Note: Descriptions are shown in the official language in which they were submitted.
WO9l/02073 "j~, PC~/~S90/040~6
r~ f l r
"Immunoassa~ ~'acuum Chamber With ~icrotiter Plate"
Field of Invention
The present invention relates to immunoassay
procedures and, in particular, to quantitative
immunoassay procedures which can be rapidly
accomplished in su~stantially less tlme than
heretofore obtainable.
BacXqround
Immunoassay procedures have for many years
: provided sensitive diagnostic tools for the detection
of a variéty of substances, generally referred to as
ligands. Such procedures are described in a number of
: articles and textsj an example of which is Reviews on
: Immunoassay Technoloqy, Ed. S. B. Pal, Pub. Chapman &
15 Hall, 1988.
~ One type of immunoassay procedure, commonly
referred to as E~ISA~ utilizes a solid support such as
the well in a plastic plate in accomplishing the
assay. A receptor for a target ligand is bound to the
: 20 ~ solid Cupport. A liquid sample containing the liyand,
having speci~icity for the bound receptor, is then
applied to the plate. Following washing and
incubation procedures, an enzyme conjugate having
binding specificity for the ligand is added to the
:
:
.
PCI`/~lS90/()qO86
well. After further rinsing, ~ substrat~ is added
which develops color on contact with the bound enzyme,
the amount of color developed ~eing dependent upon the
amount of bound con~ugate present which, in turn, i5
indicative of the àmount of target ligand bound to the
support. Thus, by measuring the amount of color
development and correlaking such against known
standards, the concentration of ligand in the sample
can be determined. In general, conventional ELISA
procedures take on the ~3rder of five hours or more.
Recently, it has been suggested that the ELISA
procedure can be accelerated by utilizing
immunofiltration (Ijsselmuiden et al., Journal of
Immunolo~ical Method, 119 (1~89) 35-43 and Eur. ~.
Microbiol. 6, (1987) 281). In the disclosed
procedurej a nitrocellulose filter is pre-coated with
an antigen. Thereafter, a solution containing the
target ligand, in this case an antibody, is drawn
through the filter followed by rinsing solutions.
Finally, either an enzyme-labeled antibody
(Ijsselmuiden (1987)) or 125I-labeled protein A
(Ijsselmuiden (1989)), both of which have binding
specificity for the target ligand, is applied and
drawn through the filter to detect the target antibody
bound to the antigen on the nitrocellulose filter.
The device used to accomplish the above described
assay is illustrated in the 1989 Ijsselmuiden artic1e.
It consists of three blocks of perspex which are
clamped together during the assay. The bottom section
has an external outlet and a valve, and constitutes a
reservoir attached to the upper sections. The middle
and top sections, designed to accommodate the
nitrocellulose filter between them, contain 32
corresponding holes with a diameter o~ 5 mm and
neoprene 1'0ll rings facing the nitrocellulose sheet to
prevent lateral flow.
WO~l/02073 PCr/US90/0~0~6
2 o~ 9,8 6
While Ijsselmuiden appears to describe an
immunoassay procedure which has the a~vantage of
rapidity over prior procedures, only the procedure
utilizing 125I detection is quantitative. The enzyme-
labeled antibody system (Ijsselmuiden (1987)) yieldsonly a qualitative determination o~ target ligand. A
quantitative procedure having the advantages of
rapidity and not necessitat:ing the use of
radioactively labeled detection reagents such as 1~5I
would be desirable. In addition, a procedure having
the foregoing attributes which also can utilize
commercially available microtiter plates and
associated readers for determination of color
development would be advantageous.
Summary of the Present Invention
In accordance with the present invention, there
is provided a procedure and related apparatus for
rapidly and quantitatively accomplishing immunoassays
without the necessity for using a radioactive
detection system. Further, in accordance with this
- invention, standard microtiter plates and associated
readers can be used to quantitate the results of the
assay.
The present invention embodies the features of
filtration, such as illustrated by Ijsselmuiden. But,
in addition thereto, it provides means for collecting
the colored reaction product o~ enzyme and substrate
in ~ fashion that permits quantitative measurement of
color development in a microtiter plate system to
achieve quantitation of target ligand~
Accordingly, the present invention provides an
apparatus which can be used for colorimetric ligand-
receptor assay procedures to quantitatively determine
the concentration of a target ligand in a liquid
.. ,. , . . , . , , . . , i
WO91/02073 l'Cr/US~0/04086
4 . '
sample. sriefly described, the apparatus contains ~
top member, a middle member and a bottom member in a
sandwiched relatl`onship. The top and middle members
are plates having holes therethrough and between which
a membrane can be placed. When the plates are placed
one on top of another the holes are in axial alignment
and the cross-sectional area o~ the holes at the
hottom surface of the top plate is greater than the
cross-sectional area of the holes at the top surface
of the middle plate. ~urthermore, the sidewalls of
the holes in the middle plate extend below the surface
of the plate. As described later, the holes in the
middle plate are preferably contained within tubes,
herein termed cannulas, which are inserted through
openings initially formed in the middle plate.
The bottom member of the apparatus is a
collection chamber which has an opening on the upper
side which faces the bottom surface of the middle
plateO The chamber contains a port through one of its
surfaces so that a YacUUm can be created within the
chamber. The chamber contains means for accepting a
microtiter plate containing a plurality of wells. In
the assembled apparatus, the ends of the sidewalls of
the holes which extend beneath the bottom surface of
the middle plate are located within the wells of the
microtiter plate. The apparatus contains means ~or
securing the three members together in a vacuum type
relationship when a membrane is positioned between the
top and middle plates.
In use, the apparatus described above is first
assembled with a liquid permeable membrane, to which
a receptor can be bound, placed between the top and
middle plates, and without the plate containing the
wells being positioned in the bottom chamber. The
membrane can either have the receptor already bound
thereto or a solution containing the receptor can be
. . . , : .
WO9l/0~073 PCr/US~0/0~086 ¦
9;8 6
added to the holes in the top plate. In the later
instance, vacuum is then created in the chamber with,
for example, a peristaltic pump, so that the solution
can be pulled past the membrane at a highly controlled
consistent rate.
Having the receptor now bound to the membrane,
the liquid sample containiny the target ligand is
added to the holes in the top plate. As above
described, vacuum is applied and the liquid is drawn
directly through the membrane, through the holes in
the middle plate, and then discharged into the
collection chamber. As the liquid sample passes
through the membrane, the ligand is bound to the
receptor on the membrane. Thereafter, a solution
containing an enzyme conjugate which has binding
specificity for the target ligand is drawn through the
membrane and is, in turn, bound to the target ligand.
This step is then followed by a washing step to remove
enzyme conjugate which did not bind to the ligand.
After the above described steps, the bottom
chamber is separated from the top and middle plates,
which remain secured together, and is emptied of any
liquid which may be present from previous steps.
Then, the microtiter plate is positioned in the bottom
chamber and the apparatus is reassembled with the
sidewall extensions o~ the middle plate now extending
into the wells of the microtiter plate.
A solution contaihing a substrate for the bound
enzyme, which on reaction therewith gives a colored
product, is then add~d ~o the holes in the top plate
and drawn through the membrane, through the holes in
the middle plate and, in turn, is deposited into the
wells of the microtiter plate. Sufficient substrate
solution is uti.lized so that on collection in the
wells the solution extends above the bottom ends of
the sidewall extensions. This feature is important
'; ~'', ~ . , , ,, ,,,'j ~,' '. . ' ' ', I '
'' ' ' ~ .::' .: -' .
' ': ' , , ' ,
'. ' ' . ' .. .
., ' . '
.... ' ' ,' ''
": ' ' . ' ' , ,
WO~1/02073 l'CT/US9~/04086
~; ,
,390
in order to insure that the volume of solution
transferred from the holes in the upper plate to the
wells in the micr~ iter plate is the same for each set
of holes and ~ells in the apparatus. In turn, this
permits measurement of well to well differences in
color intensity which correlate to differences in
bound ligand. After collection is complete, the
bottom chamber is again separated from the remaining
structure, the microtiter plate removed from the
chamber and the development of color of the solution
in the wells determined.
Brief Description of the Drawln~s
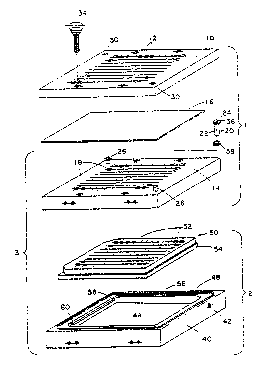
FIG. 1 is an exploded perspective view showing
the apparatus of the present invention with certain
parts omitted and with a membrane included.
FIG. Z is an exploded side elevation with certain
parts broken away and shown in section.
FIG. 3 is a cross-section taken vertically
through the apparatus.
FIG. 4 is an enlargement, partly in section, of
one of the clamps.
FIG. 5 is a top plan view of the assembled
apparatus.
FIG. 6 is a side elevation, of view of the
assembled apparatus.
Description of Preferred Embodiments
.
The apparatus illustrated in the drawings is
shown to comprise as a top member a sample application
plate 10 having a plurality of holes 12 extending
therethrough into which a liquid sample can be placed.
The middle member is a membrane support plate 14
positioned beneath the plate 10 with the liquid
.
'
.
,: .. ;:: . ~-, . ... .
WO 91/02073 PCr/US90/0408~i t
~ ,., ., .
20,B3~
7 -} ;
permeable membrane 1~, when present, being placed
between the two plates. The membrane support plate 14
has openlngs 18 into which the cannulas 20 are
inserted. As shown, each cannula has a sidewall
5 portion 22 with a hole 24 extending through the
cannula. As assembled, the holes 12 in the
application plate 10 are in axial alignment with the
holes 24 in the cannulas 20 located in the support
plate 14. In order to attain registry of the two
10 plates 10 and 14 to achieve the aforementioned
alignment, the support plate contains studs 26 which
are adapted to fit into the holes 30 of the
application plate. Four thumb screws 34 serve ~o
secure the application plate 10 and membrane support
15 plate 14 together.
Turning to the cannulas 20, as mentioned, they
contain a sidewall portion 22 and a hole 24. As shown
in FIG. 3, the sidewalls 22 of the cannulas extend
beneath the bottom surface of the support plate 14.
20 In addition, as illustrated, the sidewalls of the
cannulas extend above the top surface of the middle
plate, the top end of the cannulas containing a
flanged portion 3~ which abuts against the membrane
16.
To permit easy application of liquid to the holes
12 in the application plate, the holes are conically
shaped and, as illustrated, the cross-sectional area
of the holes 12 at the bottom surface of the plate 10
is greater than the cross-sectional area of the holes
24 at the ~op surface of the cannulas 20. The cross-
sectional area of the ~langed portion 36 of the
cannulas 20 is greater than the cross-sectional area
of the holes 12 at the bottom surface of the
application plat:e. These features, in combination,
assuro that liquid applied in the holes 12 will be
. .
W091tO~073 PCT/US90/04086
6 8
drawn through the membrane 16 and, in turn, through
the holes 24 in the cannulas 20.
In order to assure that liquid, when being drawn
through the membrane 16, does not disperse laterally
and cause cross-contamination between samples,
flexible "O" rings 38 are placed below the flanyed
portion 36 o~ the cannulas between the flanged
portions and the membrane support plate 14.
Alternatively, instead of the illustrated individual
"O" rings, other gas~.eting means can be used to
prevent lateral dispersion of liquid through the
membrane. Another example is a flexibls perforated
diaphragm, having dimensions co-extensive with the
array of holes in the top plate, which can be disposed
on either or both sides of the flanged portions of the
cannulas, a diaphragm on both sides being preferred.
Referring still to the drawings, the illustrated
apparatus is shown to contain, as a bottom member, a
collection chamber 40 for receiving liquid drawn
through the application plate 10, the membrane 16 and
the holes 24 in the support plate 14. T~ receive
liquid, the collection chamber is open on the upper
side thereof which faces the bottom surface of the
membrane support plate.
To enable vacuum to be created within the chamber
to draw liquid through the apparatus, the chamber
contains the vacuum port 42 which is attached via
tubing and valving to a vacuum pump such as a
peristaltic pump. As illustrated in FIGS. 2 and 3,
the bottom internal surface 44 of the collection
chamber 40 is sloped to permit fluid to flow to the
~acuum port and, in turn, be removed from the chamber
40. The chamber 40 also contains a port 46 which
contains a valve tnot shown) to relieve vacuum in the
chamber after each sample solution (i.e., receptor,
ligand, etc.) has been drawn through the membrane. By
. . , -,,; :
WO91/02073 Pr/US~0~04086
J r~
so doing, the succeeding solution placed in all of the
holes of the application plate can be drawn
simultaneously through the men~rane by vacuum created
by the peristaltic pump.
As indicated above, the final step in the assay
procedure described herein involves collectiny the
colored liquid reaction product of substrate and
enzyme so that color can be measured and, in turn, the
- amount of bound target ligand determined. To that
end, there is provided for insertion into the chamber
a microtiter plate 50 containing a plurality of wells
52 for collection of liquid. ~s illustrated, the
lower portion of the microtiter plate contains a skirt
54 which, when the plate is inserted into the chamber
~0, rests upon the ledge 56 in the chamber. In order
to permit the vacuum created in the chamber 40 to
communicate with the upper and middle portions of the
apparatus and thereby permit drawing of the liquid
through the apparatus, the ledge 56 is, as indicated,
discontinuous between points 58 and 60. Thus, when a
vacuum is drawn in the chamber 40, the microtiter
plate does not form a vacuum tight seal with the ledge
56.
- As shown in FIG. 3, when the microtiter plate 50
is contained within the collection chamber 40, the end
of the sidewall extension of the cannulas 20 is
located within the wells 52 o~ the microtiter plate
50. The extent to which the end of the cannulas so
extend into the wells is such that when the assay
~procedure is completed and the colored reaction
product of substrate and enzyme has been drawn into
the wells 52, the end of the cannulas are submerged
below the surface of the liquid. By so doing, when
the membrane support plate and, in turn, the cannulas
are withdrawn from the wells, the reservoir of liquid
in the wells prevents drops from being retained on the
.
~ ~ .
WO 91/02073 ~'cr/US90/O'tO86
C~,Q~! ,' - ,1~'`, 10
ends of the cannulas, which drops may vary in volume
from cannula to cannula. If drops were permitted to
remain on the cannulas, the amount of solution
transferred from the cannulas into the wells would be
different, from well to well, and precise quantitation
of bound ligand, from well to well, would not be
obtainable.
The collection chamber is secured in vacuum tight
relationship to the membrane support plate 14 by means
of conventional clampin~ and gasketing. An example of
such is shown in the drawings to include clamps 62 and
gasket 48. Turning specifically to the clamps 62,
they are shown in FIG. 4 to include a lever 64, a
bracket 66 which is secured to the collection chamber
40 by means of the screws 68, a bracket 70 which is
secured to the membrane support plate by means of the
screws 72, and a tension screw 74 adjustably mounted
in the housing 76. The housing 76 is pivotally
mounted at the base of the lever 64 which, in turn, is
pivotally mounted on the bracket 66. The upper
bracket 70 is slotted to accommodate the head of the
screw 74.
To clamp the chamber 40 to the support plate 14
using the clamps 62, the head of the screw 74 is
inserted into the slot of the bracket 70 by pivoting
the sc:rew into position. Then, the lever 64 is
raised, thus forcing the screw and, in turn, the
bracket 70 downward to, in combination with the gasket
48, to e~festively seal the chamber to the membrane
support plate. By adjusting the screw within the
housing 76, the tension can he adjusted to insure a
vacuum seal.
The following example illustrates the use o~ the
above described apparatus. All parts of the
apparatus, except for the thumb screws and clamps,
were made of plastic and a peristaltic pump was used
.
,., :: , ~ ~
WO91/02()73 PCr/~S~0/040~6
11 ,''.~.2,0C3986
to create vacuum. A commercially available
nitrocellulose membrane sold for use in immunoassays
was employed. Prior to insertion between the sample
application plate and the support plate, the membrane
was wetted with distilled water. The apparatus when
assembled measured about 6-1/2" x 4-1~2" and was
approximately 2-1/2" high. 96 conically shaped holes
were present in the application plate. The membrane
support plates contained 96 cannulas as illustrated.
A commercially availab]e microtiter plate with 96
wells was used. The procedure employed was as-
follows.
100 ~1 of distilled water was placed into each of
the 96 holes in the sample application plate. To
expel air from the system, the distilled water was
drawn by vacuum through the apparatus into the
collection chamber and removed through the vacuum
port. When the holes in the application plate are
emptied of liquid and air first contacts the membrane,
no further flow of liquid or air can occur because of
capillarity. Thus, throughout the procedure, there
remains a continuous liquid phase between the lower
surface of the membrane and the bottom of the
cannulas.
200 ~1 of human serum albumin (HSA) solution (50
~g/ml in Tris buffer~ was then placed in each of the
holes in the application plate. The peristaltic
vacuum pump was started and over a period of five
minutes the HSA solution was drawn through the
apparatus and discharged in~o the collection chamber.
The ~acuum relief valve was then spened and again
closed after which time 200 ~1 of a 3% bovine serum
albumin (BSA) solution was added to each of the holes
in the application plate. Over a five minute period,
the BSA solution was drawn through the membrane and
discharged into the collection chamber, this step
.,,....,,; . , , . . . , . : . .,, ' . ... .
.. .. . . ,: .
W09l/0~073 PC~/US90/(~086
~ q ~ S 12
serving to block the vacant ~inding sites on the
membrane. Again, the vacuum relief valve wac; opened
and closed. Next, 200 ~1 of a lolO00 dilution (in
Tris buffer) of mouse derived anti-HSA was deposited
into each of the holes in the application plate and
drawn through` the membrane and discharged into the
chamber over a five minute period. After again
opening and closing the vacuum relief valve, 200 ~1 of
a 1:1000 dilution of goat derived anti-mouse IgG
alkaline phosphatase conjugate solution was deposited
into each of the holes in the application plat:e and
over a five minute period drawn through the apparatus
and discharged into the collection chamber. After
again opening and closing the vacuum relief valve,
unbo~md conjugate was removed from the membrane by
washing the mem~rane three times with 200 ~1 of t.~ris
buffer drawn through the membrane in thirty second
intervals.
Subsequent to the foregoing washing step, the
vacuum relief valve was opened and the collection
chamber separated from the top assembly oc the
apparatus containing the application plate~ membrane
and membrane support plate. The chamber was emptied
of any liquid remaining from the foregoing operations
and the top assembly was placed on a piece of paper
toweling to remove any excess liquid off the bottom of
the cannulas. A commercially available 96 well flal~
bottomed microtiter plate was placed in the collection
chamber and the apparatus reassembled by clampinq the
collection chamher and the membrane support plate
together. As so assembled, the bottom of the cannulas
extend into the wells of the microtiter plate. After
closing the vacuum relief valve, 200 ~1 of a 0.5 mg/ml
p-nitrophenyl phosphate solution was added to the
wells and drawn through the apparatus over a five
minute period. The colored solution produced by the
W091/02073 PCr/US90/040X6
f~ 2l~6g9~86:, ,
13
reaction of substrate and enzyme was collected in the
wells of the microtiter plate with the solution
extending above the end of the cannulas positioned in
the wells.
After the above procedure was completed, vacuum
was relieved and the chamber was again separated from
the membrane support plate. The cannulas were slowly
withdrawn from the wells so that there was no
remaining liquid on the exterior of the cannulas. The
microtiter plate was then removed from the chamber and
the color in each well read using a commercially
available automatic ELISA plate reader. The amount of
color determined in each well was substantially th~
same indicating the precision of the assay since equal
quantities of mouse derived anti-HSA (the target
ligand~ were applied to each hole in the application
plate.
~ hile, in the foregoing procedure, a standard
known concentration of target ligand was employed, an
unknown amount of target ligand can be identified by
using the above protocol. In this instance, instead
of one standard known concentration of target ligand
being smployed, an entire range of known
concentrations or target ligands are utilized, thereby
yielding plotted data which correlates color
development with the known concentrations of target
ligands in a standard curve. The standard curve is
then used to translate ~he color development of an
unknown concentration of target ligand to its actual
conce~ntration. In practice, unknown samples can fill
a number of the holes in the application plate and the
balance of the holes can contain the standard
solutions for construction of the standard curve.
Although the protocol illustrated as an example
above is commonly called a direct sandwich assay, the
apparatus and methodology herein described is not
i
:
r
WV91/02073 ~cr/~ls~ 4~
39~
1~
limited to this type of assay. It can also be used in
direct assays, indirect assays, indirect sand~ich
assays, competitive assayci, etc. Essentially any
immunoassay arrangement used with standard ELISA type
techniques can bè mimicked with the apparatus and
methodology described herei.n.