Note: Descriptions are shown in the official language in which they were submitted.
w° ' WO 92/00491 2 4 6 5 3 5 9 pCT/US91/04602
-1-
PROCESS FOR INCINERATING SOLID WASTES AND A PROCESS
FOR TREATING SOLID WASTES AND A PROCESS FOR TREATING
GASES GENERATED THROUGH INCINERATION OF THESE WASTES
DETAILED DESCRIPTION OF THE INVENTION
The invention relates to a process for inciner-
ating solid wastes, such as plastics, and a process for
treating gases generated through incinerating these
wastes. Over the last several decades an increasing eco-
nomic base, and increasing urbanization, has caused a
corresponding increase in the volume of solid wastes.
Additionally, the properties of these wastes have be-
come more varied, and now include a variety of plastic
and chemical products containing a diverse cross sec-
tion of toxic and harmful products. Generally, either
burning or embedding (burial) is applied to treat such
wastes, resulting in various complicated environmental
and regional social problems. This is especially true
in treatment by embedding, making the establishment and
expansion of landfills increasingly difficult because
of higher land utilization rates, and resistance of res
idents near the landfill. Meanwhile, the problem in
creases year-by-year with the growing volume of solid
waste.
Among solid wastes the amount of plastics has
increased dramatically due to the increased use of
these substances in home appliances and automobiles,
and as a packaging material for fast foods, disposable
diapers and medical wastes. Treating these wastes is
especially difficult because they are either slow to
degrade, or are completely non-biodegradable, thus
shortening the life of a landfill. Such biodegradation
that does occur creates its own problems through the
release of component elements, such as chlorine.
WO 92/00491 ~ Q B 5 3 5 9 PCT/US91/0460''
-2-
Treatment of these wastes by incineration is even more
hazardous due to the release of these hazardous materi-
als in the form of air pollution. More recent research
has focused on processes for manufacturing biodegrad-
able plastics which can be degraded by microorganisms
present in the soil. However, many problems are yet to
be solved with respect to the effectiveness of these
new plastics.
In light of the aforesaid situation, the inven
for has focused on the fact that the plastics per se
are burnable, and other burnable wastes still exist in
an undegraded condition, intending to solve the problem
by incineration. After conducting extensive research,
the inventor has thus accomplished the present process.
The first aspect of the present invention re-
lates to a process for incineration of burnable waste,
characterized in that the energy of an explosive, and
of burnable wastes, combustible gases, oil and fat prod-
ucts and refined fossil fuels, is used to initiate the
combustion of the said burnable wastes, such as plas-
tics, embedded in a landfill while simultaneously form-
ing a combustion chamber; supplying fuels and an oxidiz-
ing gas into said chamber whereby combustion is carried
out while discharging the gases generated through incin-
eration to the surface of the land.
Another aspect of the present invention re-
lates to a process for treating gases generated through
incinerating the burnable wastes, characterized in that
the gases generated through the incineration of burn-
able wastes are oxidized in the presence of catalysts.
Thereby toxic CO, COC12 and dioxin as chlorinated bi
phenyl, and sulfur compounds such as thiophene, thiols
and carbon disulfide etc., are altogether converted
into C02, H20, HC1, and SOX, etc., which are then
treated separately.
:,
" WO 92/00491 2 p 8 5 3 5 9 PCT/US91/04602
-3-
The wastes applicable to the present invention
include those of plastics, rubbers, paper, cellulose,
wood, oils and lipids, and waste petroleum products,
medical wastes, agricultural wastes and wastes from the
food processing industry. In particular, wastes from
the medical industry and facilities are increasing in
volume and diversifying in kind, and are most often in-
cinerated or disposed of underground, sometimes without
proper treatment. These burnable wastes are placed flat
on the surface of the land or into an excavated land-
f ill. These wastes are piled up to desired depth by
means of earth-moving equipment or other mechanical de-
vices, and covered with soil; mechanical force is then
applied downwards to achieve embedding. A series of
pipes are sunk into the embedded wastes (fig. 1) includ-
ing respectively: a fuel conduit pipe _1 for supplying
fuel from the surface; an air conduit pipe 2_ for supply-
ing an oxidizing gas; and a number of discharge conduit
pipes 3 for discharging the gases generated through in-
cineration. The aforesaid conduit pipes 1, 2_, and 3 may
be set up by sinking a shaft vertically in the ground
by a device such as an auger, the diameter of the shaft
being larger than those of the conduit pipes. A casing
is then set into the shaft and the conduit pipe is in-
stalled into the casing. Both conduit pipes 1 and 2 may
be installed in a single casing. There may be many such
pipes and casings. Fuel conduit pipe 1 and air conduit
pipe 2 are, for example, set up at approximately the
center of the embedded waste, and within a few meters
of each other. At the front opening of the fuel conduit
pipe 1 the pipe is provided with a nozzle which
functions to control the jet-angle of the fuel or mix-
ture of the fuel and air, whereby the direction of
incineration of the wastes can be controlled. Similar-
ly, at the front end of the air conduit pipe 2 there is
WO 92/00491 2 0 6 5 3 5 9 PCT/US91/046r'"
-4-
a nozzle which serves the same function of controlling
the direction of incineration, and adjustment of the
angle may be coordinated with the nozzle on fuel con-
duit pipe _1, or made independently or the adjustment
may be set in a single fixed condition. Also, if de-
sired, piping may be connected respectively with fuel
conduit pipe 1 and air conduit pipe 2 so as to treat
sewage, drainage water (grey water) or steam generated
from the landfill. Prior to injection this sewage,
and/or waste water must be treated to remove metals,
especially heavy metals in the form of hydroxides and
sulf ides of metals etc., while organic materials are
decomposed through combustion. The number and position-
ing of conduit pipes 3 are defined by the size of the
area to be treated, local topography, and considera-
tions of controlling the direction of combustion.
In cases in which a smaller quantity of wastes
is incinerated, a circular or equilateral polygon
scheme may be adopted (Figure 1) . In this scheme, fuel
conduit pipe 1 and air conduit pipe 2 are sunk approxi-
mately at the center of the embedded wastes and a num-
ber of discharge conduit pipes are embedded on the cir-
cumference of concentric circles or on the circumfer-
ence of an equilateral polygon, so that the combustion
proceeds with a shape approximating an expanding circle
or equilateral polygon radiating from the origin. In
variations of this scheme, the origin may be located
off the center or near a corner of a site and incinera-
tion may proceed in a shape approximating an expanding
segment of a circle or polygon. Each conduit pipe 3 may
be connected to each other conduit pipe, and all are
eventually connected to the incineration-treatment
apparatus for treating gases from incineration.
Where a large quantity of wastes is to be in-
cinerated, it is desirable to adopt a linear scheme for
'w WO 92/00491 PGT/US91/04602
~0 65 35 9 -5-
the placement of conduit pipes (Figure 3). In this
scheme, a primary line on which fuel conduit pipe 1 and
air conduit pipe 2 are sunk. A secondary line, which is
approximately parallel to or at an angle to the primary
line, an a tertiary line that connects the fuel conduit
pipe 1 and air conduit pipe 2 and a conduit pipe 2 on
the secondary line are established. A number of dis-
charge conduit pipes 3_ are sunk on the primary, second-
ary and tertiary lines. Each of these conduit pipes is
connected to the adjacent pipes. The primary line can
have more than two secondary lines on either side or
both sides. Between the primary and secondary lines a
number of discharge conduit pipes 3 may be sunk in a
grid pattern with each of the conduit pipes connected
to adjacent pipes. All of the discharge conduit pipes
are eventually connected to the incineration treatment
apparatus for treating gases from incineration.
In actual operation, a number of factors need
to be considered with regard to placement of conduit
pipes. Schemata described above need to be modified or
combined to accommodate idiosyncracies of each incinera-
tion site.
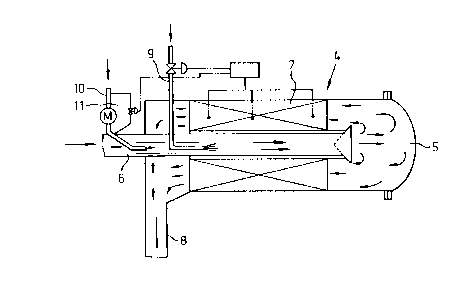
Now an embodiment of gases from incineration
treatment-apparatus 4 is illustrated in fig. 2. In said
fig. 2: 5 is a combustion chamber; 6 is a charge pipe
for gases from incineration which is connected to com-
bustion chamber 5; 7 is a catalyst-fill-layer which is
connected to combustion chamber 5, and is connected to
the side of catalyst-fill-layer 8; 9 is an oil-supply-
pipe connected to aforesaid charge pipe 6, and is pro-
vided with a flame nozzle; 10 is an air-supply-pipe
connected to charge pipe 6 for auxiliary combustion; 11
is an air blower attached to the said air-supply-pipe
10. In the gases from incineration-treatment-apparatus
4 with the aforesaid structure, gases from discharge
WO 92/00491 CT/US91/046~'"
-6-20 65 35 ~
conduit pipe 3 is introduced to combustion chamber 5.
When the reaction starts, auxiliary fuel or landfill
gas is used to keep the catalyst within an active
temperature range. For this purpose, fuel (liquid or
gas) or landfill gas is introduced via pipe 9, and
mixed with air, which may supplied via pipe 10 or
independently to pipe 9, for combustion, whereupon the
heat generated through combustion is passed through the
wall of charge pipe 6 which is thus directly or indi-
rectly heated whereby on one side the temperature of
the catalyst-fill-layer is maintained at a specified
temperature and on the other side the temperature of
the gases from incineration is elevated. The gases from
incineration are then introduced into the catalyst-
fill-layer 7 so as to be subjected to oxidative decom-
position such that the toxic components are converted
respectively to carbon dioxide, water, hydrogen chlo-
ride, and depending on what is desired, sulfur is con-
verted to sulfur dioxide or hydrogen sulfide; with both
of the latter two gases being treated separately, and
then being converted to nontoxic salts or being recon-
verted to sulfur before being discharged to the atmos-
phere. In order to control the temperature of the cata-
lyst-fill-layer 7, a temperature detector is placed
therein, whereby through the sensor of the said tempera-
ture detector the amount of oil, fuel, or gases ungra-
ded in the landfill to be supplied through oil-supply-
pipe 10 can be adjusted; additionally, air blower 11
attached to air-supply-pipe 10 can be adjusted directly
or a ventilation pipe connected to air blower 11 may be
set up whereby through the action of the valve of the
ventilation pipe adjusted to the signal switch of the
temperature sensor, the cooling effect can be in-
creased. Further, at the front end of charge pipe 6
there is set up a cone so that the gases from incinera-
WO 92/00491 2 p 6 5 3 5 9 PCT/US91/04602
tion can be readily diffused, resulting in their easy
introduction into catalyst-fill-layer 7.
The catalyst to be filled into the aforesaid
catalyst-fill-layer 7 is the oxides of vanadium, cop-
s per, lead, cobalt and cadmium whose basicity is strong-
er than that of vanadium oxide. As an alternative, a
mixture of vanadium oxide and potassium persulfate, to
which an alkal hydroxide has been added, can be used.
The oxides which act to destroy the benzene ring togeth-
er with its side chains are preferably mixed with por-
ous or non-porous carrier(s). Generally, it is prefer-
able that porous carriers in powder form, such as
silica gel and titanium dioxide, with high specific
surface areas be made into shaped carriers for use.
Furthermore, in the case of a supported catalyst, it is
preferable that the carrier be made of a-A1203,
SiC, aluminum silicate which are low in specific sur-
face area and are non-porous with a pore size of 2.20
mm. In addition, the temperature of the catalyst-fill-
layer adjacent to the inlet through which the gases
from incineration enter should be sufficiently elevated
to intensively carry out the oxidative decomposition,
expecting that a desired oxidation degree can be
reached. The process for the manufacture of the cata-
lyst may utilize impregnation, concentration-to-dry-
ness, baking, melting with a proviso that the catalyst
should be rendered active at a temperature above 200
degrees centigrade.
The primary combustion of the burnable wastes
embedded under the land is carried out at an area be
neath fuel conduit pipe 1. That is, for example,
through fuel conduit pipe _1, while injecting combusti
ble petroleum products such as LPG, heavy oil, light
oil, and heating oil, or landfill gas, into the wastes
and carrying out ignition, or placing an explosive in
20 65 35 9
WO 92/00491 PCT/US91/0460"
_g_
the wastes and igniting it, so that the burnable wastes
are ignited and a combustion chamber necessary for for
primary combustion of burnable wastes is created. For
the aforesaid explosive, trinitrophenol, trinitrotolu-
ene, glycerin trinitrate or their derivative products
may be used. Following creation of the combustion cham-
ber a flammable liquid or gas is fed through fuel con-
duit pipe 1, and an oxidizing gas -- such as oxygen --,
an oxygen-containing gas, or air is supplied through
air conduit pipe 2 so that the wastes are kept in a com-
bustive state. Landfill gas (i.e, methane) can be drawn
off via pipe 12, and fed to fuel conduit pipe 1 to
supplement, or replace, the initial fuel being used.
Further, in carrying out combustion, the combustion
rate can be controlled by regulating the amount of
oxidizing gases, with concurrent use of water or steam
being feasible. In accordance with our experience, an
alkali metal carbonate such as sodium carbonate, an
alkali earth metal carbonate such as calcium carbonate,
magnesium carbonate, or an alkali earth metal bicarbon-
ate such as calcium bicarbonate is also very effective
-- when sprayed in the form of a suspension in a liquid
fuel -- at removing from the burnt products all chlor-
ine-containing, or sulfur-containing compounds in the
form of inorganic chlorides or sulfates. These above
mentioned carbonates reduce, on a large scale, the
amount of poisonous chlorides, such as phosgene, gener-
ated upon the combustive decomposition of such plastics
as polyvinyl chloride. Further, combustion direction
can be controlled by either controlling the amount of
the gases-from-incineration discharged from discharge
conduit pipes 3 or by combining said measures using
temperature recorders and electrothermography.
The gases from incineration are discharged to
the surface. At this time, a thermal imaging method
f
"' WO 92/00491 PCT/US91/04602
2065359 .9_
using an infrared scanning device can be applied to de-
tect the size and scale of the combustion area, togeth-
er with its moving directions whereupon new discharge
conduit pipes 3 can be set up. The gases from incinera-
tion discharged through discharge conduit pipe 3 are
introduced into incineration-treatment-apparatus 4
where such toxic gases as carbon monoxide, phosgene,
and dioxins, and organic and inorganic sulfur compounds
and sulfons are converted into carbon dioxide, water,
hydrogen chloride, sulfur dioxide, and hydrogen sul-
fide, etc., which, after being treated altogether are
discharged into the atmosphere in the form of clean non-
toxic gases. In addition, allyl, aryl chloride, which
is possibly contained in the combustion gases, having
been subjected to catalytic oxidation may be treated
with steam once or twice so as to convert said allyl,
aryl chloride into very simple inorganic compounds
(i.e. carbon dioxide and hydrogen chloride). In case a
power failure occurs to the incineration-treatment-appa-
ratus 4, the supply of air through air conduit pipe 2
will be cut off immediately, thereby resulting in
automatic cessation of combustion. As an additional
safety measure, at this time, the introduction of water
or C02 into the combustion chamber could be utilized.
At the end of the incineration treatment of
wastes, fuel conduit pipe 1, air conduit pipe ~ and
discharge pipe 3 may be drawn out, while the combustion
area formed under the surface may be rolled flat and
uniform with pressure for the next embedding treatment.
35
WO 92/00491 PCT/US91/046~"
-10-
[ACTION]
2065 359
In the present invention, the energy of an ex-
plosive or petroleum products such as LPG, heavy oil,
light oil and heating oil, or landfill gas, may be uti-
lized to initiate the combustion of burnable wastes,
such as plastic, embedded in a landfill, thereby simul-
taneously forming a combustion chamber for primary com-
bustion. A fuel and an oxidizing gas are supplied into
the said chamber whereby combustion is carried out
while discharging the gases from incineration through
additional conduits. By such a simple and accurate man-
ner, incineration treatment of wastes can be carried
out on a large scale. Further, toxic components con-
tained in the gases from incineration discharged to the
surface are oxidized in the presence of a catalyst
whereby toxic components, such as carbon monoxide, phos-
gene, dioxins, and organic and inorganic sulfur com-
pounds such as carbon disulfide, carbonyl sulfide, thio
alcohols, olefin polysulfides, and sulfons, are convert-
ed into carbon dioxide, water, hydrogen chloride and
SOx, which after being generically treated are dis-
charged to the atmosphere in a clean and nontoxic form.
[EFFECT OF THE INVENTION)
The present invention is constituted as stated
above, according to the present invention based on
which it is not necessary to set up, as before, an in-
cinerator to treat burnable wastes such as: plastics,
rubbers, wooden materials, oil and lipids, cellulose,
waste petroleum products and medical wastes. These mate-
rials can be simply, conveniently and safely incinerat-
ed on a large scale. Meanwhile, the combustion chamber
formed after incineration treatment may be rolled uni-
form with pressure for its next use as a landfill, thus
°
'~WO 92/00491 ~ ~ g 5 3 5 g '~ -11- PCT/US91/04602
extending the life of the landfill. Also, gases gener-
ated upon incineration treatment can be oxidized in the
presence of a catalyst to convert them into clean, and
completely nontoxic gases, without environmental prob-
lems caused by conventional incineration treatment. The
following examples serve to illustrate the present
invention, but not to claim 7, the scope of the present
invention.
Example 1:
l0 Fuel conduit pipe 1 and air conduit pipe 2,
provided with nozzles for which the spray angle could
be adjusted, were respectively set up near the center
of embedded wastes from the surface. Meanwhile, four
discharge conduit pipes 3 were set up on the circumfer-
ence of a circle, with said two conduit pipes 1 and 2
as its center at a radius of 20 meters, such that the
pipings from said conduit pipes 3 were connected to
each other. Then the wastes were subjected to incinera-
tion treatment under the
following conditions:
Explosive used (trinitrophenol) . 5 kg
Fuel (C grade heavy oil with 5% sulfur): 1 kg/hr
Rate of gaseous fuel supply . 0 NM3/hr
Rate of air supply :20 NM3/hr
Alkali used . 0 kg/hr
The gases from incineration thus produced were
discharged through conduit pipe 3, and were then treat-
ed in the gases-from-incineration-treatment apparatus
4. After the embedded wastes had been incinerated out,
WO 92/00491 2 4 ~ ~ ~ ~ ~PCT/US91/046P"
-12-
conduit pipes 1. 2 and 3 were withdrawn, and the
combustion cavity generated under the land was rolled
uniform by means of earth moving equipment.
Example 2:
Fuel conduit pipe 1_, air conduit pipe 2, and
discharge conduit pipes 3, were set up in embedded plas-
tic wastes in a manner similar to that of Example 1.
Incineration treatment was carried out under the follow-
ing conditions, and the gases from incineration thus
generated were treated in the gases-from-incineration-
treatment-apparatus 4.
Explosive used (trinitrophenol) . 5 kg
Fuel (C grade heavy oil with 5o sulfur): 1 kg/hr
Rate of gaseous fuel supply . 60 NM3/hr
(gases generated in the landfill)
Rate of air supply . 40 NM3/hr
Alkali used (CaC03) . 10 kg/hr
Example 3:
Fuel conduit pipe 1, air conduit pipe 2, and
discharge conduit pipes 3 were set up in embedded plas-
tic wastes in a manner similar to that in Example 1.
Incineration treatment was carried out under the follow-
ing conditions and the gases from incineration thus gen-
erated were treated in gases-from-incineration appara-
tus 4.
Explosive used (trinitrophenol) . 2 kg
Fuel (C grade heavy oil with 5% sulfur) . 0 kg/hr
Rate of gaseous fuel supply :100 NM3/hr
(gases generated in the landfill)
Rate of air supply . 60 NM3/hr
Alkali used (MgC03) . 10 kg/hr
~' WO 92/00491 PCT/US91/04602
20 65 35 9
-13-
Example 4:
Fuel conduit pipe _1, air conduit pipe 2, and
discharge pipes 3, were set up in embedded plastic
wastes in a manner similar to that in Example 1. Incin-
eration treatment was carried out under the following
conditions and the gases from incineration thus generat-
ed were treated in the gases-from-incineration-treat-
ment apparatus 4.
The result of analysis on the gases from incin-
eration thus generated in Examples 1 through 4 are
shown in Table 1.
20
30
WO 92/00491 ~ p 8 5 3 ~ ~CT/US91/046~'"
-14-
Table 1 Analysis of the Gas From Incineration
Exam les
Com onents 1 2 3
C02 38.13 0 43.65 0 36.19 0 30.32 0
02 2.01 0 1.37 0 2.29 0 2.35 0
S02 0.1 1 io 0.08 0 0 13 0 0.25 0
CO 15.19 ~!0.43~ 23.84 20.66 0
Phos ene +++ +++ +++ +++
Dioxin ++ + ++ ++
Note: + denotes approximately 1 G ppm
WO 92/00491 PCT/US91/04602
2 p 6 5 3 5 9 -15-
The results obtained by treating the gases from inciner-
ation generated in Examples 1 through 4 with gases-
from-incineration-treatment apparatus 4 are shown in
Table 2 and Table 3.
For Table 2 the gases were treated at the cata-
lyst temperature of 200 degrees centigrade and with
space speed of 1000 hr-1 (volume of the reaction gas-
es/hr/volume of the catalyst, i.e. the reciprocal of
contact time). For the catalyst, V205 - K2S04 -
Si02 - K2S207 - S series was used in mole ra-
tios:
K2S04/V205=0.98,
K2S207/V205=2.02,
(K2S207+K2S04)/S03=0.55,
K2S04/S03=0.18,
and for the carrier, silica gel and diatomaceous earth
in ratio 5 to 1, was made into pellets of five millime-
ters in diameter and tree millimeters in length.
For Table 3, an example of the catalyst
composition in mole ratios is:
K2S04/V205=1.10,
K2S207/V205=1.71,
K2S04/S03=0.23,
K2S207/S03=0.35,
(K2S207+K2S04)/S03=0.58.
Gas was treated at the catalyst temperature of 400
degrees centigrade with space speed of 2000 hr-1 and
silica gel was used as carrier with powdered pumice
added in the ratio five to one. The pellet size was the
same as above. At lower temperatures emission of S03
from the catalyst series decreases and the acidity of
the catalyst increases, so that the potential becomes
decreased. Therefore, when the sulfur content of the
sample gas is high, the sulfur content was removed and
the amount of total S03 was adjusted before use.
PCT/US91 /(146r
WO 92/00491 2 0 6 5 3 5 9
-16-
Table ~ Analysis of the Gas Before and After Treatment
Before treatment After treatment
Com onents
H2S 300 ppm undetected
CH3SH 86 p m S p m
CS2 70 p m 2 pm
Benzene 318 ppm 5 m
Example of Catalyst Composition for Table
V20s 10.80
K2SOa 10.20
K2S20~ 30.40
Sn02 4.SOro
Carver and others44,10
a
Bulk Density ~ 0.63
~' WO 92/00491 PCT/US91/04602
17-
2065 359
Table 3 Analysis of Gas Before and After Treatment
Betore treatment Atter treatment
Components
C2Hs-SH 300 ppm undetected
Toluene 86 ppm 5 ppm
CH2CL2 70 ppm 2 ppm
CO 318 ppm S ppm
COC12 109 ppm undetected
Example of Catalyst Composition for Table 3
V205 14.50
K2S04 15.30
0
K2S20~ 34.60
0
Cu02 3.80
Carrier and others 31.80
Bulk Uens~ty ( ~~.65
Analysis of Example of Carrier for Table 3
Si02 89.68
A 1203 6. 43 7
F e203 1 475
K2G 0.35io
Ca0 0.291 I
Ti02 0.45
Ignition LOJJ 0.43
Total I 99.10
PCT/US91 /046r"'
WO 92/00491 2 0 6 5 3 5 9
-18-
Brief Description of the Drawincts
Fig. 1 depicts an arrangement status of fuel
conduit pipe 1, air conduit pipe 2 and discharge con
s duit pipes 3.
Fig. 2 depicts an arrangement status of gases-
from-incineration-treatment apparatus 4.
Fig. 3 depicts an arrangement of conduit pipes
that is an alternative to that shown in Fig. 1.
In Fig. 1, Fig. 2 and Fig. 3, the numerals 1
to 11 have the following meanings.
1... fuel conduit pipe
2... air conduit pipe
3... discharge conduit pipes
4... gases-from-incineration-treatment apparatus
5... combustion chamber
6... charge pipe
7... catalyst-fill-layer
8... side of catalyst-fill-layer
9... oil-supply-pipe
10... air-supply-pipe
11... air blower
12... discharge conduit pipe
35