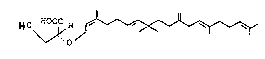Note: Descriptions are shown in the official language in which they were submitted.
20~244~
HOECHST AKTIENGESELLSCHAFT HOE 91~F 069 Dr. SIJfe
Description
A pro~ess for the preparation of MA
Moenomycin A is the main component of Flavomycin- which
is used in livestock nutrition. Like other known phospho-
glycolipid antibiotics, it inhibits the bio~ynthesis of
the peptidoglycan framework of the bacterial cell wall.
More detailed investigations have found that the trans-
glycosylation reaction of the penicillin-binding protein
lb of E. coli is inhibited by these substances ~Huber G.,
Antibiotics, V-1, pp. 135 - 153, (1979)]. Attempts at
specific enzymatic or microbial degradation of phosoho-
glycolipid antibiotics initially ~ailed.
European Application EP 0 355 679 describes a process for
the degradation of moenomycins (= phosphoglycolipid
antibiotic) to MA, MB and MC catalyzed by the enzymes
moenomycinase and MBase from Bacillus sp. DSM 4675.
Moenomyfins
Moenomycinase
MC~MB
J! MBase
MA
Examples of antibiotics in the moenomycin group are
pholipomycin", the prasinomycins2), the diumycins
(macarbomycins)3 esanchomycin, prenomycin and teichi-
mycin, and other structurally related substances which
have a corresponding functionalized phosphoglyceric acid
~ 1) S. Takahashi et al., Tetrahedron Lett. 1983, 499
2) F.L. Weisenborn et al., Nature 213, 1092 (1967)
3) S. Takahashi et alO, J. Antibiot. 26, 542 (1973)].
. . . : . ~ . : , :
. .
.
2~2~46
-- 2 --
In addition, EP 0 355 679 describes the aerobic fermen-
tation of ~acillus spec. DSM 4675, the cleavage products
resulting from the degradation o~ the moenomycins, the
enxymes catalyzing the degradation, and the use of the
degradation products as synthetic building blocks for the
preparation of transglycosylase inhibitors tMA~ or as
substance with antibiotic activity (MB).
The process in the abovementioned application gives a 1%
yield of MA because it is directed at the biologically
active, i.e. antibiotically active, MB.
However, there is a distinct need to optimize processes
for the preparation of MA because MA is a valuable
building block for novel MA analogs, i.e. for novel
transglycosylase inhibitors.
The invention thus relates to:
1. A process for the preparation of MA of the formula I
~0 ~OOC H ~ ~ I
by enzymatic degradation of phosphoglycolipids,
wherein the enzymatic catalysis takes place in a
glycine/NaOH bu~fer.
2. A process as claimed in claim 1, wherein the culture
medium for 3acillus sp. DSM 4675 i8 optimized with
respect to the titer~ of moenomycinase and MBase by
addition of phosphate.
5 3. A process as claimed in claim 1, wherein acid or
alkaline phosphatase is employed as substitute for
the enzyme MBase.
4. A process as claimed in claim 1, wherein the fil-
trate of the biomass i9 extracted with ethyl acetate
'
20624~
-- 3 --
and then with acetone, and the biomass itself is
extracted by stirring with acetone.
The invention is described in detail hereinafter, especi-
ally in the preferred embodiments. It is furthermore
defined in the claims.
Unless otherwise indicated, percentage data relate to
weight.
Bacillus sp. was deposited with the number DSM 4675 under
the conditions of the Budapest Treaty at the Deutsche
Sammlung von Mikroorganismen und Zellkulturen GmbH in
Braunschweig, Germany, on June 23, 1988.
The growth of the microorganism Bacillus sp. DSM 4675 and
the production of the enzymes necessary for the
degradation reactions according to the invention is
particularly ~ood in a nutrient medium with the main
components: citric acid, sodium gluconate, glycerol,
peptone, phosphate and a vitamin solution. The concen-
tration of the phosphate, for example potassium phos-
phate, is preferably 50-100 mM. The nutrient medium can,
however, also be employed without phosphate or with
phosphate in any desired physiological concentration. The
content of gluconic acid or salt thereof i9 1-2%, prefer-
ably 2%.
The fermentation is carried out aerobically, that is to
say, for example, submerged with shaking or stirring in
shaken flasks or fermenters, where appropriate introdua-
ing air or oxygen. The fermentation can be carried out in
a temperature range from about room temperature to 50C,
preferably at about 35 to 37C. The culture time is
generally 8 to 48 bours, preferably 16-18 hours.
As described in EP 0 355 679, when Bacillus cells are
used it is advantagsous for them to be permeabilized, for
example with cetyltrimethylammonium salts, or to be
.
.
" ' :
.
.
2~2446
-- 4 --
lyophilized. It is likewise possible to operate with
protein isolates from the Bacillus cells or with enzyme
extracts which have been partially concentrated by
salting out or chromatography, or naturally with the
purified enzyme. It is furthermore possible to employ the
enzyme in free or immobilized form.
Lyophilized cells are preferably employed as source of
enzyme for the enzymatic cleavage of the moenomycins to
MA in the process according to the invention.
It is evident from the diagram on page 1 that two enzymes
are necessary for the preparation of MA. One enzyme is
needed for the cleavage of the phosphoglycosidic linkage
of moenomycin A, and this was called moenomycinase by the
inventors. Moenomycinase is associated with the cyto-
plasmic membrane of Bacillus 9p. DSM 4675 and can be
obtained from the microorganism by methods known per se
for enzyme isolation.
MBase can likewise be isolated from the microorganism byknown methods. For example, the cells are disrupted with
ultrasound, and the resulting crude extract is further
concentrated either by ammonium sulfate fractionation
(~5-5S% saturation) or ultracentrifugation. This i9
followed by dialysis. The moenomycinase and MBase are
finally separated by chromatography.
In the process according to the invention the enzymatic
cleavage, i.e. the conversion of the moenomycins into MA,
preferably takes place in one mixture (Example 2).
The cleavage of the moenomycins is carried out with
lyophilized cells or enzyme isolates, but preferably with
lyophilized cells.
The reaction takes place in glycine~NaOH buffer. The pH
of the buffer is preferably pH 8.0-8.5, otherwise in the
range pH 7.5-10. The reaction takes place at 34-39C,
.
.
2~624~6
preferably at 37~C. The pH of the en~yme reaction i~ in
the range pH 7.0-9.0, preferably 7.8. The reaction time
is generally 5-48 hours, preferably about 24 hours. The
substrate concentration ought to be in the range from 0.1
to 5%, preferably 1 to 2%.
It is still possible likewise to carry out the reaction
at higher or lower temperatures or pH values than stated.
However, the enzyme activity is then lower.
It is possible to employ for the degradation of MB to MA
besides MBase, as described in EP 0 355 679, also
phosphatase. Preferably used are the acid phosphatase
from potatoes and the alkaline phosphatase from calf
intestine. The enzymes are commercially available
(Sigma). Both enzymes can be employed in immobilized and
non-immobilized form.
The MA of the formula I obtained by the cleavage reaction
is subsequently isolated and purified. This is carried
out by extraction of the filtrate of the biomass or of
the biomass itself with organic solvents. Preferably
employed as solvent is acetone in a ratio of 0.2-1,
preferably 0.3, by volume. The purification by chroma-
tography takes place by using a petroleum ether/acetone
or petroleum ether/ethyl acetate mixture as washing
liquid. Methanol is employed as MA eluent.
The resulting reaction product MA can be used as
synthetic building block ~or transglycosylase inhibitors.
The invention is described further by means of examples.
Example 1
Maintenance of the Bacillus sp. DSM 4675 strain
The maintenance of the strain and the culturing of the
preculture are described in European Patent Application
.. . .
~ - 6 - 20~2~6
0 355 679 (Examples 1 and 2~.
a) A 12 1 laboratory fermenter containing 9 1 of medium
of the following composition serves as main culture
stage:
Peptone 12.5 g/l
Glycerol 20.0 g/l
Citrate 2.0 g/l
Na gluconate 10.0 g/l
K2HPO4 10.0 g/l
MgSO4 x 7H2O 0.5 g/l
FeCl3 x 6H2O 0.04 g/l
Vitamin solution 1 ml
Vitamin solution:
Nicotinic acid 0.35 g/l
Thiamine HCl 0.30 g/l
D-Biotin 0.01 g/l
p-Aminobenzoic acid 0.20 g/l
Pyridoxal HCl 0.10 g/l
Ca pantothenate 0.10 g/l
Vitamin B12 0.05 g/l
This ls incubated with 500 ml of preculture at 37C,
300 rpm and an aeration rate of 0.5 w m for 16-18 hours.
The fully grown culture is centrifuged and then
lyophilized.
~he novel medLum and the shortened ~ermentation tlme
result in a doubling of the biomass yield. The biomas~ i9
characterized by mean~ of the optical den~ity. ~he
measured OD = 7. In addition, the resulting cells degrade
moenomycins to MA with a high yield.
In a test mixture with 100 ~1 of crude extract, 12 mg of
moenomycin A and 900 ~1 of potassium phosphate buffer
(pH 8.0) 50 mM, 50% of the substrate employed is degraded
within 7-24 hours at 37C. The reaction products found
` - 7 - 2062~46
are MA, Ms and MC.
b) If the medium described under a) is used but without
the addition of phosphate there is a distinct reduction
in biomass. The measured OD = 3.
Example 2
Conversion of the moenomycins into MA (enzymatic
eleavage)
Lyophilized cells of Bacillus sp. DSM 4675 are used for
the conversion.
a) 90-200 g of lyophilisate are suspended in 9 1 of
glycine/NaOH buffer, 100 mM, pH 8.5, and, after addition
of 135 g of moenomycin mixture or after addition of MB,
1.8 g of Na azide and 214 mg of CoCl2 ~ incubated at 37C
and 190 rpm for 6-48 hours.
The course of the reaction, which is followed by TLC
analysis, shows that up to 80% of the substrate is
degraded to MB and MA. Of the cleavage produets, about
10-20% comprises MB and about 80-90% eomprises MA.
This way of earrying out the reaetion makes large-seale
produetion of MA possible.
b) A yield of 60% MA is obtained when a buffer of
identieal eomposition but with pH 9.0 i8 employed.
e) The yield of MA is likewise redueed to 60% when a
tris-HCl buffer tl00 mM tris, pH 7.8, otherwise the
eomposition eorresponds to the buffer stated under a)] is
used.
``` 206244~ -- 8 --
Example 3
Enæymatlc cleavage of MB using phosphatases
The use of acid phosphatase from potatoes and alkaline
phosphatase from calf intestine for the preparation of MA
from MB was investigated.
The conversion of MB (5 mg/ml) into MA is about 50% with
acid phosphatase (10 U/ml of mixture) at pH 4.8 and room
temperature, and more than 90% with alkaline phosphatase
(50 U/ml) at pH 8.0 within 144 hour~. The conversion rate
of the alkaline phosphatase i~ distinctly increased
(c 24 h) in the presence of 0.1 mM ZnCl2 and MgCl2 at
pH 10.5 (glycine/NaOH buffer, 100 mM) and 37C.
It is likewise possible to use immobilized alkaline
phosphatase. In this case 35 U/ml under the above-
mentioned conditions convert more than 90% of the sub-
strate within 28 h.
Example 4
Isola,tion of the Flavomycin degradation product MA
The solids present in the suspension resulting from the
enzymatic conversion are removed by centrifugation.
The resulting biomass is extracted by stirring several
times with the same volume of acetone at room temperature
until MA i9 no longer detectable in the organic phase by
thin-layer chromatography. The MA-containing extracts are
combined.
The filtrate obtained after removal of the biomass is
initially extracted once with 1/3 of its volume of ethyl
acetate. The aqueous-organic, colloidal solution i~ then
extracted with 1/3 of its volume of acetone until MA is
no longer detectable by TLC in the organic phase which
`` 9 20~2~
can be separated off in each case.
The MA-containing extracts are combined with the acetone
extracts of the biomass, and the solvent is removed in
vacuo.
The Flavomycin component MB can be isolated from the
remaining aqueous reaction solution by extraction several
times with n-butanol. The crude MB obtained after removal
of the solvent by distillation in vacuo can be used anew
for the enzymatic reaction for preparing MA.
On average, about 110 g of crude M~ and about 85 g of
crude M~ are obtained by the extraction process described
above from about 500 g of the Flavomycin A/C complex.
Example 5
Purification of MA by chromatography
The MA obtained after evaporation of the solvent is
subsequently purified by column chromatography on silica
gel.
For this, about 20 g of crude MA are dissolved in the
minimum amounts of a 1:1 petroleum ether/acetone mixture
and loaded under 7-10 bar at a flow rate of 5 l/h onto a
steel column containing about 2.1 kg of silica gel 60 (pH
- 7.5) as stationary pha~e. Washing i~ then carried out
with about 10 l of a petroleum ether/acetone ~6:4~
mixture under the same conditions. The washing liquid i9
collected in a single fraction. Elution i9 then carried
out with about 5 l of pure methanol.
The methanol eluate is collected in fractions each of
0.1 l. The MA-active pure fractions detected by TLC are
combined, and the solvent i9 removed in vacuo. MA is
obtained as a pale yellow highly viscous oil. Marginal
fractions can ~e rechromatographed. About 13 g of pure
`` 2~62~46
-- 10 --
substance are obtained from about 20 g of crude MA.
Precoated silica gel plates are used for the detection of
MA by thin-layer chromatography. The mobile phase used is
a solvent mixture composed of chloroform/methanol/ acetic
acid t80: 10: 1). Detection is effected by staining the
developed plates with PMS and subsequently drying them at
130C. Also applied as comparison substances are MA, MB
and the Flavomycin A/C complex.
~' , ' ' ~ ` . .
,, - .~ :