Note: Descriptions are shown in the official language in which they were submitted.
W091/0~698 PCT/AU90/00~8
- 1 - 2064809
`
~ ~ :
IMPROVED SEWAGE TREATMENT PROCESS
This invention relates to an improved process for
the treatment of sewage or other wastewater containing
organics to provide a clear li~uid effluent which may be
disposed of safely, e~g. into the ocean or a waterway and
a dense sludge suitable for landfill or other uses.
Australian Patent Specification No. 79700/87, the
complete disclosure of which is incorporated herein by
reference, describes a process or treating sewage in
which a particulate mineral or clay material (referred as
a "coagulant/adsorbent") is mixed with the raw sewage
WO9l/02698 PCT/AU~/0~3~8
20~j~809 f,'''.' `,
-2
under slightly acidic conditions in conjunction with the
addition of a coagulant. After separation of the
clarified sewage, a concentrated slurry containing the
organic matter originally present in the raw sewage can
be produced by the addition of alkali to the separated
particulate mineral or clay material and by passing the
mixture through a solid separation device. The
concentratad slurry is then treated by anaerobic
digestion for about one day to produce an effluent which
is passed to a settling pond where the particulate
biomass eventually settles out. The overflow from the
set~ling pond is suitable for recycling for washing
purposes in the plant.
The concentrated slurry is high in water content and
causes problems if it is disposed of by landfill without
anaerobic digestion. Anaerobic digestion is a slow and
uncertain process susceptible to environmental and feed
water changes. The need for further treatment in
settling ponds increases the area of land needed and
hence the capital cost of the sewage treatment plant as
well as the time required for treatment.
Surprisingly, we have now found that the
concentrated slurry can be further concentrated by the
addition of acid to lower the pH of the slurry to 4 or
less whereby a dense sludge is quickly formed. The
supernatant liquid may be easily separated and used to
acidify -the incoming raw sewage. The sludge being high
in solids can be readily dewatered before disposal to
landfill or reuse.
According to the present invention, there is
provided a process for the treatment of sewage or other
wastewater containing organic matter which comprises the
steps of:-
. ' '
; ' , ` ~
.: ., . :, ~ . .
WO91/02698 ~ i; PCT/A~9~ 3~
` -3~ 20~48~9
(a) mixing sewage or other wastewater containing organic
matter with a coagulant/adsorbent which is a finel~
divided particulate mineral or clay material the
S individual particles of which have a thin
hydroxylated surface layer, under conditions whereby
at least a substantial proportion of the organic
material in the sewage or other wastewater
containing organic matter becomes attached to the
coagulant/adsorbent;
(b) separating the coagulant/adsorbent with attached
organic material from the mixture to leave a treated
- liquid effluent;
(c) treating the separated coagulant/adsorbent from step
(b) with alkali thereby to release the organic
material therefrom, separating the
coagulant/adsorbent from the resultant concentrated
slurry containing the organic material; and
(d) adding acid to the concentrated slurry of organic
material to lower the pH to less than 4 and thereby
to obtain a sludge which separates under gravity and
a supernatant liquor which optionally may be
recycled to acidify he incoming sewage or other
wastewater containing organic matter.
The coagulant/adsorbents which may be used in
accordance with the present invention may be f two
notionally different types, i.e.: (I) those in which the
hydroxylated layer is derived directly from the substance
of the particles; and (II) those in which the layer is
derived from another substance.
The preferred coagulant/adsorbent materials are
those of t~pe I and these can be derived from a wide
.. . .
':
.
W091/02698 PCr/A~90/~0~4~
~o6~ 9 4 ~"
variety of minerals and clays provided the nature of the
mineral is such as to permit the ready formation of the
hydroxylated surface. In this respect oxides and
silicates are particularly useful.
Examples of such minerals include zinc oxide, silica
and siliceous materials such as sand and glass and clay
minerals such as mica, china clay and pyrophillite. This
list is not exhaustive, however, and many other minerals
are suitable for use in this invention.
In the most preferred embodiment of this invention,
the particulate material is a magnetic or magnetisable
material. For this purpose iron oxides, such as gamma
iron oxide or magnetite, which are eminently suitable, or
ferrites, such as barium ferrite or spinel ferrite, can
be used.
The coagulent/adsorbent particles should have a
particle size of 50 microns or less, preferably 1 to 10
microns more preferably 1 to 5 microns.
- ,, .
The preparation of finely divided
coagulant/adsorbent particles of type I to give each a
thin hydroxylated surface layer is easily carried out,
usually by suspending the particles in a basic,
preferably an alkali, solution for a short period of
time, preferably in the presence of air. Sodium
3~ hydroxide is suitable, but potassium hydroxide, lime or
aqueous ammonia may also be used. Generally, alkali
concentrations should be at least O.OlM, preferably about
0.05M to O.lM, at which level the treatment is effective
after abou~ 10 minutes. Shorter treatment times can be
achieved by the use of elevated temperatures and/or
higher alkali concentrations. A suggested temperature
. . . . . ..
. . ,
,
', ' ' ':,''' ',~ ~' ' ' , '
' ~ , : , :
~091/02698 PCT/AU90/00~8 '~
f ' ! ,.~ ;, . '. i . '
-5- 20~809
range is 40-600C. For example, a satisfactory material
is produced using either O.lM sodium hydroxide at room
temperature (i.e. about 200C) for ten minutes, or 0.05M
sodium hydroxide solution at about 600C for five minutesO
Because the hydroxylated layer of the type II
coagulant/adsorbent is provided by a different substance,
to the material of the mineral or clay particle the range
of starting materials is broader. A wide variety of
minerals and clays can be used provided the nature of the
mineral or clay is such as to permit the ready deposition
of a hydroxide gel on its surface. In this respect
oxides, sulphates, silicates and carbonates are
particularly useful. Examples of such minerals include
calcium sulphate, calcium carbonate, zinc oxide and
ba ium sulphate. This list is not exhaustive, however,
an_ many other minerals are suitable for use in this
invention. In some cases, pre-treatment of the surface
of the mineral may be required to produce a satisfactory
deposition of the hydroxide layer. Yet another
alternative is to use hollow microspheres, e.g. of glass
for the production of gel particles which can be
separated from the liquid effluent, after the adsorption
of the organic material in step (a), by flotation rather
than sedimentation.
The hydroxylated layer of the coa~ulant/adsorbent
particles of type II can be provided by any of a number
of metal hydroxides, the requirements being substantial
insolubility in water and a metal valency preferably of
three or more.
Suitable metals with this characteristic are iron,
aluminium, zirconium and thorium. Ferric hydroxide is
preferred because it is cheap, and exceptionally
', ' ' ' '' ': ,
;
W~91/026~8 PC~/AU90iO0~
~,e~4~
~ 6- ~"
insoluble, over a wide pH range. For example, it does
not readily dissolve at high pH, as does aluminium
hydroxide.
s
The preparation of the coated particle of type II :is
also easily carried out, usually by suspending the
particles in water, adding a salt of a suitable metal
followed by an alkaline material, preferably in aqueous
solution which will precipitate the metal hydro~ide which
then forms a coating on the particle. Typically,
chlorides, sulphates, nitrates or other minera~ acid
salts of the metals are suitable; ferric chloride or
aluminium sulphate are examples. The alkaline material
may be sodium hydroxide, calcium hydroxide, ammonia or
similar soluble material. The concentration and
temperature at which the preparation is carried out is
generally not critical.
In the case where magnetite or other iron oxide
materials are used as the basis for type II particles,
the metal salt which is employed to produce the hydroxide
layer may be obtained by~-first adding acid to the
suspension of the particles (to give ferric and/or
ferrous salts in solution from the iron oxide) and then
adding the alkaline material.
After preparation, it is best if the coated
particles are not permitted to dry out. This can be
avoided by keeping them under water. The thickness of
the hydro~ylated layer on the particles is not important
since the flocculation or coagulation is a surface
effect.
An important advantage of the process of the present
invention is that ~he coagulant/adsorbent particles can
- , . ,
,. , :
- . ' ~ : '
; , ,
,
WO91/02698 PCT/~U90/~ 8
(` 7 206~8~9 . I
be recycled many times. To achieve this, the adsorbed
material is removed by raising the pH of a suspension of
the adsorbent in water. In the case of type I
coagulant/adsorbents, the coagulating properties may be
regenerated by treatment with alkali solution; these two
treatments may be combined.
As in the "Sirofloc" process of water clarification
described in the applicant's Australian Patent No.
512,553, the process of the present invention may be
enhanced by the addition to the li~uid under treat~e~t o*
a suitable coagulant, such as polyelectrolyte (cationic,
anionic or non-ionic) and/or an inorganic coagulant which
provides multi-valent cations such as FeZ~ (e.g. ferrous
sulphate). More usually the multi-valent cations will
have a valency of three or more, such as Fe3~ or Al3~,
(e.g. from alum or ferric chloride). These coagulants
are not essential but when both types (i.e.
polyelectrolytes and the inorganic coagulants) are
present they complement each other. The polyelec-trolyte
may be present in the range 0 to lO mg/L, preferably from
2 to 5 mg/L. The inorganic coagulant may be present in
the range 0 to 500 mg/L, preferably 20 to 50 mg/L.
The separated coagulant/adsorbent from step (c) of
the process may be optionally recycled to step (a).
Any strong acid may be used to reduce the pH in step
(d) of the process. Acids of this type include mineral
acids, such as, sulphuric acid or hydrochloric acid and
organic acids, such as, fluoroacetic acid.
The sludge produced by the process of the invention
contains me~al hydroxides and organic matter and
optionally may be dr.ied by any conventional technigue,
WO91/0269~ - PCT/A~0/~
2~ 09` - 8- ~ -
including centrifugation, belt-pressing or microwave
radiation.
S Alternatively, the organic material may be removed
from the sludge by the process known as "wet air
oxidation" leaving only a small volume of metal oxides
for disposal.
The preferred coagulant/adsorbent is magnetite and
the following detailed description will refer to that
material. It will be appreciated however, that reference
to magnetite includes mutatis mutandis reference to other
coagulant/adsorbents.
Reference will now be made to the accompanying
drawing in which:
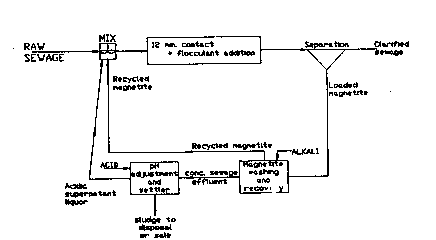
Figure l is a flow diagram showing the process of
the invention in its basic form.
Sewage or other wastewater containing organic matter
is mixed with finely-d~vided cleaned recycled magnetite
particles which have been regenerated by suspension in a
solution of caustic soda to produce a thin hydroxylated
surface la~er, and the mixture is stirred to provide good
contact of sewage or other wastewater containing organic ~,
matter with the regenerated magnetite particles which are
preferably in the size ranga l to lO microns. The pH
level may be adjusted by acid addition to be in the range
5 to 9, preferably 5.5 to 6.5 and the addition of an
inorganic coagulant and/or a coagulant aid (e.g. a
polyelectrolyte) may also be necessary to achieve a
satisfactory effluent quality depending on the strength
35 and composition of the input feed. After 2 to 20 ~ -
minutes, preferably lO to 15 minutes, of contact the
magnetite slurry is separated from the treated effluent
~ .
~, ' , ':
' .: , ' ' :'
- ' ~ ' . ~ .
.
WO 91/02fi98 PCT/AU90/00348
, .,
which may go to polishing ponds for final treatment
before discharge~ The magnetite particles, which now
have attached to them most of the organic material
originally present in the sewage or other wastewater, are
then cleaned b~ stripping off the organic material using
a dilute solution of sodium hydroxide, ammonia, or
potassium hydroxide or, for example, a lime slurry which
also regenerates the magnetite. The magnetite can then
be recycled while the liquid slurry produced in the
stripping or regeneration step is acidified to a pH level
below 4 by the addition of sulphuric acid. Acidification
causes a sludge of organic matter to separate out by
gravity. This sludge is formed within a few minutes of
acid addition but takes abou~ 30 minutes to fully settle
out from the superna~ant liquor. The sludge can also be
rapidly separated by dissolved air flotation. The sludge
thus produced can then be readily dewatered by a number
of standard techniques, for example, centrifugation, belt
press filtration, and then dried on sand beds to produce
a granular cake suitable for disposal by landfill. The
acidic supernatant li~uor can be recycled to acidify the
incoming sewage or other wastewater.
The invention is further described in and
lllustrated by the following examples. These examples
are not to be construed as limiting the invention in any
way.
The following abbreviation is used:-
COD - chemical oxygen demand
Exam~le 1
Preliminary experiments were performed in which 50
ml of the sewage concentrate (old stock, stored 1 week in
. ' '
:
.~ . ' ' ~
W091/02698 PC~/AU90/OOY48
~6~S , ,,, " ~ 10-
.. .
a cool room) was acidified from pH 10 down to about pH 70
However, at p~ 7 little coagulation occured.
Polyelectrolyte was then added to the sewage concentrate
S after pH reduction to 7 and again, no further coagulatio~
was observed. The pH of the sewage concentrate was
further reduced by slowly adding acid. Coagulation was
observed to commence at a pH of about 3.5, the amount of
acid added being 30mmole H~/litre of sewage concentrate.
Exam~le 2
A set of experiments were performed to investigate
the effect of pH on fresh sewage concentrate. The
lS amounts of acid added were 30, 40 and 50mmole H~/litre of
sewage concentrate, respectively. The results are shown
in Table 1. The pH of the sewage concentrate drops to
3.5, 3 and 2.5 respectively, while the percentage COD
removed from the sewage concentrate increased from 80 -to
85%. The sludge volume after 45 minutes settling was 35%
of the total volume. This sludge volume could be reduced
further in a thickener.
Example 3
Experiments were performed to investigate the effect
of temperature and acid dosage on the coagulation by
acidification process. The temperatures chosen were 30,
40, 50 and 600C. The results of these experiments are
3U shown in Table 2. The same batch of sewage concentrat~
(2 days old) as used in Example 2 was employed. The
results show that the percentage COD removal was
relatively independent of temperature and acid dosage
(above 40 mmole H~jlitre of sewage concentrate) and that
fresh sewage concentrate can be coagulated better than
old sewage concentrate. The results are also shown in
Figure 2.
~091/02698 PCT/AU90/003q~ ,
2 0 S ~ `
Exam~le 4
The effect of higher doses of acid on both the
release of iron into solution and the capture of organics
from both fresh and stored sewage concentrate is
demonstrated by the results in Table 3 which were
obtained using the method of Example 2. Since the COD
removal is relatively unaffected by temperature (see
Example 3) the experiments were performed at room
temperature (20C). Between 50 and 70% of the iron
present in the sewage concentrate can be solublised by
the addition of between 80 and 100 mmole H~/litre
concentrate. At this acid dose about 90% of the COD in
the sewage concentrate was precipitated. Visually the
flocs formed at higher acid doses were larger and faster
settling. The higher iron removal has advantages where
the clarified supernatant is recycled to acidify the
incoming sewage.
Example 5
Jar tests were performed to determine the effect of
pH on the release of aluminium and COD into solution
during the acidification of sewage concentrate derived
from the "Sirofloc" process for sewage treatment.
Aluminium sulphate was being used as the coagulant in the
"Sirofloc" plant and the sewage concentrate from the
plant was used for these tests. The results of tests
using increasing amounts of sulphuric acid to reduce the
pH of the sewage concentrate are shown in Table 4.
The settling characteristics of the formed floc and
the clarity of the final treated sewage concentrate
varied with the pH of the test. At pH 7.1, the floc
formation was not good and the resultant solution was
.... . ~ .
':
~ .
WO9l/02698 PCI/AU90/0~8
~5~9 ; - 12- ~'
very turbid which resulted in high COD in the treated
sewage concetrate. The aluminium concentration in
solution was also high as would be expected at this pH.
S At pH between about 3.0 and 5.2 the floc formation was
good since alu~inium solubility was low however, the floc
formed was slow settling. The resultant clarifed
solution was clear and of low COD. At pH less than 3OO,
the solubility of aluminium increased and the resultant
floc formed was faster settling and settled to a lower
volume than at the higher pH. COD released into solution
increased as pH decreased. There was obviously a
compromise between release of COD into solution and -the
recovery of aluminium into solution.
.
WO 91/02698 PCr/AUgO/OD348
--13-- ~ r
,Z
~ ~ ~ m
U~d~ ~
~r; c~ D
Z u~ O u
~ , , .
~ D. ~ . -`'
V :~ 000
~: E ~ t`~ 'P u7 ~ ;
,~
,, '.
~3 0 ~ O
E~ o
~:
Lt~ O
: . ~ ` , ` :
:, - :. , . , ' '
.~ .
~ , , .
WO 91/026~8 pcr/hu9~/oo3~8
206~go9 ~ -14-
:~
o~
C~ Z t~ ~ o ~ o a~ m o ~ 0 ~'
~n ~t~oo~ : . :
a -: .
C: ~
~1 ~ ~I O ,~ ~ .:
~:~~p ~; ~
E~ ~ ~ . ''
o :~
~ Z o . ~ .
o ct~ In o u~ ~ o u, ~ o u, a:
~n~ ~D~D~OD~C~C`~
~ . '~.
o ~ ~ o ~I ~ o ~
.
~ . . ' '
Q ~ oooooooo~ooo
" ._ ~u~m~
OooooooooooO
E~ o
~ m
~ ~ ~ co o~ ~ 'I ~
U~
.
: . .. . . .
WO 91/0269X PC~/AU90~003'18
--15-- . 1
f 20~q ~09i
5 o o o o o o o o o
W OD I ~ ~ ~ ~ t~
~_ ~ ~ ~ o ~ c~ a~
o ~
~ ,, o o ~,
~ g o o o o o O
O ~ 1 1 ~ ~ ~
O 0~
U~ ,~
t~ o P~ t:n o
3 to~ 3 ~
u~ o o o o ~ m ~ 3 ~ ~o a
O ~ co ,~ o ~ o ~ 3
~ ~ ~ a) ~ a
,~ ~4 ,~ E 3 3 ~ 3
~ o
Z ~L~ ~ h O ~I h
1~; Ln ~ 0 ~a4tO X'1dP
~:1 ,~ ~ ~ ~ t` O o~ 1 4 0
~: :: E~ ,; ~
E~ U) U~ ta Q~ U1 M .C U~
a~ 3 3 3
3o ~ 3
~ o4 :~t o
h ,
m ~p ~ ~ ~u~ ~ ~ ,~ a) hc ~ h
I . . . . . . .
r~ r~ C 4 U '
~ o ~ ta
:~ tl '~ 3U E~ 3
. a~
t~
h
H 3 0 3 a) 3
~4 ~ u
D; o ~D ~ O O a~ c~ I I _
O
U~
E~
U~
= O ~
a~ o 3 a) o 3
~d ~ ~ ~ a
n O ;~ E~
~ a :~: O o U~ O O O O O O O
P~ a ~ ,~ D OD ~
P~ ~ o ..
~ ~ . ~
~: ~3 :~
o ~ o
r~ r~1
, ~.
.
WO91/02698 PCT/AU90/00~8
16-
TABLE 4
ACID ADDED FINAL SUPERNATANT CONCENTRATION (mg/l)
S (mmolH+/l) pH COD Al
0 10.3 4820 234 ,
7.1 3617 190
5.2 713 11
32.5 4.7 651 15 I :
4.2 618 28
37.5 3.9 685 51
3.5 745 69
3.0 800 140
2.6 863 177
lS lO0 2.0 996 210 __
,' ' ' ' ~ ' '' ~ ' " '
. .:
. , : .