
0 F~ ti.
~ ~ O
,
.
n
V UVUV
'~
'~
aa
a..
~..
7
r1 H > M
.1 ~ .~
.1
CA 02062183 2002-12-02
fd N P1
O~ , N ,
N p, . . . .
,
.
I~ X a
~
ao t~'f
.a
W
U
m a
u1 . rn .
r x . p . . . ,
p
N t'1
..-I C
E
> o CD ~O v O ~D
~O N ~O a
1~ ~ ~ If1 N t1W D
~f1 vD ~f1
If1 Iff
y ~
al
U
U
W 0 vn u~ o
0
3 > o u~ 0 0
o
~"~ E y N ~ N N u1 e~-~
c'1 v' rh
"C ,,~ ~ E ~ N M a' N .-1
N ~ N t'~
G N
~
_ C
,
fd
~ d, ,..~ .E
H
W
~ ~ N o 0 0~ O~
rn tn e~ a
y0 w ' ~
v O y ~ aD ~t7 W G vwG
N ~ th
N c'~ N en N
r~ N N .-r
1~ G7 ra
i
~ 1~ ~lr G
_
,ri
. w ~ 3 y
~ ~' N N r1 N N t7
N N ~
N
.~ N
~ ,C O N a 01 01
b ~ N N .~-i ~O
U W o ~ ao ac co m o,
d .-~ rn co 00
H ~ ~ 0 0 0 0 o .-i
o 0 0
,fir !O p" o 0 0 o 0 0
',~ 0 0 0
o
N
-..
U
~
N ~'j n u~ u~ un ~mn
...
U1 H
H . U w u
~3r <i <i .~ e1
~-1 'i .1 N
N
H l0 x o 1
H pq a
, ro
H W r-i y m
ri C
_ ~ ~ N a E
H ~ m a~
y
~ O O O O N O
' N O o
. .i .-i .-~ ri
.-s .-i .1
r-i .-i
m 3 ~ y m
.
0 U to
p .~
A 1r U y
.
O U > .~ .>
C N
'
'C., m
C
V C r- (17
~
''~ U LL
H ~ ~ N cf v~ e~f
t1 a u1 ~ u~
. . . .
.
'~ ~ O . . m ~ ~ V CL
. . .d
ei ~ .-~ e1
e-1 .--~ .-i
.-i .~
, y r1
~"~ fp E
~ .m r1
~~
G 3
o 0 ~ ..~ 0
.c ~
C7 O. ~ U
H fs.
OC OG OG W W
W W W W E
a ~
H
H
U
U
m E x9~Ha u~
~xa
C~~a
C
m C1 W t4 tJ~ ,~ pq (1. U
E ~ ,~ ,~ 4 ,~ Y~ H fs,
sC
W W w ~, w w
w w w
m ~ z z z ,~ ,~
,~ ,~ ,~ ,~
v aooaaaaaa