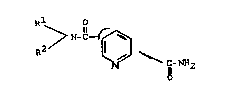Note: Descriptions are shown in the official language in which they were submitted.
%~fi3177
HOECHST AKTIENGESELLSCH~FT HOE 91/F 079 Dr.FI/pl
Description
Mixed pyridine-2,4- and -2,5-dicarboxamides, a process
for preparing them, the use thereof and pharmaceuticals
based on these compounds
Compounds which inhibit proline hydroxyla~e ~nd lysine
hydroxyla~e effect a very selective inhibition of
collagen biosynthesis due to influencing collagen-
~pecific hydroxylation reactions. In the course thereof,protein-bound proline or lysine is hydroxylated by the
enz~mes proline hydroxylase or lysine hydro~ylase
respectively. If this reaction is snppre~sed by
inhibitors, the resulting collagen molecule is unable to
function, is insufficiently hydroxylated and can be
released by the cells only in small ~mounts into the
ex~racellular space. The insufficiently hydroxylated
collagen cannot, moreover, be incorporated in the
collagen matrix and i8 VQry easily broken down by
~0 proteolysis. The conseque~ce of these ef~ects i8 an
overall reduction in the amount of collagen deposited in
the extracellular ~pace.
It is known that inhibition of proline hydroxylase by
known inhibitors such as ~ dipyridyl result~ in
inhibition of Clg biosynthe~is by macrophages (W. Muller
et al., FEBS Lett. 90 (1978), 218; Immunobiology 155
t1978), 47~. This results in the classical pathway of
complement activation becG~ing inoperative. Hence
inhibitors of proline hydroxylase act as immunosuppres-
sants, for example in immune complex disea~es.
It is known that proline hydroxylase can be effectivelyinhibited by pyridine-2,4- and -2,5-dicarboxylic acids
(K. Mayama et al., Eur. J. Biochem. 138 (1984) 239-245).
However, in cell culture, these compounds are effective
inhibitors only in very high concentrations (Tschank, G.
2~3~77
-- 2 --
et al., ~iochem. J. 238 tl987) 625-633).
DE-A 34 32 094 describes pyridine-2,4- and -2,5-
dicarboxylic disesters with 1-6 carbon atoms in the ester
alkyl moiety as pharmaceutical~ for the inhibition of
5 proline hydroxylase ~nd ly~ins hydroxylasa.
These lower al~ylated die~ters have ~he disadvantage,
however, that they are too rapidly clsaved in the body to
the acid~ and do not reach their site of action in the
cell in sufficiently high concentration and thus are less
suitable for poqsible admini~tration a~ pharmaceuticals.
DE-~ 37 03 959, DE-A 37 03 362 and DE-A 37 03 963
describe in a general form mixed estexsJ~mides, higher
alkylated dies~ers and diamides of pyridine-2,4- and -
2,5-dicarbo~ylic acids which are effec~ive inhibitors of
collagen biosynthesi~ in animal models.
Thus, DE-~ 37 03 959 describes, inter alia, the ~ynthesis
of N~ N '-bis(2-methoxyeth~l~pyridine-2,4-dicarboxamideand
N,N'-bi~3-isopropoxypropyl)pyridine-2,4-dicarboxamide.
German Patent Application~ P 38 26 471.4 and P 38 28 140.6
propose an improved process for preparing N,N'-bis~2-
methoxyethyl)pyridine-2,4-dicarboxamide. Germa~ Patent
Application P 39 24 093.2 proposes novel N,N'-
bis(alkoxyalkyl)pyridine-2,4~dicarboxamides.
The object to be achieved was thus to find compound
which are suitable in a much improved manner than those
hitherto disclosed for the inhibition of proline hydroxy-
la~e and lysine hydroxylase. The ob~ect has been achieved
by pyridine-2,g- and -2,5-dicarboxamides of the formula
O
R -N-~- ~
Rl N tI)
~-N~2
2 ~
-- 3 --
in whicA
Rl is Cl Cl2-alkyl, C2~C12~alkenyl or C2-Cl2-alkynyl, which
are un~ubstituted or ~ubsti~uted once or, in the
case of the C2-C12-alkyls, C2-C12-alkenyls and C2-C12-
alkynyls, al80 several times by
halogen, hydroxyl, cyano, amino, carboxyl,
alko~y, alkoxycarbonyl, alkylcarbonyloxy, alkyl-
or dialkylam~no, where the alkyl radicals have 1-
4 carbon atoms, or b~
indolyl or phenyl, which is unsubstituted or
subs~ituted once, twice or three times by halo-
gen, ni~ro, Cl-C4-alkyl or Cl-C4-alkoxy, it al~o
baing possible in the case of multiple substitu-
tions for the substituents to be independently
different from one another,
or R1 i~ saturated C5-C7-c~cloalkyl which i5 option-
ally benzo-fused,
: or R1 i8 aryl or heteroaryl, which i~ unsubstitu~ed
or in turn 3ubstituted once, twice or three times
by halo~en, nitro, cyano, C~-C4-alkyl or Cl-C4-
alkoxy, it also being possible in the ca~e of
multiple substitutions for the substituents to be
independently dif~erent from one another,
or provided that R2 is H, R1 is amino which is
unsubstituted or mono- or disubstituted by Cl-C4-
alkyl, phenyl or Cl-C3-alkylcarbonyl,
and
R2 is hydrogen or R1, where R2 and Rl are identical or
different,
:
20~3.~7~
- 4 -
or where the radicals Rl and R2 form, ~ogether with the
nitrog~n atom~ a radical of the fonmula
~ R3
-N\ ~ X
(CH2)n
in which
n is 1 to 3 and
X is 0, S, CH2 or N-R3,
where
R3 is ~ydrogen, phenyl or Cl-C6-alkyl, C2-C~-alkeIIyl or
C2-C6-alkynyl, where these phenyl, alkyl, alkenyl and
alkynyl radicals are unsubstitu~ed or sub~ti~uted
one or more tlmes by:
phenyl which in turn i~ unsub~tituted or substituked
one or more times by one or more substituents
selected from: halogen, nitro, cyanot carboxyl,
hydroxyl" methyl, ethyl, methoxy, e~hoxy and
trifluoromethyl,
or
N(R4~2, where
R4 is H or C1-C3-alkyl,
or
CoGR5, where
R5 is H or C,-C3-alkyl,
or
CON ( R6 ) 2 or CONHR6, where
R8 is H or Cl-C3 alkyll or where (R6)2 is a C4-C
~$~
-- 5 --
alkylene chain in which zero or one CH2 group
which is not directly ad;acent to the nitrogen
atom is replaced by O, S or N-R4,
or where
R3 is C~-C4-alkoxycarbonyl or C3-C7-cycloalkyl,
and the physiologically tolerated salts, which likewise
effectively inhibit lysinP hydroxylase and prolin~
hydroxylase in animal models.
The invention particularly relates to pyridine-2,4- and
-2,5-dicarboxamides of ~he formula I in which
Rl is Cl-Cl2-alkyl which is unsubstituted or substitllted
once or, in the case of the C2-Cl2-alkyls, 180
several times by
phenyl, hydro~yl, alkoxy, amino, alkoxycarbonyl,
lS alkyl- or dialkylamino, where the alkyl radicals
have 1-3 carbon atoms,
or R1 is phenyl which is unsubstituted or in turn
substituted once by halogen, nitro, cyano, methyl
or methoxy,
or, provided that R2 is H, Rl is amino which i8
unsubstituted or mono~ubstituted ~y Cl-C3-alkyl,
phenyl or Cl-C3 alkylcarbonyl,
- and
R2 i8 hydrogen,
or where the radicals R1 and RZ form, together with the
nitrogen atom, a radical of the formula
r~
-N X
6 2~3~77
in which
X is O, CH2 or N-R3,
where
R3 is hydrogen, or Cl-C3-alkyl, and the physiologically
tolerated salts.
The meanings of halogen are fluorine, chlorine, bromine
and iodine, those of aryl are, phenyl and naphthyl, and
those of heteroaryl are 5- and 6-membered aromatic rings
with l, 2 or 3 nitrogen and~or oxygen and/or sulfur
atoms, which can also be benzo-fused where appropriate,
the heteroaryl radicals are~ in particular, pyridyl,
pyridazyl, pyrimidyl, pyrazyl, 1,3~5-triazyl, pyrrolyl,
pyrazolyl~ imidazolyl,~ tria 701yl ~ thienyl, oxazolyl and
thiazolyl radicals and, where appropriate, the benzo-
fused compounds thereof.
"Substituted several times~ means hereinbefore andhPreinafter that at least 2~ not more than 4, hydrogen
atoms present in the alkyl, alkenyl, alk~nyl, het roaryl
and aryl radicals are replaced by the ~ubstituents
mentloned. In the case of multiple substitutions the
substituents can also be independently different from one
another.
All alkyl and alkenyl radicals mentioned with more than
2 carbon atoms and all alkynyl radicals with more than 3
carbon atoms can be ~oth ~traight-chain and branched.
~he invention further relates to the compounds of the
formula I for use as pharmaceuticals. The invention
additional~.y relates to the compounds of the formula I
for use as fibrosuppressants and immunosuppressant~ and
for the inhibition of proline hydroxylase and lysine
hydroxylase and for influencing the metabolism of
2 ~ s7 7
-- 7 --
collagen and collagen-like substances and the
biosynthesis of Clq. Inhibitors cE proline hydroxylase are
sui~able tools in the therapy of diseases in which the
deposition of collagens makes a crucial contribution to
the clinical picture. The~e include, inter alia, fibroses
of the lungs, liver and skin (scleroderma) and
~therosclerosis.
The pyridine-2,4- and -2,5-dicarboxamides o~ the formula
I which are substituted exclusively in position ~ or S in
the amide group show a considarable and surpri~ing
improved activity in inhibiting proline hydroxylase and
lysina hydroxylase in animal experiments comparad with
the pyridine-2,4-dicarboxamides substituted in position
5 also by carboxamide groups from ~E-A-3 707 429 and
compared with the pyridine-2,4- and -2,5-dicarbo~clmides
substituted in both amide groups of DE-A 37 039 59.
The invention furthsr relates to a process for the
preparation of compounds of tha formula I, which com-
prises reacting
~0 a compound of the formula II'
\ c5~
(II')
N 2CH3
with a compound of the formula III
~,1
H-N (III)
--R2
where R1 and R~ have the meanings indicated for formula I,
and Y is halogen, especially chloriney
and subsequently converting the resulting compound of the
formula IV
2~$317~
C - N RlR2 (IV)
1,
ll ~l
N C2CH3
with ~H3 into a compound of the formula I
- N RlR2
N ~1 - NH2
followed, where appxopriate, by co~ersion into its
ph~siologically tolera~ed salts.
The following reaction diagram shows the preparation
: route ~tages 5 and 6), including the synthesi~ of the
precursors (1 to 4)
2~63 ~ 7rl
Reaction diagram
C02H C02CH
NCO2H N C2CH2
Stage 2
C02CH2~ C02CH2~
;; NC2CH3 N CO2H
:~ Staye 4
C02H CONRlR2
Stage 5
: . ¦ (II) ~ ¦ (IV)
- ~ ~ C02CH3 H~Rl NC2CH3
N~3/
MeOH Stage 6
~ ~ .
CONRlR2
( I
CONH2
2 ~ 7 7
-- 10 --
In ~tage 1, commerclally availabls pyridine-2,4-
dicarboxylic acid is converted into its dicarbonyl
dihalide, preferably its dichlorid~, and reaoted with an
optionally ~ubstituted benzyl alcohol to give dibenzyl
pyridine-2,4-dicarboxylate.
In stage 2, the diester is ~electlvely hydrolyzed in
position 2, for example in the presence of a copper salt
as described by Delaxge, J.: Phar. Acta. Helv. ~4 ~10),
637 (1969).
The free acid functionality in position 2 i~ subseguently
converted in stage 3 into the corresponding acid chloride
and reacted with an alcohol such as, for example, methyl
or ethyl alcohol to give the corre~ponding 2-carboxylic
e~ter.
The remaining benzyl protective group in position 4 is
elLminated by hydrogenolysiR in stage 4 tor example with
H2/Pd, Houben-Weyl- Vol. IV/lc (1980), pp. 381 ~ 82).
The free acid in position 4 (foxmula II) is converted
into its acid halide, preferably chloride. ~he acid
chloride can now be convQrted with the amine of the
foxmula (III) into the mixed pyridine-4-carboxamide-2-
carboxylic ester (IV).
The mixed diamide of the formula (I) is prepaxed from the
2-carboxylic ester (IV) with alcoholic ammonia ~olution
(for example in methanol).
The said process, which has been described in the reac-
tion diagram for the compounds substituted in position 4,
also applies to the compounds correspondingly sub~tituted
in position S.
It is possible where appropriate for the products to be
worked up, for example, by extraction or by chromato-
graphy, for example on silica gel. The isolated products
7 7
11 --
can be recry~tallized and, wher~ appropriate, reacted
with a suitable acid ~o give a physiologically tolerated
salt. Examplas of ~uitable acids are:
mineral acids such as hydrochloric and hydrobromic acid
5 and sulfuric, pho~phoric, nitric or perchloric acid or
organic acids such as formic, acetic, prop~onic,
succinic, glycolic, lactic, malic, tartaric, citric,
maleic, fumaric, phe~ylacetic, benzoic, methane~ulfonic,
toluenesulfonic, oxalic, 4-aminobenzoic, naphthalene-1,5-
disulfonic or ~scorbic acid.
The starting compounds of the formula ~III) which cannotbe bought can be synthesized s~raightforwardly (for
example Organikum, Organisch Chemisches Grundpra~tikum,
15th edition, VEB Deutscher Verlag der Wi~senschaften,
1976; a summary of the various possibilities is to be
found in the me~hods index, p. 822).
The compounds of the formula I according to the invention
have valuable pharmacological properties and, in parti-
cular, display activity as inhibitors of proline hydroxy-
lase and lysine hydroxylase, as fibrosuppressant and
immunosuppressant.
The activity of the fibrogenase can be determined by
; radioimmunological determination of the N-terminal
propeptide of collagen type III or of the N- or C-
terminal crossl~nking domain of collagen type IV (7s
collagen or type IV collagen NCl) in ~erum.
For this purpose, the hydroxyproline, procollagen III
peptide, 7s collagen and type IV collagen NC
concentrations were measured ln the liver of
a) untreated rats (control)
b) rats given tetrachloromethane (CCl4 control)
c) rats given first CCl4 and then a compound according to
the invention
(this test method is described by Rouiller, C.,
3~ experimental toxic injury of the liver; in The
'7 7
Liver, C. Rouiller, Vol. 2, pp. 335-476, New York,
Academic Pre~s, 1964).
By reason of these pha~macological propertie~ the
compounds according to the invention are suitable for the
treatment of di~orders of the metabolism of collagen and
collagen-like substances and for ~he trea~ment of dis-
orders of the biosynthesis of Clg.
The invention therefore further relates to the use of the
compounds of the formula I according to the in~ention,
and of the physiologically tolerated salts thereof, for
the treatment of the abovementioned metabolic disorders.
The compounds can be used a~ pharmaceuticals either alone
or mixed with physiologically tolera~ed auxiliari~s or
vahicles. They can be administered for this purpose
orally in doses of 0.01 ~ 25.0 mg/kg~day, preferably 0.01
- 5.0 mgfkg/day or parenterally in dose~ of 0.001 - 5
mg/kg/day/ preferably 0.001 - 2.5 mg/kg/day, especially
to 0.005 - 1.0 mg/kg/day. It is also possible ~o increase
the dosage in severe cases. However, lower do~es al~o
suffice in many cases. These data relate to an adult
weighing about 75 kg.
The invention also embraces the use of the compounds
according to the invention for preparing pharmaceutical~
which are employed for the ~reatment and prophylaxis of
the abovementioned metabolic disorders.
The invention addit onally relates to pharmaceuticals
which contain one or more compounds of the formula I
according to the invention and/or their physiologically
tolerated salts.
The pharmaceuticals are producing by processes known per
se and familiar to the person skilled in the art. As
pharmaceuticals, the pharmacologically active compounds
(= active substance) according to the invention are
2 ~ 7 ~
- 13 -
employed either as ~uch or, preferably, in combination
with suitable pharmaceutical auxiliaries or vehicles in
the f Qrm of tabletR, coated tablets, capsule~,
suppositories, emulsions, suspensions or solutions, where
S the content of active substance is up to 95 %
advantageou~ly between 10 to 7S ~.
Suitable auxiliaries and vehicle~ for the required
pharmaceutical formulation are, for example, besides
solvents, gel formers, suppo~itory bases, tableting
auxiliaries and other active ~ubstancQ vehicles, also
antioxidant6, ~ispersants,` emulsifiers, foxm
suppressants, f~avorings, preservatives, solubili2ers or
colorants.
The active substances can be administered orally,
parenterally or rectslly.
~ha active compounds are mixed with tha additives
suitable for this, such as vehicles, ~abilizers or lner
diluents, and converted by the usual methods into
suitable ~osage forms such as tablets, coa~ed tablets,
hard gelatin capsules, aqueous alcoholic or oily
suspen~ions or aqueous or oily solutions. Examples of
inert vehicles which can be used are gum arabic,
magnesia, magnesium carbonate, potassium phosphate,
lactose, glucose or ~tarch, especially corn starchn The
preparation can be carried out either as dry or as wet
granules. Examples o~ suitable oily vehicles or solvents
are vegetable or animal oils, such as ~unflower oil or
fish liver oil.
For subcutaneous or intravenous administxation, the
active compounds are, if required, converted into a
solution, suspension or emulsion with the substances
suitable for this purpose, such as solubilizers,
emulsifiers or other auxiliaries. Examples of suitable
solvents are physiological saline or alcohols, for
example ethanol, propanol, glycerol, as well as sugar
~ v ~ ~ ~
solutions ~uch a~ glucose or mannitol ~olution~, or el3e
a mixture oP the various solvent mentioned.
The inventien i8 explained in more detail hereinafter by
means of example~.
General procedure for the preparation of the compounds
1 mmol of methyl pyridine-4~carboxam1de-2-carboxylate
(IV) is di~solved in 30 ml of sa~urated methanolic
ammonia solution and tirred at room temperature for 2
hours. The solution is concentrated and the residue i5
stirred with diisopropyl ether and filtered off with
suction.
Example 1
4-N-Ethylpyridine-2-carboxamide-4-carboxamide
~elting point: 197C
Exampl~ 2
4-Norpholinocarbonylpyridine 2-carbox~mide
Melting point: 12BC
Example 3
4-N,N-Diethylpyridine-2-carboxamide-4-carboxamide
Oil, MS = 222 ~M + H+) molecular mass C11~15N302 (221)
Example 4
4-N-(2-methoxypropyl)pyridine-2-carboxamide-4-carboxamids
~elting point: 116 - 120C
- 15 - ~ 7~
Example 5
4-N-~3-methoxypropyl)pyridine-2-carboxamide-4-carboxamide
Melting point: 14~C
~xampl~ 6
4-N-(3-hydroxypropyl)pyridine-2-carbox~mide-4-~arboxamide
~eltin~ point~ 154 - 156C
Example 7
4-N-alanylp~idine-2-carboxamide-4-carboxamide
Melting point: 124 - 125C
Example 8
4-N-(O-benzylalanyl~pyridine-2-carboxamide-4-carboxamide
Melting point: 138 - 140C