Note: Descriptions are shown in the official language in which they were submitted.
WO91/00727 za ~ PCT/US~0/03761
SUBSTITUTED 2-AMINOTETRALINS
Back~round of the Invention
Field of the Invent on
The invention relates generally to substituted 2-
aminotetralins and to processes for preparing such
compounds. More particularly, the invention relates to
compounds for therapeutic use, in particular in treating
disorders of the central nervous, cardiovascular and
endocrine systems. The compounds of this invention are
also useful for alleviating glaucoma, parkinsonism, and
schizophrenia, and for inducing anorexia and weight loss in
mammals.
Backaround of the Prior Art
It is known that various hydroxylated 2-aminotetralins
of the general formula
~ ~ 2
(OH)n
where Rl and R2 are saturated alkyl groups and n i5 1 or 2,
are dopamine receptor agonists (McDermed et al., J. Med.
Chem. 18, 362 (1975); Feenstra et al., Arch. Pharmacol.
313, 213 (1980).
.. . ~ . . , ~, ~ , ;, , " .:
- - : , :: . .
. ~ . . . .
- : . : ,: , , ~ ,' ,. , -
- - - - - . .
WO 91/007'~7 PCr/( IS90/0376~
5~
--2--
Many structure-activity relationship studies have been
conducted to find compounds with high dopamine-receptor
stimulating activity. A survey is contained in
Katerinopoulos, H. E., et al., "Structure-Activity
Relationships for Dopamine Analogues," Druqs of the Future,
Vol. 12, No. 3, 1987, 223-253. Based upon the high
activity of apomorphine, many derivatives and simplified
structural analogues have been tested and found to have
dopaminergic activity. For instance, some of the bicyclic
analogues of dopamine, 2-amino-5,6- and 2-amino-6,7-di-
hydroxytetralin, and their N-alkylated derivatives were
tested and showed activity.
In addition, studies have shown that the 5-hydroxy
derivatives of 2-aminotetralins possess high potency almost
equivalent to that of the 5,6 catechols, with the
additional advantage o~ increased stability, selectivity
and duration of biochemical action. These studies have
also shown that in bicyclic compounds the size of the two
nitrogen substituents controls activity. ~or instznce, the
N-butyl and N,N-dibutyl derivatives of 2-amino-5,6-
dihydroxytetralin, dopamine and norapomorphine have little
or no dopaminergic activity, while analogues having at
least one N-ethyl or N-n-propyl group possess high
activity~
Further studies have shown that the D2 receptor potency
of dopamine agonists is at a maximum when one of the two N-
substituents fits into a receptor niche which, becaus~ of
size constrain~ max.l~ ccommodates an n-propyl grollp.
Conversely, activity drops off when the propyl group is
replac~d by the smaller groups ethyl or methyl. When the
,, . . , , , ::
- . . . : :
. ~ . , ~ :
.: - - . . . . . .. : .. ..... . .
wosl/oo727 2~ 5~ PCT/~S90/03761
compound contains no N-substituent at least as small as n-
propyl, activity is small or non-existent.
; However, the structural requirements for the second N-
substituent in such compounds have not been established.
Consequently, the search continues for new and better N-
substituents to enhance both dopamine receptor binding and
activity, especially as shown by in vivo studies designed
to test the dopaminergic activity of compounds, such as
contralateral turnlng studies in 6-OH-DA-lesioned rats.
See Seiler, Max P., et al., "Structure-Activity
Relationships o~ Dopaminergic 5-~ydroxy-2-aminotetralin
Derivatives with Functionalized N-Al~yl Substituents." J.
Med. Chem. 1~86, 29, 912-917.
.,
The receptor site into which this second N-substituent
is thought to interact appea~s to accommodate a wide
variety of large, bulky groups having different
functionalities without lcss of activity. See McDermed, J.
D., et al., J. Med. Chem. 1975, 18, 362; Cannon, J. G., et
al., J. Med. Chem. 1977, 20, 1111; and Wikstroem, H., et
al., J. Med. Chem. 1982, 25, 925. However, the
dopaminergic activity and potency conferred upon the
compound by the choice of the second N-substituent is at
present, unpredictable so that the search continues for new
and better dopa~ine receptor agonists, especially for
compounds showing a high degree of selectivity and
specificity as either Dl or D2 receptor agonists.
,.. .... .. .. . . .... .. . . . . . .
;, , , . , . ,,, ~ ,, :
WO91/00727 PCT/US90/03761
-
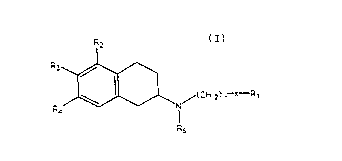
summarv of the Invention
There has now been discovered certain novel compounds
having dopaminergic activity and having the structural
formula
,~
_ ~ .
where R2, R3 and R~ are each selected from the group con-
sisting cf H and OA with the provision that at least one of
R2, R3 and R4 is H, that R2 and R4 are not both OA; A is H
or is selected from the group consisting of hydrocarbyl
radicals, for example lower alkyl radicals optionally
substituted with aromatic residues (i.e. methyl, ethyl,
O O
propyl, benzyl, etc.), as well as -~-R5, -CNHR5, and
-C-OR5.
R5 is selected from the group consisting of alkyl and
aromatic residues having between 1 and 12, preferably
between 1 and 6, carbon atoms, for example alkyl,
optionally substitued with aromatic residues, and aromatic
residues optionally substituted with alkyl radicals; n is
an integer between 1 and 4; R6 is an alkyl chain comprising
between 1 and 4 carbon atoms, X is selected from the group
consistiny of -CH2-, oxygen, sulfur, and nitroaen. ~ith the
provision that when X is not -CH2-, R1 is selected from the
group consisting of
. , . .. , . ............ . : . . . .
: ~ . - ~ ~.. ., ,: - . . , , . , -. . :
. WO91/00727 2 ~ 5 ~ PCT/US90/03761
(Y)a ~ N ~
z N~(Y)a
(Y)
wherein Z is oxygen, nitrogen or sulfur, wherein Y is
selected from the group consisting of hydroxy, nitro,
cyano, azido, amino, acylamino, carboxyamido, trifluoro-
methyl, sulfate, sulfonamido, halogen, hydrocarbyl, and :
hetero ato~-substituted hydrocarbyl radicals, wherein said
heteroatoms are selected from the group consisting of
halogen, nitrogen, oxygen, sulfur and phosphorus and said
hydrocarbyl radicals comprise from 1 to 12 carbon atoms,
and a is an integer of from zero to ~; and~with the further
provision that when X is -CH2-, R1 is either -C-Aryl or
-C~-Z-Aryl ~R8
IR8 Rg
Rg
wherein R8 is hydrogen, aryl, or R6; and further wherein Rg
O O
is aryl, R6, -OH, -NH2, -OR6, -C-OR6, -OCR6 or -NtR6)2;
or Rl is
(`t' )
S~lf3STlTUTE SHEE~ ~
- - . - . . . . .
- , . ~ . ,
. ` . .. ~ . . , . . . ~ ... . . . ..
. `~ .---, . ~ , :. . ':
.
. . . . . . :
.. . . .
-: . . :. , . .- . : ,
WO91/00727 PCT/US90/03761
Z~5~5')
wherein B is oxygen, sulfur or two hydrogen atoms, and
pharmaceutically acceptable salts thereof.
Preferably, R2 is OA and A is H.
It is essential that the compounds in the present
invention be an optically active compound or racemic
mixture thereof having substantial affinity and selectivity
for binding to dopamin~ D2 receptors, e.g., in a human. In
particular, it is found that 2-(N-n-propyl,N-2-
~phenyloxy]ethylamino)-5-hydroxytetralin is especially
preferred for its hish affinity and selectivity for binding
to dopamine D2 receptors.
Detailed Description of the Invention
The compounds used in the present invention are selected
from the group of steroisomers or mixtures thereof of
compounds having dopaminergic activity represented by the
formula:
.... .
~ /(cn2)
where R2, R3 and R4 are each selected from the group
consisting of H and OA with the provision that at least one
of P~. R~ and R4 is H, that R2 and R4 are not both OA; A ic
H or is selected from the group consisting of hydrocarbyl
radicals, for example lower alkyl radicals, optionally
w091/00727 ~ PCT/US9~/03761
substituted with aromatic residues (i.e. methyl, ethyl,
o o o
Il 11 ll
propyl, be~zyl, etc.), -C-R5, -CNHR5, and -~OR5.
R5 is selected from the group consisting of alkyl and
aromatic residues having between 1 and 12, preferably
between 1 and 6, carbon atoms, for example alkyl residues
optionaliy subs~i~uted with aromatic residues and aromatic
residues optionally substituted with alkyl radicals; n is
an integer between 1 or 4; R6 is an al~yl chain comprising
between 1 and 4 carbon atoms; X is selected from the group
consisting of -CH2-, oxygen, sulfur, and nitrogen, with the
provision that when X is not -CH2-, R1 is selected from the
group consisting of
)~ ~ (Y)a ~ (Y)a ~ N
(Y ja
'Y' ~,11 ,~ (Y'a l~ ,~
wherein Z is oxygen, nitrogen or sulfur, wherein Y is
selected from the group consisting of hydroxy, nitro,
cyano, azido, amino, acylamino, carboxyamido,
trifluoromethyl, sulfate, sulfonamido, halogen, hydrocarbyl
and hetero atom-substituted hydrocarbyl radicals, wherein
said heteroatoms are selected from the arolln ~nC;c~ina of
halogen, nitrogen, oxygen, sulfur and phosphorus and said
hydrocarbyl radi~.als comprise from 1 to 12, preferably 1 to
6, carbon atoms, and a is an integer of from zexo to 3, for
~ U8 5-iiT U ~ E ~-~_E~I
. . . .: , . . .
.
.
wo 91/00727 2~;.~'-i~ PCI/IJS90/03761
instance zero to 2; and with the further provision that
when X is -CH2-, R1 is either -C-Aryl or -C-Z-Aryl
Rg Rg
wherein R8 is hydrogen, aryl, or R6; and further wherein Rg
O O
Il 11
is aryl, R6, -OH, -NH2, -OR6, -C-OR6, -OCR6 or -N(R6)2;
or R1 is O
_~ \F B
,~
~1
(Y)a
wherein B is oxygen, sulfur or two hydrogen atoms, and
pharmaceutically acceptable salts thereof.
Preferably, R2 is OA and A is H.
A is preferably H or is selected from the group
consisting of phenyl and alkyl radicals having from 1 to 12
carbon atoms, and more preferably R5 is an alkyl or aryl
radical that would serve to extend the activity of the
compound in the body, for example phenyl, methyl, t-butyl,
o-methylphenyl, o-, m- or p-methoxyphenyl, p-isopropyl-
phenyl or nonyl.
The more preferxed groups represented by R1 are
thienyl, phenyl, hydroxyphenyl, furanyl and naphthalenyl,
e.g., 2-thienyl, 3-thienyl, 3-hydroxyphenyl,
4-hydroxyphenyl, etc.
In the more preferred compounds for use in the present
invention n is 2, X is oxygen or ~CH2- and R2 is OA; and
most preferably A is H and R6 is propyl.
~5~ ~ 5~1T ~
.. . .. . ....... :
. . . ....
WO91/00727 ~ PCT/US90/037fi~
~ 3
_g _
It is essential that the compounds herein be an
optically active or racemic mixtures capable of binding
selectively to one or more dopamine D2 receptors, e.g., in
a human. In particular, 2-(N-n-propyl,N-2-[phenyloxy]
ethylamino)-5-hydroxytetralin is an especially preferred
compound because of its high affinity and selectivity for
binding to D2 dopamine receptors. Due to their high
affinity for binding to D2 dopamine receptors, it is
believed that the compounds herein will be useful in the
treatment of disorders of the central nervous,
cardiovascular, and endocrine systems. In particular it is
believed that the compounds herein are useful in the
treatment of such conditions in humans as elevated
intraocular pressure, schizophrenia and parkinsonism, and
for inducing anorexia and weight loss in humans and other
mammals.
Particularly preferred compounds are as follows:
Compounds wherein X is oxygen, nitrogen or sulfur and R
is selected from the group consisting of radicals
represented by the general formula:
~ (Y)a
wherein specific preferred compounds of this group include:
2-(N-n-propyl-N-2-[2-thienyloxyJethylamino)-5-hydroxy-
tetralin,
2-(N-n-propyl-N-2-[3-thienyloxy]ethylamino)-5-hydroxy-
tetralin,
2-(N-n-propyl-N-2-[2-furanyloxy]ethylamino)-5-hydroxy-
tetralin,
: . : , , . , ., .: : ~ , .
.
WQ91/00727 PCT/~IS90tO3761
;~r~-4s~ 1
--10--
2-(N-n-propyl-N-2-[3-furanyloxy]ethylamino)-5-hydroxy-
tetralin,
2-(N-n-propyl-N-2-[2-(4-methyl)thienyloxy]ethylamino-5-
hydroxytetralin,
2-(N-n-propyl-N-2-[2-(3,4,5-trimethyl)thienyloxy]
ethylamino)-5-hydroxytetralin,
2-(N-n-propyl-N-2-[2-(5-chloro)thienyloxy]~thylamino)-5-
hydroxytetralin,
2-(N-n-propyl-N-2-[2-(4-bromo-5-methyl)thienyloxy]
ethylamino)-5-hydroxytetralin,
2-(N-n-propyl-N-2-[2-(4-methyl-5-ethyl)thienyloxy]
ethylamino)-5-hydroxytetralin,
Compounds wherein X is oxygen, nitrogen or sulfur and
wherein R1 is selected from the group of radicals
represented by the formulae: `
--~(Y~ (Y~
More preferably, in these compounds Y comprises no more
than 5 carbon atoms and a is an integer from 0 to 2.
Specific preferred compounds of this group include:
2-(N-n-propyl-N-2-[2-naphthalenyloxy]ethylamino)-5-
hydroxytetralin,
2-(N-n-propyl-N-2-[4-indolyloxy]ethylamino)-5-hydroxy- :~
tetralin,
2-(N-n-propyl-N-2-[2-benzothienyloxy]ethylamino)-5-
hydroxytetralin, and
2-(N-n-propyl-N-2-[3-benzothienyloxy]ethylamino)-5-
hydroxytetralin;
- ~ - - -
.
:, . .
,- . : : - . , .:
- ~ . . . . . . . .
.... , . ~ . . . . . . .
- WO 91tO~727 ~ 5~ PCT/US90/03761
Compounds wherein R1 is phenyl and/or substituted
phenyl and is selected from the group of radicals repre-
sented ~y the formula:
~ ~ (Y)a
and wherein x is oxygen, nitrogen or sulfur. Specific
preferred compounds of this group include:
2-(N~n-propyl-N-2-[phenyloxy~ethylamino) 5-hydroxy-
tetralln,
2-(N-n-propyl-N-2-[4-hydroxyphenyloxy]ethylamino)-S-
hydroxytetralin, and
2-(N-n-propyl-N-2-[3-hydroxyphenyloxy]ethylamino)-5-
hydroxytetralin,
2-(N-n-propyl-~-2-[phenyloxy]ethylamino)-5-
methoxytetralin
2-(N-n-propyl-N-2-[phenylamino]ethylamino)-5-
hydroxytetralin
2-(N-n-propyl-~-2-[4-hydroxyphenylamino]ethylamino)-5-
hydroxytetralin
2-(N-n-propyl-~-3-[phenyloxy]propylamino)-5-
hydroxytetralin
~ 2-(N-n-propyl-N-2-[2,6-dimethylphenyloxy]ethylamino)-
: 5-hydroxytetralin
2-(N-n-propyl-N-2-[3,5-dimethylphenyloxy)ethylamino)-
5-hydroxytetralin
- Compounds wherein X is -CH2- and R1 is selected from
the group consisting of radicals represented by the
following formulae:
~ ~ -C Aryl, or -C-Z - Aryl
R ~R8
/) ' "
~1~
(Y '`
~ 3sTllTuTE SHEET
~.. . . . ~ . ............ . .
.: `- '' . ' .. ~ . ` `:` `
:. : ,' ~ -
wosl/oo727 PCT/US90/03761
;~r~ 5-~ -
-12-
and wherein B is 0, S, or H2. Specific preferred compounds
in this group include:
3-[2-[propyl(1,2,3,4-tetrahydro-5-methoxy-2-
naphthalenyl)amino]ethyl]-1(3H)-isobenzofuranone
3-[2-[propyl(1,2,3,4-tetrahydro-5-hydroxy-2-
naphthalenyl)amino]ethyl]-1(3H)-isobenzo~uranone
6-[[2-(1,3-dihydro-1-isobenzofuranyl)ethyl]propylamino]-
5,6,7,8-tetrahydro-1-naphthalenol
2-(N-n~propyl-N-3,3,3-triphenylpropylamino)-5-hydroxy-
tetralin
2-(N-n-propyl-N-2,2,2-triphenylethylamino)-5-hydroxy-
tetralin
2-(N-n-propyl-N-3,3-diphenylpropylamino)-5-hydroxy-
te.tralin
2-(N-n-propyl-N-2,2-diphenylethylamino)-5-hydroxy-
tetralin
2-(N-n-propyl-N-2-phenylpropylamino)-5-hydroxytetralin
2-(N-n-propyl-N-2-phenylpropylamino)-5-metho~ytetralin
2-(N-n-propyl-N-2-(2-methoxy)phenethylamino)-5-hydroxy-
tetralin
2-[N-n-propyl-N-2-~2-phenyloxy)propylamino]-5-hydroxy-
tetralin
The invention is further illustrated by the following
examples which are illustrative of various aspects of t~e
invention, and are not intended as limiting the scope of
the inventions defined by the appended claims. :
- , : - . . ' , . . :, . . ' :
wosl/0o727 ~ PCr/US90/03761
EXA~IPLE 1
Preparation of 2-[N-n-propyl,N-2-(phenyloxy)ethylamino]-5-
methoxytetralin.
A mixture of 2-(N-n-propylamino)-5-methoxytetralin (7.0 g,
0.0319 mol; prepared according to J. Che~. Soc., 1965, pp
26-36), phenoxyacetic acid (4.86 g, 0.0319 mol), and borane
trimethylamine complex (2.33 g, 0.0319 mol) was refluxed in
xylenes overnight. The cooled reaction ~ixture was
extracted with NaHCO3 and the organic layer was dried over
MgSO4, filtered and concentrated unde- reduced pressure.
The resulting oil was subjected to flash ch~omatography
(silica; 9:1 pet ether/EtOAc) and product was isolated:
NMX of the free base (300 MHz, CDC13): ~ 7.3-6.6 (m, 8H),
4.0(t, 2H), 3.7(s, 3H), 3.1-2.5(m, 5H), 2.1(~, lHj, 1.7-
1.4(m, 3H), 0.9(t, 3H). The free base thus isolated was
converted to a hydrochloride sal_ by the addition of dry
ether-HCl.
EXAM~LE 2
Preparation of 2-[N-n-propyl,N-2-(phenyloxy)ethyla~ino]-~-
hydroxytetralin.
A mixture of pyridine hydrochloride and the product of
Example 1 was heated in an oil bath at 200C. When the
reaction was complete (TLC), it was cooled, diluted with
H2O, made basic with NH40H and extracted with ether. The
organic layer was dried over MgSO4, filtered and
concentrated under reduced pressure. The residue was
subjected to flash chromatogr~phy ~ re~
ethertEtOAc). The product was dissolved in ether and
converted to a hydrochloride salt by the addtition of dry
! ,' ~ , ' . ' . `, , . , ' .
WO91/00727 PCT/U~90/0376l
ether-HCl. Anal. Calcd. for C21H27NO2-HCl: c, 69.69; H,
7.80; N, 3.87. Found: c, 69.54; H, 7.90; N, 3.87. NMR of
the ~ree base (300 MHz, CDC13): ~ 7.3-6.6(m, 8H), 4.0(t,
2H), 3.1-2.5(m, 9H), 2.1(m, lH), 1.7-1.4(m, 3H), o.s(t~
3H).
EX.~PLE_3
The product of Example 2 was also obtained by dissolving
the product of Example 1 in dry dichloromethane and adding
a solution of boron tribromide in dichloromethane dropwise
at room temperture under nitrogen. After completion, the
reac~ion was poured into a beake~ containing NU~OH and ice
and stirred for 0.5 h. The organic layer was separated,
and the product was purified as in Exa~,ple 2.
EXAMP_E
Preparation of 2-[N-n-propyl,N-3-(phenyloxy)pr~pylamino]-s-
methoxytetralin.
In Example 1, phenoxyacetic acid can be replace~ by 3-
phenoxy-propionic acid.
.
EXAMPLE 5
Preparation of 2-[N-n-propyl,N-3-tphenyloxy)propylamino]-5-
hydroxytetralin.
The product of Example ~ can be used as the startin~
material for E~ample 2.
... .. . . . , . " .. , .,. , :.. . i: :. .:. .. , ,. :, : :, .
WO 91/00727 PCr/l lS90/03761
t5~11
EX.~MPLE 6
Preparation of 2-[N-n-propyl,N-2-(1-naphthalenyloxy)
ethylamino]-5-methoxytetralin.
In Example 1, phenoxyacetic acid was replaced by (1-
naphthoxy)-acetic acid. The resulting oil was sujbected to
flash chromatography (silica; pe~ ether) and the product
was isolated; characteristic peak of N~R (300 M~2, CDC13);
8.3-6.7(m,lOH), 4.2(m, 2H), 3.85(s, 3H), l.O(t, 3H).
E~MPLE 7
Preparation of 2-[N-n-~rop~-l,N-3-(~-naphthalenyloxy)
propylamino]-5-hydroxytetralin.
The product of Example 6 was used as the starting material
in Example 2. The resulting oil was subjected to flash
chromatography and the isolated product showed
characteristic peaks at : NMR (CDC13) ~ 8.3-6.6(m, lOH),
4.2(m, 2H), O.9(t,3H).
EX~PLE 8
Preparation of 3-~2-[propyl(1,2,3,4-tetrahydro-5-methoxy-2-
naphthalenyl)amino]ethyl]-1(3H)-isobenzofuranone.
In Example 1, phenoxyacetic acid was replaced by phthalide-
3-acetic acid. The resulting oil was subjected to flash
chromatography and the isolated product showed distinct
peaks at: NMR (CDC13) ~ 8.0-6.7(m, 7H), 5.7(m, lH), 3.8(s,
3H) O.9(t, 3H). Anal. Calc. for C24H29~03 HCl: C,
69.~ ., ;, 3.37. Found: C,-69.0~ , 7.~ .2~.
WO91/1)0727 PCl/U590/03761 1
5~
-16-
EXAMPLE g
Preparation of 3-[2-[propyl(1,2,3,4-tetrahydro-5-hydroxy-2-
naphthalenyl)amino]ethyl]-1(3H)-isobenzofuranone.
The product of Example 8 was used as the starting material
for Example 3. The resulting oil after purification showed
dis.inct peaks at: NMR(CDCl3) ~ 7.9-6.6(m, 7H), 5.7(m,
lH), 0.95(t, 3H). Anal. Calc. for C23H27N3 ~ HCl C,
68.73; H, 7.02; N, 3.48. Found: c, 68.62; H, 7.09; N,
3.35.
EX.~ F 1 0
Preparation of 6-[[2-(1,3-dihydro-1-isobenzofuranyl)
ethyl]propylamino]-5,6,7,8-tetrahyàro-1-naphthalenol.
The reduction of the product of Example 9 will result in
the desired product.
EX~PLE 1i
Preparation of 2-[N-n-propyl,N-2,2-diphenylethylamino)-5-
methoxytetralin.
In Exàmple 1, phenoxyacetic acid was replaced by dipheny-
lactic acid. The puri~ied product showed characteristic
peaks at: NMR(CDCl3) ~ 7.8-6.65(m, 13H), 4.1(t, lH),
3.8(s, 3H), 3.2(d, 2H), 0.7(t, 3H).
EXP~PLE_12
Preparation of 2-[N-n-propyl,N-2,2-diphenylethylamino]-5-
.ydroxytetralin.
.
.' :. ' - ' ' ' ' ' . ' . ;' ,., ' "...... '' ~ , : . '
W091/00727 PCT/US90/0376]
-17-
The product of Example 11 was used as startin~ material in
Example 3. After purification the product showed distinct
peaks at: NMR(CDC13) ô 7.8-6.65(m, 13~), 4.1(t, lH), 3.2
(d, 2H), 0.7(t, 3H). Anal. calc. for C27H31NO o HCl: C,
76.84; H, 7.64; N, 3.32. Observed: C, 77.00; H, 7.69; N,
3.21.
EX~MPLE 13
Preparation of 2-~N-n-propyl,N-3,3-~iphenylpropylamino]-5-
methoxytetralin.
In Example 1, phenoxyacetic acid was replaced by 3,3-
diphenylpropionic acid. The purified product sho~ed
distinct peaks a': NMR(CDC13) o 7.~-7.2(m, llH), 6.65(m,
2H), 4.05(t,1H), 3.8(s, 3H), 0.9(s, 3H).
EX~PL- 14
Preparation of ~-~N-n-propyl,N-3,3-diphenylpropylamino]-5-
hydroxytetralin.
The produc' of Example 13 was use- 2S sta~ting material for
Exa~ple 3. The isolated product showed distinct peaks at:
NMR(CDC13) ~ 7.4-7.0(m, lH), 6.7-6.5(m, 2H), 4.0(t, lH),
0,9(t, 3H)-
- .- . . - . . ~ .. . .
. : : : : . ~ . .-.:,. . . . . . . . . .
...... . : -. . ; ,.:.. : : , :
- .-, : : .: . . :
-: : : . : . : . :
:.: . . . : , ~ . . . .. - :
. .
WO91/00727 PCT/US90/03761 ~
2~?-$~
-18-
EX~MPLE 15
Preparation of 2-[N-n-propyl,N-2-(2-phenyloxy)propylamino]-
5-methoxytetralin.
In Example 1, phenoxyacetic acid was replaced by DL-2-
phenoxypropionic acid. The purified product showed
distinct peaks at: NMR(CDC13) ~ 7.3-6.65(m, 8H), ~.45(m,
lH), 3.8(s, 3H), O.g(t, 3H).
EXLM~1E 16
Preparation of 2-[N-n-propyl,N-2-(2-phenyloxy)propylamino]-
5-hydroxytetralin.
The product of Example 15 was useà as starting material for
Example 2. The isolated produc. showed characteristlc
peaks at: NMR(CDC13) ~ 7.3-6.65(m, 8H), 4.45(m, lH),
0.9(t, 3H).
EX.3~lPL~ 1/
P-eparation of 2-~N-n-propyl,N-3,3,3-t~iphenylpropylami~o]-
5-methoxytetralin.
In Example 1, phenoxyacetic acid was replaced by 3,3,3-
triphenylpropionic acid. The purified product showed
characteristic peaks at : NMR(CDC13) ~ 7.4-6.65(m, 18H),
3.8(s, 3H), O.9(t, 3H).
.
..
W V 91/00727 PC~/~;S90/03761
5~
_~9_
EXAMPLE 18
Preparation of 2-[N-n-propyl,N-3,3,3-triphenylpropylamino]-
5-hydroxytetralin.
The product of Example 17 was used as starting material in
Example 3. After pu-ification the product sh~wed distinct
peaks at: NMR(CDCl3) ~ 7.4-6.65(r, 18H), O.9(t, 3H).
EX~PLE 1g
Preparation of 2-[N-n-propyl,N-2,2,2-triphenylethylamino]-
5-methoxytetralin.
In Example 1, pehnoxyacetic ~cid can be replaced by
triphenylacetic acid.
EX~MPLE 20
Preparation of 2-[N-n-propyl,N-2,2,2-triphenylethylamino]-
5-hydroxytetralin.
The produc- of Exa..~le 15 can be used as th_ starting
material fo~- Example 3.
,
E~PLE 21
Preparation of 2-[N-n-propyl,N-2-[2-methoxy]phenethyl-
amino]-5-methoxytetralin.
In Example l, phenoxyacetic acid can be replaced by ~-
methoxyphenlyacetic acid.
. . ... .
. i . ,., .. , ,, " . " . .. , , . , ., . " , ,, , ,~, . . . . . . ... ..
.
- . - - , . . .
.: . . . . . .: .: .
: :: -, : . - , , . ,~,
W O 91/00~27 PCT/US90/0376]
Z~$~
-20-
EXAMPLE 22
Preparation of 2-[N-n-propyl,N-2-(2-methoxy)phenethyl-
amino]-5-hyroxytetralin.
The product of Example 21 can be used as the starting
material for Example 2.
EXAMPLE 23
Preparation of 2-[N-n-propyl,N-(2,3-cihydro-lH-inden-l-
yl)methylamino]-5-methoxytetralin.
In Example 1, phenoxyactic acid can be replaced by 1-
indancarboxylic acid.
EX.~M~L~ 2~
Preparation of 2-[N-n-propyl,N-(2,3-dihydro-lH-inden-l-
yl)methylamino]-5-hydroxytetralin.
The product of Example 23 can be used as the starting
material fo- Example 3.
EXAMPLE 25
Preparation of 2-[N-n-propyl,N-(tetrahydro-2-naphthyl)
methylamino]-5-methoxytetralin.
In Example 1, phenoxyacetic acid can be replaced by
1,2,3,4-tetrahydro 2-naphthoic acid. .:
: , : . .
. - : , . ~
: . ~ , . . : . ' . .
WO91/007~7 ~ 5~ PCT/US90/03761
-21-
EXAMPLE 26
Preparation of 2-[N-n-propyl,N-(tetrahydro-2-naphthyl)
methylamino]-5-hydroxytetralin.
The product of Example 25 can be used as the starting
material for E~ample 3.
EX~M~LE 27
Preparation of 2-[N-n-propyl,N-2-(3,5-dime~hylphenyloxv)
ethylamino]-5-methoxytetralin.
In Example l, phenox~acetic acid can be replaced by 3,5-
dimethylphenoxyacetic acid.
EX}~
Preparation of 2-[N-n-propyl,N-2-(3,5-dimethylphenyloxy)
ethylamino]-5-hydroxytetralin.
The product of Example 27 can be used as the startins
ma_erial for Example 3.
EX~MPLE 29
To test the selectivity and specificity of the present
compounds for binding to dopamine receptors tests were
conducted using the following standard procedures.
To test binding to dopamine receptors, the bovine
caudate nuclei assay was employed. ~ovine brains were
obtained fresh from a local slaughterhouse. The caudate
nuclQi ~_ ~ d ~ ut and homosenl~ed ir. DuL~er ~ (~û
mM Tris; 1 mM Na2-EDTA; 5 mM KCl; 1 mM MgCl2; 2 mM CaCl2;
pH 7.4) using a Brinkman Polytron. The homogenate was
~.. . . - : . .. .
.:, : -: . , . : :
; , . .
WO91/00727 PCT/US90/03761
-22-
centrifuged at 40,000 x g for 20 minutes and washed on~e.
The pellett was resuspended in Buffer A, incubated at 37C
for 15 minutes, then centrifuged. The pellet was washed
once more, resuspended to a protein concentration of 5-lO
mg/ml in Buffer A and frozen at -70C until used.
To test binding of the compounds to ~2-adrenergic
receptors, the rat cerebral cortex assay was employed.
~ale Sprague Dawley rats were killed by decapitation and
the brains removed. The cerebral cortices were homogenized
in 50 mM Tris; 2mM ~gCl2 (pH 7.4), and centrifuged at
40,000 x g for lO minutes. The pellet was washed once,
resuspended in Tris/MgCl2 and incubated with 8 units/ml
adenosine deaminase at 37C for 30 minutes. The homogenate
was centrifuged, washed once, resuspended to a protein
concentration of 5-lO mg/ml and frozen at -70C until use.
The following tritiated drugs were used as radioligands
for each of the receptors tes~ed: [3H]-Spiperone 21-24
Ci/mr.ol for D2 receptors, [3H]-scH2339o 75-85 Ci/mmol for
Dl receptors, and [3H]-Para aminoclonidine 48-52 Ci/mmol
for ~2-adrenergic receptors. The radioligands were
incubated with various concentrations of competing drug and
the appropriate membrane source for periods of time as
follows: 75 minutes at room temperature for D2 receptors,
15 minutes at 37C for D1 receptors, or 30 minutes at room
temperature for ~2 receptors. Specific binding was defined
using l~M butaclamol (D2), 1 ~M SCH23390 (Dl), or l ~M
yohimbine (~2) In addition the D2 assays contained 30 nM
:._.aserin in order to bloc~ the blr,ding of 3n-~p ~ peron~ IO
5HT2 receptors.
.
.. ~ . .
- ~' '. ~: . ' , . : , ,' . . : ,
WO91/00727 ~ ~ PCT/US90/03761
The assays were ter~inated by filtration using a 24-
port Brandell cell harvester over filters that had been
previously soaked in 0.1~ polyethyleneimine, and the
filters were washed three times by filtration of cold
buffer. The filters were then placed in 5 ml scintillation
vials to which 4 ml of Beckman Ready-Protein was then
added, and each vial was counted for 2 minutes in a BecXman
3801 scintillation counter calibrated for conversion of cpm
to dpm. Binding data were analyzed using the Ligand
program of Munson and Rodbard (1980). The results are
presented as Ki values if the data were best fitted to a
one-site model, or as ~H and ~L values if a two-site model
produced the better fit.
Results of the binding tests ~re summarized in Table 1
below:
Table 1
RECEPTOR AFFINITIES (Ki, nM) - -
Example D2 (KL)D1(KL) ~2
Cor~ound
14 3401,350 2,600
2 12S12,000 500
12 47 8,96~ 11,000
9 66 2,130 100 -
N-0437 1101,000 190
This table shows high dopamine D2 receptor affinities of
compounds chosen from the examples above, with unexpectedly
high degrees of selectivity and specificity. The compound
N-0437, a potent dopamine n~ ;cl ;s included as a
reference compound for comparative purposes.
. , . .. .. , ~, . .. ,. ,, . , ,, , , . .. . .. - . . . .
, . ., . ~ . - ..
- ~ - . .. : . ,. ~ .
~: ; , - . , ~ . . , , : :
: - ~ : .~ - .. , - . .
.. . ..
007~7 PCT/US~0/03761
r~
While particular embodiments of the invention have been
described it will be understood of course that the
invention is not limited thereto since many obvious
modifications can be made and it is intended to include
within this invention any such modifications as will fall
within the scope of the appended claims.
., - , . ~ , . ~ . :
:. . ... ~ . . :
.. . . ~ . ..
- : . :
~ . ~ , . .
.. .. . . . .. . .
.