Note: Descriptions are shown in the official language in which they were submitted.
` 2064768
COMPOSITION CONTAINING AN EXTRACT OF COLEUS
FOR SKIN PIGMENTATION.
05 The present invention essentially relates to a cosmetic
or pharmaceutical composition, particularly a dermatological
composition, containing an extract of Coleus for skin
pigmentation, or combined with various active principles for skin
and hair pigmentation, and process for the preparation thereof.
It also relates to the use of an extract of Coleus for
preparing said cosmetic or pharmaceutical composition for skin
and/or hair pigmentation.
The different species of Coleus belong to the family of
Labiaceae, in the same way as the Plectranthus which are very
close thereto, and are often mistaken one for the other, since
they are mainly originated from the same regions. Thus, the Coleus
barbatus specie is generally designated under the name
Plectranthus barbatus, and the Coleus aromaticus specie is also
called Plectranthus aromaticus. However, L.H. CRAMER, in his
article published in Kew Bull. 1978 volume 32 No. 3, pages
551-561, discusses the differences between these two groups of
species.
There are nearly two hundred species of Coleus which
are found in the tropical and subtropical regions of Asia, Africa,
Australia and the Pacific Islands. About 9 species are catalogued
in India. The ten principal varieties of Coleus are catalogued in
various Indian dictionaries and in particular : "The Wealth of
India, a dictionary of Indian Raw Materials and Industrial
Products, Raw Materialsn, volume II, Dehli 1950, pages 308-309;
the book entitled ~Indian Materia Medica" by Dr. K.M. Nadkarni's,
third edition revised and completed by A.K. Nadkarni in two
volumes, volume I, page 372 ; the book entitled "the Flora of
British Indian, by Sir J.D. Hooker, C.B., K.C., S.I. volume IV
entitled "Asclepiadae to Amarantaceae", pages 624 to 627.
More recently, the extracts of Coleus, in particular the
" 2064768
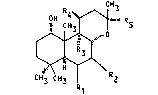
Coleus forskohlii (syn. Coleus barbatus) have revealed substances
having the carbon network of Labdanes, and more specifically, that
of the 8,13-epoxylabd-14-en-11-one of the following formula :
CH3
05 ~ ~ R5
~ (I)
CH3 CH3 I R2
R1
In particular, the 1~ ,6 ~,9~ -trihydro-7 ~ -acetoxy, or
forskoline derivative whose extraction process was described in
Hoechst patent application FR-2 336 138 was isolated. The
1c~,7~ ,9 ~ -trihydroxy-6 ~-acetoxy derivative, or coleforsine
(HOECHST, FR-2 364 211) and the 1 ~ ,6 ~ dihydroxy-7p -acetoxy
derivative, or 9-desoxyforskoline (HOECHST, EP-A1-0 243 646) were
also described and isolated.
These documents describe a certain number of
pharmacological properties of said substances, such as a
hypotensive and calming activity on the central nervous system.
Document EP-A-O 293 837 also discloses a composition for
treating hair comprising a combination of at least one coumpound
selected from the group comprising forskoline and its derivatives
and peptides having a structure of the basic network, alone or
combined with a compound selected from the aliphatic acids,
alcohols and derivatives having an uneven number of carbon atoms.
This composition is applied on hair in order to activate the
melanocytes of the radix pili and to improve the melanine
synthesis of said hair so as to prevent grey hair from appearing
and to promote restoration of the natural color.
It should be noted that between the melanocytes present
in the hair follicles and those present in the skin, there are
significant differences, notably at the metabolic level.
Therefore, there is not necessarily any relationship as
regards activity between a compound activating the melanocytes of
206~768
the hair follicles and a compound activating the melanocytes
present in the skin. This is due in particular to the fact that to
enable activation of the melanocytes in the skin, the compound
must also be capable of going through the superficial layers of
05 the epidermis so as to penetrate through the level of the
melanocytes.
The present invention is based on the discovery that the
extracts of Coleus, in particular Coleus forskohlii, have
interesting biological properties useful in the pharmaceutical and
cosmetic ,fields by unexpectedly presenting a melanogenesis-
promoting activity at the level of the melanocytes present in the
skin, thus making it possible to promote skin pigmentation, as
well as the carrying out of a treatment for treating the skin
pigmentation disorders by promoting more particularly the melanine
biosynthesis.
The object of the present invention is therefore to
solve the new technical problem consisting in providing a new
cosmetic or pharmaceutical composition, particularly a
dermatological composition, intended to promote skin pigmentation.
2û Another object of the present invention is to solve the
new technical problem consisting in the preparation of a novel
formulation of a cosmetic or pharmaceutical composition presenting
a good melanogenesis-promoting activity at the level of the
melanocytes present in the skin.
Yet another object of the present invention is to
provide a solution to the new technical problem consisting in
providing a plant extract particularly easy to obtain, which
presents in itself a good melanogenesis-promoting activity at the
level of the melanocytes in the skin, without having to isolate
any active substance, these isolation processes being in general
long and expensive.
Yet another object of the present invention is to solve
the new technical problem consisting in providing a novel
formulation of a cosmetic or pharmaceutical composition presenting
a good melanogenesis-promoting activity at the level of the
206~768
melanocytes present in the hair follicles, while promoting hair
pigmentation.
All these new technical problems are solved for the
first time by the present invention in a satisfactory way usable
05 on an industrial scale.
Thus, according to a first aspect, the present invention
relates to the use of an extract of Coleus for preparing a
cosmetic or pharmaceutical composition, particularly a
dermatological composition, intended to promote skin pigmentation
and/or to the treatment of skin pigmentation disorders.
According to a particular characteristic of the
invention, the extract of Coleus is an extract of the Coleus
forskohlii specie. It is preferably an extract of roots.
According to another characteristic, an organic extract
of Coleus is used, preferably of rootC of Coleus, advantageously
obtained by a process comprising at least one step of extraction
with a solvent selected from the group constituted by ethyl
acetate, methanol, ethanol and dichloromethane. In a general way,
organic solvents, such as aromatic hydrocarbons, halogenated
aliphatic or aromatic hydrocarbons, dialcoylic ethers,
dialcoyl-Ketones, alcanols, carboxylic acids and their esters or
other solvents, such as dimethylformamide, dioxane,
tetrahydrofurane and dimethylsulfoxide can be used. Among the
aforesaid solvents, preferred solvents are benzene, toluene or
xylene, methylene chloride, chloroform, ethyl acetate, methanol or
ethanol. The ratio of the plant matter to the extraction agent is
not critical and is generally ranged between 1:5 and 1:20 parts by
weight, and it is preferably about 1:10 parts by weight. The
extraction is effected at temperatures included between ambient
temperature and the boiling point of the solvent used for
extraction. An advantageous technique of extraction is the
technique known as the technique of extraction employing Soxhlet.
It may be advantageous, and in certain cases necessary, to
evaporate the solvent, for example by lyophilization, and to take
up the crude extracts for purification purposes. According to the
206~76~
s
present invention, extraction by alcohol is particularly
advantageous, notably at the end of the process for obtaining the
extract due to the usually hardly toxic nature of the alcohols. A
particularly advantageous alcohol is, for example, ethanol.
05 Another particularly advantageous solvent is ethyl
acetate because it provides an extract rich in labd-14-ene
derivatives.
Particular variants of process are described in the
litterature, in particular in the documents set forth in the
preamble of the previous description.
Generally, the concentration of the extracts used
according to the present invention, for preparing a cosmetic or
pharmaceutical composition, expressed by dry weight is ranged
between 0.001% and 2% by weight, preferably between 0.01% and 0.5%
by weight, with respect to the total weight of the composition.
The cosmetic or pharmaceutical, particularly
dermatological compositions, according to the present invention,
may be applied by topical route to promote skin pigmentation, in
particular in compositions in the form of creams, gels or lotions
intended for application on the skin.
Thus, the cosmetic or pharmaceutical, particularly
dermatological compositions, according to the invention, have
various applications in cosmetology or dermatology, in particular
when an increase in pigmentation is sought. For example, these
compositions can be used as solar products for activating or
intensifying the suntan which, in addition to the aesthetic
advantage often sought, enables strengthening of the natural
defenses ~against ultraviolet radiation by increasing the melanine
rate in the epidermis. Moreover, in dermatology, the compositions
according to the present invention can be used as therapeutical
agents, alone or combined with other drugs, in particular in
topical administration for treating melanogenesis disorders.
According to an advantageous embodiment, a cosmetic or
pharmaceutical composition according to the invention comprises in
addition a xanthine, such as in particular IBMX or theophylline,
2064768
preferably at a weight concentration ranged between 0.01% and 2%,
and still preferably between 0.01% and 0.5% with respect to the
total weight of the composition.
According to another embodiment, a cosmetic or
05 pharmaceutical composition according to the invention further
comprises tyrosine or one of its derivatives, preferably at a
weight concentration ranged between 0.001% and 10%, with respect
to the total weight of the composition.
According to yet another embodiment, a cosmetic or
pharmaceutical composition according to the invention further
comprises at least one other active substance, at an efficient
concentration, selected from vitamins, in particular vitamins B,
quinine or its derivatives, rubefacients, such as methyl
nicotinate, a supernatant of culture of fibroblasts of papillae,
as defined in document EP-A-272 920, keratin hydrolysates,
oligo-elements such as zinc, selenium, copper, 5-C~-reductase
inhibitors, such as progesterone, cyproterone acetate, minoxidil,
azelaic acid and its~derivatives, a 4-methyl-4-azasteroid, in
particular the 17- P -N,N-diethylcarbamoyl-4-methyl-4-aza-5-~ -
androstan-3-one, or still an extract of Serenoa repens.
According to a second aspect, the present invention also
relates to a process for the preparation of a cosmetic or
pharmaceutical composition, particularly a dermatological
composition, intended to promote skin pigmentation, characterized
in that it comprises the incorporation of at least one extract of
Coleus as described previously in a cosmetically or
pharmaceutically acceptable excipient, vehicle or support.
According to a third aspect, the present invention
further relates to a process for treating the skin so as to
promote pigmentation, characterized in that it comprises the
application in an efficient quantity to produce a pigmentation, of
at least one extract of Coleus such as described previously,
incorporated in a cosmetically or pharmaceutically acceptable
excipient, vehicle or support.
According to a fourth aspect, the present invention also
- 20~768
relates to the use of an extract of Coleus combined with an
efficient quantity of at least another active substance, selected
from xanthines, such as in particular IBMX or theophylline,
quinine or its derivatives, rubefacients, such as methyl
05 nicotinate, a supernatant of culture of fibroblasts of papillae,
keratin hydrolysates, oligo-elements such as zinc, selenium,
copper, 5-d -reductase inhibitors, such as cyproterone acetate,
minoxidil, azelaic acid and its derivatives, a 4-methyl-4-azaste-
roid, in particular the 17-~ -N,N-diethylcarbamoyl-4-methyl-4-aza-
5-C~-androstan-3-one, or else an extract of Serenoa repens, for
the preparation of a cosmetic or pharmaceutical composition,
intended to promote hair pigmentation.
According to particular variant embodiments, the
preferred combinations relate to the combination of an extract of
Coleus, in particular an extract of Coleus forskohlii, preferably
of root, with a xanthine, such as in particular IBMX or
theophylline.
According to a fifth aspect, the present invention also
relates to a process for the preparation of a cosmetic or
pharmaceutical composition, intended to promote hair pigmentation,
characterized in that it comprises the incorporation of at least
one extract of Coleus as described previously in combination with
at least another active substance as defined above with regard to
the use, in a cosmetically or pharmaceutically acceptable
excipient, vehicle or support.
Finally, according to a last aspect, the present
invention also relates to a process for treating hair so as to
prevent gray hair from appearing and/or to restore the natural
color of hair, characterized in that it comprises the application
on hair of an efficient quantity in order to produce hair
pigmentation, of at least one extract of Coleus as described
previously in combination with at least one active substance as
defined previously, incorporated to a cosmetically or
pharmaceutically acceptable excipient, vehicle or support.
For each one of the preceding aspects intended to
` 2~64768
promote hair pigmentation, the concentration used is generally the
same as that used to pigment the skin.
Other purposes, characteristics and advantages of the
invention will appear more clearly from reading the following
05 explanatory description given with reference to several examples
given solely by way of illustration and which consequently could
not in any way limit the scope of the invention.
In the examples, the percentages are expressed by
weight, unless otherwise indicated.
EXAMPLE 1
Preparation of an extract of Coleus from Coleus forskohlii
12 kg of dried and ground roots of Coleus forskohlii are
extracted twice with each time 20 l of petroleum ether.
The roots are then repeatedly extracted with 15 l of methylene
chloride till they are exhaustively extracted. 60 l of methylene
chloride are used.
The extracts are combined with methylene chloride and
are then filtered or centrifuged and concentrated under a reduced
pressure.
The obtained residue is extracted twice each time with 3
1 of methanol.
The combined methalonic extracts are filtered and the
methalonic filtrate is evaporated under reduced pressure, thus
obtaining the extract of Coleus forskohlii called extract I1.
EXAMPLE 2
12 kg of dried and ground roots of Coleus forskohlii are
extracted twice with 15 1 of petroleum ether.
3û The roots are taken up in order to proceed to repeated
extractions with 25 1 of methanol until complete extraction. The
combined methalonic extracts are filtered and are evaporated in
vacuo.
An extract of Coleus forskohlii called extract I2 is
thus obtained.
2064768
EXAMPLE 3
12 kg of dried and ground complete plants of Coleus
forskohlii are repeatedly extracted and ground with a total of 100
l of methanol until the extraction is completed. The combined
05 methalonic extracts are filtered and evaporated in vacuo.
The obtainsd extract of Coleus can be used.
However, according to a variant embodiment, the residue
(about 650 9) is partitioned by using a chloroform/water solvent
mixture (2/1.5 v/v). Ths chloroformic layer is recovered after
decanting. The aqueous layer is repeatedly extracted at the same
time as the intermediate layer each time with 1.5 l of chloroform
and the obtained chloroformic layer is recovered every time.
The combined chloroformic extracts are then filtered,
dried over anhydrous sodium sulfate in order to eliminate the
residual water and evaporated in vacuo. About 300 9 of an extract
of Coleus called extract I3 is obtained.
EXAMPLE 4
Dried and finely ground roots of Coleus forskohlii
(1 kg) are repeatedly extracted with benzene (3 x 5 l) preferably
according to the extraction technique using Soxhlet at 70C for
several hours. The obtained benzene extracts are filtered and
concentrated in vacuo in order to eliminate the benzene. The
obtained extract of Coleus is called I4.
EXAMPLE 5
60 9 of dried and ground roots of Coleus forskohlii are
extracted with Soxhlet with 600 ml of solvent. Extraction is
effected at the boiling temperature of the solvent with an
ever-renewed solvent.
The mean yield of extraction is as follows, expressed in
dry weight :
a) Ethanol/water mixture (80/20 v/v) : about 9.2 to 9.5 9,
(Extract 5A)
206~7~8
b) Ethyl acetate : about 1.5 9, (Extract 5B)
c) Dichloromethane : about 4 9, (Extract 5C)
EXAMPLE 6
05 Measurement of the activity of an extract of Coleus forskohlii
according to the invention on cultured melanocvtes
Protocol :
Murine melanocytes are conventionally cultured in an
appropriate medium.
On day D = O, the product to be tested is introduced in
the culture medium.
On day D = 5, cells are removed and isolated by
centrifuging and the cellular residue is recovered and dissolved
in soda 0.5 N.
A reading of the optical density is then effected on a
spectrophotometer at 475 nm, which makes it possible to assess the
quantity of melanine formed by comparison to the optical density
of a melanine solution of known concentration.
Numbering of the cells is also e ff ected and the rate of
melanine formed per cell is determined, with respect to a control
culture without adding a product to be tested.
Test :
The extract Coleus 5B, according to example 5b is
compared with the forskoline alone, used at an equivalent
concentration with a positif control consisting in 10 8 mole of
~ -MSH. Forskoline at a concentration of 0.1 >g/ml is thus
introduced on day D=O in the culture medium.
In another culture medium, the extract of Coleus is
introduced at a determined concentration such that the forskoline
rate is also of about û.1 ~g/ml.
Table I hereinafter shows the obtained results.
2064768
11
TABLE I
- i
¦ ¦ Control ¦ Extract ¦ Forskoline ¦ ~-MSH ¦
05 1 ¦ ¦ of Coleus l
¦ Melanine
¦ formed in >g
¦ for 106 ¦27 + 1 ¦116 + 2 192 + 3 ¦ 70
I cells
¦ A-activity
¦ in % ¦ 0 ¦207 1151 1 100
.1 1 1 1 1 1
The A-activity of the stimulation of the melanogenesis
by the products according to the invention is determined by the
following formula :
q - q
A = x 100
q+ qo
-
in which the sizes q represent the quantities of melanine formed
qp = culture medium receiving the product to be tested
q+ = culture medium receiving the ~MSH
qO = control culture receiving no product.
It is clear from table I that the extract of Coleus
according to the invention promotes the production of melanine in
a significantly greater proportion than forskoline alone, which
represents an unexpected result for the man skilled in the art.
2064768
Furthermore, extracts of Coleus 5A and 5C, respectively
obtained by extraction by a ethanol-water mixture 80:20 and by
dichloromethane were tested in similar conditions. However it
should be noted that a culture medium receiving some ~-MSH at a
05 concentration of 2.10 8 M was taken as positive specimen.
The activity of these extracts, determined according to
the aforesaid formula, is shown in table II hereinafter.
TABLE II
¦ Tested product Concentration ¦ Melanine formed in ¦ A -activity¦
¦ ¦ >9 for 106 cells ¦ in %
¦Extract 5A 1 >g/ml ¦ 121 + 3 ¦ 46
I n 2.5 n n ¦161 + 5 ¦ 83
I n 5 n n ¦188 + 9 ¦ 108
¦ Extract 5B 1 n 1~ ¦ 89 + 3 ¦ 23
I n 2.5 n ~I 1114 + 7 ¦ 45
I n 5 ..... n ¦ 171 + 1 ¦ 95
I n 10 n n I 197 + 6 ¦ 118
¦~ -MSH 2.10 8 M ¦ 179 + 4 ¦ 100
¦ Cbntrol
¦ (no product) ¦ 71 1 3 ¦ 0
20~768
13
EXAMPLE 7
Measurement of the activity of a combination according to the
invention of Coleus forskohlii with the 3-isobutyl-l-methylxanthine
(IBMX), on cultured melanocytes.
05 The procedure is that of example 6. The combination IBMX
plus extract of Coleus is tested in comparison with the IBMX and
the extract of Coleus alone, as well as with the ~ -MSH at a
concentration of 2.10 8 M taken as positive specimen.
The results on day D = 4 and D = 5 are shown in table
III hereinafter.
It is noted on day D = 4 and D = 5 for the combination
IBMX + extract of Coleus a much more important activity, which is
greater than the amount of activities corresponding to each of the
two constituents tested separately.
This proves a very definite reinforcement of the
activity of the extract of Coleus by the IBMX.
TABLE III
1 1 1 J = 4 J = 5
PRODUCT 1 Concen- 1 Melanine >9 1 A 1 Melanine >91 A
1 1 tration 1 for 106 1 1 for 106
1 1 >g/ml ¦ melanocytes ¦ ¦ melanocYtes¦
¦Ncne
¦(control) 1 0 1 29 + -3 1 0 1 51 + -l I ¦
1 ~-MSH
1 2.10 8 M 1 1ll9 + -7 1 + lûO 1 200 + -2 1 + lOO¦
1 l/ 1 lO 164 + -O 1 ~ 39 1 103 + -2 1 + 35
206~768
TABLE III (continued)
.
l l I J = 4 J = 5
05 ¦ PRODUCT ¦ Concen- ¦ Melanine >g ¦ A ¦ Melanine >9¦ A
¦ ¦ tration ¦ for 106 1 ¦ for 106
¦ >g/ml _ l melanocytes ¦ ¦ melanocytes¦
2/ 1 0.6 1 53 + -2 1 + 27 185 + -4 1 + 23
3/ 1 10 + 0.6 1 120 + -61 + 101 1 186 + -13l + 91
1.
1 : IBMX
2 : extract of Coleus 5B
3 : IBMX + extract of Coleus 5B
N.B. The A-activity of the tested products is determined as
indicated in example 6.
EXAMPLE 8
Measurement of the activity of an extract of Coleus forskohlii
according to the invention on thq cutaneous pigmentation in the
guinea pig
Protocole :
The study is made on a batch of 10 three-colored guinea
pigs,
Before and during the experimentation, the right and
left flanks of the guinea pi98 are carefully shaved, every day for
the first 5 days (period of exposure to U.V.), then every 2 days
until the end of the study.
For each animal, comparably pigmented marks are found on
each flank, mainly of light brown appearance. On one of the two
flanks taken at random, about 0.5 9 of product to be tested or of
control product, depending on the batch, is applied 10 mins.
2064768
before exposure to ultraviolet rays, the other flank being exposed
"bare" by way of control.
The application of the products to be tested is effected
as from the first day of exposure until the animal is sacrificed.
05 Exposure to ultraviolet radiation is effected by means
of a solar simulator delivering 86% of U.V.A. and 14% of U.V.B.
during the first 5 days of the experiment, at a rate of 5 mins.
the first day, 10 mins. the second day, 15 mins. the third day and
20 mins. the fourth and fifth days.
The animals ars sacrificed 12 days after the last
exposure, and a cutaneous biopsy is effected.
A fragment of skin is thus removed from the non-treated
but exposed flank as well as from the other treated and exposed
flank.
A histological examination is then made of the cutaneous
fragments.
This examination comprises : on the one hand, the study
of the melanogenesis by the Argentaffine de Fontana method on
sections of 4 ~ m (Techniques d'histologie, Professor Chevreau, Ed.
Maloine, 1977, page 157), on the other hand, the assessment of the
thickness of the epidermis on sections of 4~ m colored in
accordance with the trichomic method of Masson.
The study of the thickness of the epidermis and of the
intensity of the melanogenesis makes it possible to assess a
suntan effect ~or more exactly the activation of the process of
melanogenesis.
To study the melanogenesis, two zones taken at random
from 8 pigmented mark are examined, in which zones 25 malpigian
cells are noted and among these the "activated" melanocytes, i.e.
containing melanine in a cluster. The activation of the process of
melanogenesis is then expressed in percentage of activated cells
from the average of these two values.
On these same zones, the quantity of melanine in the
other layers of the epidermis is examined and this quantity is
assessed overall in a five value scale varying from O to 4
206476~
16
depending on whether the quantity of melanine formed is zero, low,
average, large or very large.
Table IV shows the results of the histological study
giving the percentage of activation and making it possible to
05 assess the variation in the quantity of melanine formed (average
values on the scale defined hereinabove), of the thickness of the
epidermis (expressed in ~ m).
The product to be tested is constituted by an extract of
Coleus with ethyl acetate according to example 5b.
TABLE IV
¦ ¦ Control ¦ Flank treated with¦
I I flank I an extract Df
l l ¦ Coleus
I
1% Activation ¦ 31 ¦ 90
¦Quantity of melanine ¦ 0.71 1 2.42
20¦Thickening of the epidermis in ~m ¦ 8.96 ¦ 9
From table IV it may be ascertained that the extract of
Coleus is active on melanogenesis. A reading of the histological
sections of the treated flanks shows a very high rate of activated
melanocytes presenting rhizomic forms, and a considerable
migration of the grains of melanines in the epidermis.
These results therefore clearly confirm the activity of
the extracts of Coleus in accordance with the invention on
melanogenesis in animal skin.
206476~
17
EXAMPLE 9
Measurement of the activity of an extract of Coleus forskohlii
according to the invention on the cutaneous pigmentation in man.
The influence of the products according to the invention
05 were measured on the appearance and intensity of the suntan in man.
Protocol :
The experimentation was made on 12 subjects of the II or
III skin type, which skin is not yet tanned.
The products to be tested were first of all applied
twice a day (morning and evening) on the inside face of a forearm
of each subject, during a first period of one week (7 days) ; the
applied quantity being that conventionally used.
These products were then applied in the same quantities,
i.e. only once a day (morning) during a second period of two weeks
during which irradiation takes place.
The minimum erythemale dose (MED) was determined for
each subject by irridiation of the arm opposite the one to which
the products were applied.
The products to be tested were applied on four separate
zones of the forearm. These four zones are exposed to U.V.
supplied by a solar simulator once a day (afternoon) at a daily
dose which is lower than MED. When a slight tan appears, the
radiation dose administered for 5 to lû seconds according to the
skin type is increased while avoiding an occurence of an erytheme.
The exposures are effected every day until a definite
tan is obtained, i.e. 8 days (twice 4 days separated by an
interval of 2 days).
Measurements are effected on the skin which is not
exposed to U.V. and on the treated zones.
A mark of 0 to 5 is given depending on the intensity of
the tan. The more greatly tanned area is marked 5, the area
without tan is marked 0, while the mark given to the other area is
fixed between 0 and 5 depending on the intensity of the tan.
Several resdings are effected by different persons and the average
206~768
18
given mark is retained. The standard deviation is determined and a
t test is effected in order to check if the results are
significant.
Three products were tested :
û5
product A : extract of Coleus 5B, according to the invention, at
0.025% in a gelled excipient,
product B : extract of Coleus 5B, according to the invention, at
0.05% in the same excipient,
product C : the gelled excipient.
The results shown on table V respresent for the three
products A, B and C the average marks of the intensity of the
tan during 8 days of exposure to U.V.
TABLE V
¦ Product ¦ Reading average ¦ Significance with respect
l l ¦ to the U.V. alone
¦ A 1 3.55 + 0.88 I S (~= 0.05)
¦ B ¦ 2.85 1 0.76 .I S ( n
¦ C ¦- 2.53 + 0.58 I NS ( n
¦ U.V. slone ¦ 2.14 + 0.67 ¦ S (with respect to the
l l ¦ control without U.V. ¦
¦ control
¦ without U.V.¦ 0 + 0
206a~768
These results clearly show that an extract of Coleus in
accordance with the invention makes it possible to intensify the
cutaneous tan in man. This confirms the test results obtained on
melanocyte in culture medium or in vivo on the aforementioned
05 guinea pigs.
Various examples of formulation of cosmetic or
pharmaceutical composition, particularly a dermatological
composition having an activity in the treatment of cutaneous
pigmentation disorders will be given hereafter.
EXAMPLE 10
Tan gel for the face
Extract of Coleus (dry weight) according to example 1 0.0284 9
Ethanol 40.- 9
Distilled water 2û.- 9
Carbopol 940 R gel at 1% qsf 100.- 9
EXAMPLE 11
Tan solar cream
Extract of Coleus (dry weight) according to example 2 0.03 9
Isocetyl stearate 8.- 9
Hydrogenated peanut oil 10.- 9
Lanoline oil 3.5 9
Cetylic alcohol 5.- 9
Stearylic alcohol 2.5 9
Light paraffin oil 10.- 9
Phosphoric monoester of the
neutralized OE cetylic alcohol 3.- 9
Cinnamate octylmethoxy 5.- 9
This phase is emulsified with an aqueous phase qsf 100 9
containing :
Pantothenol 0.1 9
Preserving agents 0.2 9
206~7~8
EXAMPLE 12
Lotion for strengthening the natur~l solar protection
Alcohol 42.5 9
Propylene glycol 3.- 9
05 Menthol 0 05 9
Hydroxypropylmethylcellulose 1.5 9
Extract of Coleus (dry weight) according to example 5b 0.03 9
Perfumed aqueous excipients qsf 100 9
This lotion is applied locally, preferably twice a day,
and daily for 3 to 8 days preceding prolonged exposures to the sun.
The daily applications can be continued during the
exposure period.
EXAMPLE 13
Tonic hair lotion preventing gray hair from appearing
Extract of Coleus according to example 5c û.02 9
IBMX 0.1 9
Alcohol 30,_ 9
Water 69.- 9
Perfumed excipients qsf 100 9
This lotion can be applied on the hair and on the scalp
twice a day in a cure of 3 months.
EXAMPLE 14
Dermatological gel intended to promote skin pigmentation
Extract of Coleus according to example 5b 0.03 9
Ethanol 30.- 9
30 Distilled water 20.- 9
Carbopol R gel qsf 100 9
This gel is used once or twice a day in local
application on the zones of the skin to be treated.
206~768
21
EXAMPLE 15
Hair lotion preventing gray hair from appearing
Extract of Coleus according to example 5b 0.03 9
L-tyrosine ethyl ester, HCl 1.- 9
05 Ethanol 40 _ 9
Perfume 0.5 9
Cremophor 0.2 9
Water qsf 100 9
This lotion, applied daily on the hair and on the scalp,
makes it possible to retard the appearance of grey hair.
EXAMPLE 16
.
Tan gel for the skin
Extract of Coleus 0.03 %
IBMX 0.1 %
Absolute ethanol 30 %
Water 19.86 %
Carbopol 940 R gel at 1.25% qsf 100 %
Application : once a day on the zones of the skin to be
treated.
-" 20~768
EXAMPLE 17
Treating gel for preventing the appearance of grey hsir
Extract of Coleus 0.025 % )
Absolute ethanol 30 % ) A
05
Bidistilled water 19.925 % )
Methyl nicotinate 0.05 % ) B
Carbopol 940 R gel at 1.25% qsf 100 %
After solubilization of the active principles in A and
B, introduce A into B, stir 30 mins. at ambient temperature on the
bar magnet. Add the gel, homogenize with Raynerie.
To be applied daily on the hair and on the scalp.
EXAMPLE 18
Gel promoting cutaneous pigmentation
Extract of Coleus 0.01 % )
Absolute ethanol 28 % ) A
Theophylline 0.01 % )
Water qsf 100 % ) B
Carbopol 940 1 %
This gel is prepared according to the process of example
17 and is applied once or twice a day on the zones of the skin to
be treated.
Naturally, the invention comprises all the means
constituting technical equivalents of the described means as well
as various combinations.