Note: Descriptions are shown in the official language in which they were submitted.
r ~
Henkel 30.8.1989
Xommanditgeqellqchaft
auf Aktien
D 8663
S p 13481/89 M/MH (#27)
Proceqs for the preparation of alkali metal salts of
ether-carboxylic acid~
The in~ention relates to a proce~s for the
preparation of alkali metal ~alts of ether-carboxylic
acidq of the general ~ormula I
R-(OCmH~)n-O-CH2COOM (I)
in which
R denote~ an alkyl group having 1 to 22 carbon atom~,
an aryl group or an aralkyl group,
m denotes the number 2 and/or 3,
n denote~ a number in the range from 0 to 20 and
M denotes an alkali metal from the group formed by
lithium, sodium and pota~sium,
by oxidation of ether-alcohol~ of the general formula II
R-(OC~H~)n-OcHzcH2oH (II)
in which
R, m and n are a~ defined abQve,
in the aqueous phase with oxygen or gases contalning
oxygen at elevated temperatures in the presence of alkali
met~l hydroxldes and noble metal catalyst~.
Alkali metal ~alts of ether-carboxylic acids are
compounds which have interestinq surface-active proper-
ties and are employed in the form of their aqueous
olution~, or example in co~met~cs formulation~.
It i~ ~nown that alkali metal salts of ether-
c~rboxylic acids of the general formula I can be prepared
by catalytic oxidation of the corre-qponding ether-alco-
hols of the general formula II, compare EP-~-0,039,111,
EP-B-0,018,681, EP-B-0,073,545, US-C-3,342,858,
DE-C-2,816,127, DE-A-3, 135,946, DE-A-2,936,123 and
DE-A-3,446,561.
-- 2 .~ ,
However, only dllute solutions of the alXali
metal salts of the ether-carb~xylic acids can be prepared
by the ~nown catalytic proce~qe~. In particular, if
oxygen or a gas containing oxygen i~ passed into a
S relatively highly concentrated ~olution of the ether-
alcohol~ in water in the presence of the catalyst-~, the
viccosity of the reaction mixtur~ increases greatly a~
the conversion increases, pas~es through a maximum at
about 30% conversion (about 30~ of sodium salt of the
ether-carboxylic acid and about 70% of ether-alcohol) and
then drops greatly again aY the conv~r~ion becomeq
higher; compare DE-C-2,816,127. The rate of reaction
becomes so slow during thi~ procedure, becauYe the ma~
tran~fer i~ impeded by the vi3cosity, that the proce~s i~
uneconomical since the reaction tLme i3 then too long; in
the ~xtreme case, th~ reaction here can even qtop com-
pletely. Low concentrations of an organic sub~tance (that
is to say total weight of ether-alcohol and sodium salt
of the ether-carboxylic acid) ar~ therefore used in the
known proces~es in order to avoid a high increa~e in tho
viscosity. However, thi~ require~ 3ubsequent concentra-
tion.
It is indeed possible for the dilute aqueous
solution~ of the alXali metal salts of the ether-car-
boxylic acid~ obtained after the oxidation to be con-
centrated by removing some of the water contained in the
-solution~ by distillation or by acidifying the solution~
with ~trong acids, for example with sulfuric acid,
liberating the ether-carboxylic acid and precipitating
it, isolating the ether-carboxylic acid and preparing
concentrated aqueous ~olution~ after renewed conversion
into the alkali metal salts. However, these processe~
have the following disadvantagess
1. Aqueous solutions of alkali metal 3alt~ of ether-
carboxylic acid3 foam greatly during removal of the
water by di~tillation. The profitability of the
process i~ moreover greatly reduced by the energy
required for removal of water by distillation.
2. During precipitation of the ether-carboxylic acids
~3
-- 3 --
with acids, the aqu~ous phase which remains i
polluted by a hiqh salt load, residual ether-car-
boxylic acids and unreacted ether-alcohol; it~
disposal i~ uneconomical. Renewed conver~ion of the
ether-carboxylic acids into their alkali metal salt~
also leads to an additional increase in the co~ts of
the reaction product.
An additional hindrance occurs in particular if
air i~ used a~ the oxidizing agent. In thi3 case, the
oxidation procedure is made difficult by the foam formed
a~ a result of the surface-active properties of th~
starting substances and end products. The foam i~sue~
'rom the reactor with the waste gas and must be recycled
from there back into the reactor after its destruction.
The rate of foam formation is always high if air i5
r~ dispersed in the solution, as i8 the case, for example,
~ I in ~ reactor~ or bubble column rsactors. In
I ~ btirrcd ]cottl~ reactors in particular, the reaction
solution can be converted into a foam-like state by the
stirring action, so that mass tran~fer of the oxygen i9
prevented and the reaction is inhibited; compare
DE-C-2,816,127.
The invention relates to a proces~ for the
preparation of alkali metals salts of ether-carboxylic
acids of the abovemsntioned type, in which the above-
men~ioned di~advantages in respect of the increa~e in
vi~c08ity and the foaming of the reaction mixture are
avoided and highly concentrated aqueou~ solutions of
alkali metal ~alts of the ether-carboxyllc acid~, for
exampl~ having a concentration of 20 to 50% by weight,
ba~ed on tha total weight of the solution, can be
obtained.
According to the inventlon, thiJ ob~ect is
achieved by bringing an aqueous solution, containing an
alkali metal hydroxide ~olution, of the ether-alcohol~ in
a thin layer on a solid support or in the form of fine
particlss or droplet~ into con~act with oxygen or tha
gase~ containing oxygen as the continuous phase, the
concentration of the ether-alcohols in the aqueou~ phase
- 4 ~
bein~ in the range from at lea~t 0.1, in particular from
0.5 to 15% by weight, ba~ed on the total weight of the
aqueous phase. Below the stated range the rate of reac-
tion iq generally too low qo that the concentration
should fall below this range only towards the end of the
reaction, when the addition of ether-alcohol has ended.
Alkali metal salts of ether-carboxylic acid~ of
the general formula (I) in which the group R can be a
straight-chain or branched alkyl group having 1 to 22
carbon atomq can be prepared by the process according to
the invention; typical examples of such alkyl groups are
methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl,
octyl, nonyl, decyl, dodecyl, tetradecyl, hexadecyl,
octadecyl, eicosyl and docosyl. The proce~Y according to
the invention i~ particularly suitable for the prepara-
tion of alkali metal 3alt~ of ether-carboxylic acids in
which the radical R i~ derived from Clz-ClO-fatty alcohol~,
or indu3trial mixtures thereof, obtainable from animal
and/or vegetable fats and oils. The group R c~n also be
an aryl radical, for example a phenyl group, or an
aralkyl radical, for example a phenylalkylene group
having 1 to 3 carbon atom~ in the alkylene radical.
If n > 0, the compound of the general formula II
i8 an addition product of ethylene oxide or ethylene
oxide and propylene oxide on alcohols of the formula ROH,
it being po~ible, in the case of the ethylene oxida/pro-
~ylane oxide adducts of the formul~ II, for the propylene-
glycol radical~ to be in random or block di~tribution in
the alkoxylate ch~in, but a terminal ethyleneglycol
radical always being present. Addition products of
ethylene oxide on alcohols of the formula II are pre-
ferred in the context of the invention, 80 that m ~ 2 i9
a preferred mean~ng for the compounds of the formulae I
and II.
The increa~e in visco~ity which occurs at higher
concentrations of ether-alcohols i~ avoided if, according
to the invention, ~he reaction i~ started with only ~ low
ether-alcohol concentration at the beginning and ether-
alcohol i3 metered into the reactlon ~olution
- S _ foJ ~ t~ 2
continuou~ly or in portions a~ the reaction pregres~e~
further, and in particular at a rate such that the con-
centration of the ether-alcohol~ in the reaction mixture
does not exceed the value of 15~ by weight.
S The abovementioned problem of foaming i avoided
or con~iderably reduced by the reaction solution being
present in the form of thin layers on a solid ~upport or
in the form of fine particles in a contlnuou~ phase of
oxygen or gases containing oxygen.
According to an advantageous embodiment of the
process according to the invention, the oxidation i~
carried out at a tempsrature in tha range from 40 to
130C, in particular 60 to 85-C. The rate of reaction is
too low below the stated range. Although the reaction can
al~o be carried out above the 3tated range, this gives
only an in~ignificant increase in the rate of reaction.
According to another advantageou~ embodlment of
the procesq according to the invention, the oxidation i~
carr~ed out under an oxygen partial pre~sure of 0.1 to
5 bar. With oxygen-containing gase~ in particular,
foaming is suppre~sed more and more as the system pres-
sure increases and the effective gas throughput thus
decreases. The rate of reaction furthermore increa~es
under certain circumstance~ as the oxygen partial pre~-
~ure increase~.
According to another advantageouff embodiment of
th~ invention, tha oxidation i~ carried out with air.
This is another con~iderable advantage over the processes
known from the prior art, in which oxygen i~ in general
used in order to prevent th~ nitrogen content of air a~
the oxidizing agent promoting undesirable foaming, and in
order to carry out the reaction without a waste ~as.
Useful catalysts for use in tha proces~ according
to the invention are the nobla metal catalysts known from
the abovementioned prior art, in particular those ba~ed
on pl~tinum or palladium. P~lladium catalysts, for
example palladium-un-charcoal, have proved to be par-
ticularly suitable for the process according to the
invention. The cataly3~ i~ preferably introduced into the
; ~
- ' , " ' .
- 6 ~ r~d
procQs~ in the form of a ~u~pen~ion in thQ aqueou~
solution of the ether-alcohol~. However, it i3 al~o
poc~ible for the cataly~t to be located on a solid
support material, over which the aqueous solution of the
S ether-alcohols ic passed. Pos ible support material~ for
thi~ purpose are, for example, active charcoal, graphite,
kieselguhr, ~ilica g~l, spinel-~, aluminum oxid~ or
ceramic materials. The catalyst-q can furthermore also
contain combination3 of a plurality of noble metals
instead of one noble metal, for example mixtures of Pd
and Pt, and moreovar 3uitable activator~, such as lead,
bismuth or cadmium, in the form of their metal-R or their
compounds, including combination~ thereof. Suitable
cataly~t~ are described in the abovementioned litera~ure
and in US-B-4,607,121.
According to another advantageous embodiment of
the proce~s according to the invention, the cataly3t i8
employed in the form of a suspension in a concentration
of 0.2 to 3% by weight, based on the total weight of the
suspen~ion containing the ether-alcohols and water.
The process according to the invention is in
general carried out at pH valua~ of at lea~t 8. Par-
ticularly adv~ntageous pH value~ are at least 9, in
particular in the range from 9 to 11. Surprisingly, it
has been found that, in contrast to the doctrine of
US-C-4,607,121, in spite of these h~gh pH value~ neither
~d1s~olving of the catalyst nor oxidative chain degrada-
tion or by-product formation occurs when air i~ u~ed a~
the oxidizing agent; the end product of the process
accordinq to the invention i~ Pd-free and the cataly~t
can be reused after waYhing with hot water and treatment
with hydrogen.
According to another advantageous embodiment of
the in~ention, the oxidation of the ether-alcohol~ is
carried out in a reactor in which the oxygen or the ga~es
containing oxygen and the aqueou~ phase containing the
ether-alcohol, alkali metal hydroxide ~olution and if
appropriate the catalyst are introduced at the top of the
reactor, the reaction mixture containing ether-carboxylic
,
'
i I ~ er~ ~_
-- 7 --
acid salt~, unreacted ether-alcohol and if appropriate
the cataly~t i9 removed at the bottom part of ths re~ctor
and the reaction mixture i~ recycled to the upper part of
the reactor for renewed oxidation of a~ yet unreacted
ether-alcohol. It iR preferable here for alkali metal
hydroxide ~olution, for maintaining the pH of at least 9,
in particular 9 to 11, and ether-alcohol, for maintaining
the ether-alcohol concentration of at least 0.1, in
particular 0.5 to 15% by weight in the reaction mixture,
10 to be added continuou~ly to the reaction mixture removed
at the lower part of the reactor before recycling to the
upper part.
According to another advantageou embodLment of
the invention, packed column~ of the usual con~truction,
15 such a~ are described, for example, in Ullmann,
Enzyklopadie der technischen Chemie, 4th edition, volume
c~ r 3, pages 390 to 392 (1973), Verlag Chemie, WeinheLm, a3
beunte-r curron~ packed cOlumn8~ are used for carrying out
the proce~.
The packingR to be employed in the packed cOlumn8
advantageou~ly have a high intermediate volume 80 that
the gas speed and the rate of foaming does not become too
high. Typical examples of ~uitable packing~ are known
from Ullmanns Enzyklop~die dsr technischen Chemie, 4th
25 edition, volume 2, page 529 (1972) and 5th edition,
volume B3, pages 4-82 to 4-83 (1988); the use of Pall
rings, Novalox saddle~, Berl saddle~, Intralox ~addles
and Interpack bodies is particularly preferred. Ordered
packing~ such as are described in volume 2 of the 4th
30 edition of the abovementioned encyclopedia, page~ 533 -
534, for example of the Sulzer packing type, can further-
more al~o be employed. Finally, it is al80 possible to
employ bulX cataly~t~ or catalyst fixed beds in~tead of
~ulk packing. Ordered cataly~t packing~, for example in
35 honeycomb form, can al~o be employed.
According to another advantageous embodiment of
the invention, the reaction mixture removed at the lower
end of the column i~ recycled, after the pH and the
ether-alcohel concentration ha~ been ad~usted, to the
.. ~
,
.
:.
- 8 - ~ J
upper part of the column for renewed reaction until a
concentratlon of the ether-carboxylic acid ~alt of 20 to
50~ by weight, ba~ed on the total weight of the solution,
i~ reached and the ether-alcohol metered in ha3 reacted.
5The invention is illustrated in more detail below
with the aid of the drawing and a preferred exemplary
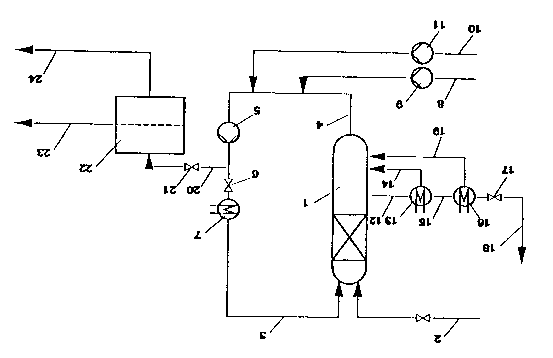
embodiment. The drawing show~ a ~chematic representation
of an installation for carrying out the proce~ according
~o the invention.
10The in~tallation compri~e~ a packed column 1
which is provided at it~ upper end with a feedlin* for
oxygen or gases containing oxy~en, in particular air. A
line 3 ~erves to feed in the water~ether-alcohol mixture,
containing the ~uspended catalyst if appropriate. A line
154 is located at the lower end of the packed column for
removal of the oxidized reaction mixtura; the reaction
mixture can be recycled to the top of the packed column
via a circulating pump 5, a valve 6, which i8 open during
the reaction and clo~ed only during the filtrat$on
20di~cussed below, and a heat exchanger 7 and via line 3.
Aqueous -~odium hydroxide solution 18 fed in via line 8
and a metering pump 9, and the ether-alcohol to be
oxidized i9 fed in via line 10 and a metering pump 11.
The waste air flowing out of the packed column 1 is
25removed laterally via line 12 and passed to a waste gas
heater 13. In this, the foam formed, for example during
-incorrect operation of the installation, and entrained
with the wa~te air can be destroyed and recycled to the
reactor as a liquid via line 14. The waste air which has
30been freed from the foam i~ fed via line 15 to a cooler
16 and i~ removed from the sy~tem via a valve 17 and line
18; any entrained droplet~ of liquid or condensate
obtained are likewise recycled to the reactor via a line
19. The aqueou~ su~pension containing the end product is
35removed via a line 20 and a valve 21 and fed to a filter
unit 22 where the aqueous solution of the process pro-
ducts and the su~pended cataly~t are sep~rated, these
each being removed via lina~ 23 and 24 respectively. The
catalyst i~ introduced at a point not 3hown in line 4.
: ' ~
: : ,
-- 3 --
The in~tallation ~hown in Figure 1 i4 oper~ted a~
follows:
In the packed reactor flu~hed with nitrogen, the
su~pen~ion of the pulverulent noble metal catalyst in
water is recycled from the bottom of the reactor to its
top by mean~ of the circulating pump S. When the solution
ha~ been heated to th~ reaction temperatur~ by the heat
exchanger 7 in the ~olvent circulation, a small amoun~ of
ether-alcohol and sodium hydroxide solution is metered in
the form of an aqueou-q solution into the circulating
su~pension by mean~ of the metering pump~ 9, 10. The
nitrogen is then di~placed by oxygen or a gas containing
oxygen and immediately after the desired pressure has
been reached, the gas throughput required for the oxida-
tion and the metering in of sodium hydroxide solution andalcohol via the metering pumps 9 and 11 are ad~uqted.
During the reaction, which i8 detectable by an
oxygen uptake, alcohol and aqueou~ sod~um hydroxide
solution ars metsred in continuously. The metering rate
20 i3 ad~usted or varied and m~tched to the rate of reaction
80 that a ~mall amount of ether-alcohol which ha~ not yst
reacted and therefore a low viscosity i8 en~ured in the
solution throughout the entirs course of the reaction.
When the required amount~ of ether-alcohol and
~odium hydroxide solution have been metered in, the
metering i9 stopped; the reaction i~ then continusd until
~t~e oxyqen uptake decrease~ ~ignificantly.
When thQ reaction h~ ended, the gaJ feed i~
interruptsd and the catalyst i~ ~eparated off from the
~olution by filtration.
Example 1.
A cu~tomary packed column of internal diameter
50 mm and haight of the packing of lO00 ~m was used for
thi~ exemplary smbodiment; the column wa~ charged with
packing of the typ~ Interpack 15/40.
The feed material wa~ a commercially availabls
fatty alcohol ethoxylate (addition product o~ about 4 mol
of ethylens oxlde on an indu~trial fatty alcohol of chain
- ~ .
-- 10 --
length Cl2-Cl~, molecular weight: 369). A palladium cata-
ly~t containing 5~ of palladium-on-charcoal (Degus~a),
which had been reduced with hydrogen before u e in the
form of an aqueou~ suspen~ion, wa3 used a~ the catalyst.
The installation wa~ initially charged with a
suspension of, based on the dry ~ub~tance, 35 g of
cataly~t in 2400 g of demineralized water. To start the
reaction, 30 g of ether-alcohol (about 1.2% by weight)
and 3.3 g of NaOH (a~ 25~ strength sodium hydroxide
solution) were metered into the in~allation. The par-
ticular content of free ether-alcohol wa~ determined
mathematically from the amount of oxygen taken up by the
reaction solution by the period up to each point in time
of the reaction and the amount of ether-alcohol metered
in by thsn.
In dstail, the proce~s parameterq were as fol-
low~s
Sy~tem pressures 1.9 bar ab~olute
Suspension temperatures 75-C
Air throughput (reactor outlet)s 30 Nl/hour
Solution circulations 170 l/hour
Metering rate o ether-alcohol~ about 140 g/hour
~0.379 gmol)
Metering rate of NaOH (calculated a~ 100S strength NaOH~s
15.2 g/hour ~O.379 gmol/hour)
Total amount of ether-alcohol metered in~ 720 g
~1.95 gmol)
Total amount of NaOH metered ln (a~ 100% ~trength NaOH)s
78.0 g (l.g5 gmol)
Average or maximum content of ether-alcohol which ha~ not
yet reacteds 3 and 4~ by weight re~pectively
Duration of the reactions 6.3 hour~
Conversion (determined mathemxtically from the 2 uptake
and the NaOH con~umption~: about 97%.
When the reaction has ended and the catalyst has
been removed by filtration, an approximately 23% ~trenqth
solution of the sot~um salt of the ether-carboxylic acid
formed, ba~ed on the total weight of the solution, was
obtained with a pale yellow color. N~R analy~is of the
- -
proces~ product ~howed an average degree of ethoxylation
which wa~ lower than that of the ether-alcohol by one
unit. ~tomic spectroscopy analysis of the filtrate on
palladium -~howed a content of < 1 ppm.
The experiment was performed seven timeq using
the ~ame catalyst without los~es in activity being
detectable. Before each use, the catalyst wa~ washed with
hot water, ~uspended in water and reduced with H2 at room
temperature.
Example 2.
An addition product of on average 5 mol of
ethyleneoxide on 1 mol of the indu~trial fatty alcohol
described in Example 1, of chain length C~2-Cl~, wa~
oxidized by a proce~s analogou~ to that of Example 1.
After a reaction time of 6.7 hour~, a 23.5% strength
~olution of the ~odium ~alt of the corre~ponding ether-
carboxylic acid wa~ obtained in a conversion of about
97%, calculated from the oxygan consumption. An addition
product of on average 9 mol of ethylene oxide on 1 mol of
an indu~trial fatty alcohol of chain length C~2-C1~ wa~
oxidized in the same manner. After a reaction time of
6.2 hours, a 19.5~ strength solution of the ~odium salt
o the corre~ponding ether-carboxylic acid was obtained
at a conver~ion, calculated from the oxygen consumption,
of about 105%.
- In deviation from the procedure of the exemplary
embodiment explained above, it i~ also possLble to use
star~ing concentrations other than 1.2~ by weight of
ether-alcohol. Thus, for example, the reaction can also
be started without initial addition of ether-alcohol,
that i8 to ~y the ether-alcohol i~ metered in only at
the ~tart of the introduction of air. However, thi
procedurQ can lead to a premature discontinuation of the
reaction and mu~t therefore be regarded a~ unstable.
Higher initial concentrations, for example of 7S by
weight of ether-alcohol, are al~o possibla, but offer no
ad~antages in re~pect of the dur~t~on of the reaction in
the case of the ether-alcohol employed in the pre~ent
3 .i J
exemplary embodiment.
I t has proved advantageou~ for an adequato rate
of reaction to choo~e the NaOH metering so that at each
point in tLme the acid formed up until that point ha~
been bonded completely; at this equivalence point, the
~olution ha~ a pH of about 9. However, a ~ignificantly
higher rate of reaction i~ obtained if the total amount
of NaOH metered in at each point in time i~ equivalent to
the to~al amount of ether-alcohol metered in, that is to
say at pH value~ of more than 9, for example of 10 to 11.
No oxidative chain degradation on tAe ethoxylate groupa
and no dissolving of the catalyst were to be found even
at the~e higher pH values.
Finally, it is also possible for the entire
amount of NaOH to be initially introduced from the
beginning; however, thi~ ia not an advantage in reapect
of the duration of the reaction compared with the reac-
t;.on procedure de~cribed above in the exemplary embodi-
ment.