Note: Descriptions are shown in the official language in which they were submitted.
~007~~~.
- 1 -
CONDUCTIVE POLYMER COMPOSITIONS
Conductive polymer compositions are known which comprise a polymer or
a mixture of polymers throughout which is dispersed a powder comprising
conductive particles. Commonly the powder is carbon black.
It has been known for some years that. some conductive polymer
compositions comprising conductive carbon black dispersed in a polymer or a
mixture of polymers exhibit a positive temperature coefficient of resistance
which itself increases with temperature in a discontinuous manner so that the
compositions have characteristics akin to a semi-conductor. For example, the
resistance may increase quite slowly with increase of temperature below a
certain level, called the critical temperature, but above that critical
temperature level there is a rapid increase of resistance. Indeed, the
increase may be so rapid that the composition becomes effectively non-
conducting at temperatures above the critical temperature. This phenomenon
is described, for example, in U.S. Specification No. 3,243,753, which
describes
an electrical resistor in the form of a plastic e.g. a thermoplastic or
suitable thermosetting material, preferaby a polyolefin plastic, containing a
finely divided conductive powder, preferably carbon black, intimately
dispersed
through the plastic matrix. In that Specification it is suggested that below
the critical temperature there is particle-to-particle contact throughout the
composition, which thus has a relatively low resistance. Above the critical
temperature, however, the substantial difference between the thermal
coefficients of expansion of the conductive particles and the plastic results
in breaking down the contact between the particles, with resultant sharply
increased resist ivity. U.S. Specification No. 4,560,498, however, refers to
other theories which have been proposed to account for the positive
temperature coefficient <PTC) phenomenon, including complex mechanisms based
upon electron tunnelling though inter-grain gaps between particles of
conductive filler or some mechanism based upon a phase change from
crystalline to amorphous regions in the polymer matrix. The Specification
refers to a discussion of a number of theories in "Glass Transition
Temperatures as a Guide to the Selection of Polymers Suitable for PTC -
Materials" by J. Meyer in Polymer Engineering and Science, November, 1973,
Vol. 13, No. 6.
20 877 2 1
- 2 -
According to the specification of U.S. patent
No. 4,560,498, known PTC materials generally comprise
one of more conductive fillers such as carbon black or
powdered metal dispersed in a crystalline thermoplastic
polymer. According to the specification, such known PTC
materials are unreliable and either do not provide the
sharply increased resistance which is intended at the
critical temperature or have a variable critical
temperature, particularly over a number of cycles. Such
unreliability has also been noted by the present
inventor.
Japanese Patent Application No. Sho 60-124654
published in 1985 discloses an electroconductive resin
composite comprising ingredients (a) to (c), with an
amount of (a) in the range of 96 to 50 parts by weight
with respect to (a + b), an amount of (b) in the range
of 4 to 50 parts by weight with respect to (a + b), and
an amount of (c) in the range of 10 to 100 parts by
weight with respect to 100 parts by weight of (b), where
(a) is a thermoplastic resin, (b) is a carbon black with
BET specific surface area larger than 850 m2/g and (c)
is a carbon black with BET specific surface area smaller
than 100 m2/g. One or more inert fillers) may also be
added. The composites are said to have excellent
electroconductivity, moulding processability, mechanical
properties and moulding appearance.
Japanese Patent Application No. Sho 60-135441
published in 1985 discloses a semiconductor resin
composite made of ethylene-series polymer with added
electroconductive carbon black and heat-conductive
carbon black with particle size larger than that of said
electroconductive carbon black. The amount of heat-
conductive carbon black may be 5 to 20 parts by weight
with respect to 100 parts by weight of the polymer. It
is said that such a type of semiconductor resin can
2os~~ 2 ~
- 2a-
realize easily any desired electrical resistance in the
range of 103 to 106 ohms/cm.
According to this invention, a conductive
polymer composition having positive temperature
coefficient characteristics comprises at least one
polymer providing a matrix throughout which is dispersed
a mixture of conductive carbon blacks each having a
structure level, as measured by DBP technique, of 40 to
150 cc/100g, the polymer matrix constituting from 20 to
98 per cent by weight of the composition, and the
mixture constituting from 2 to 80 per cent by weight of
the composi~ion and comprising a first conductive
B
_ 3 _
carbon black and a second conductive carbon black, each of the carbon blacks
constituting 1 to 40 per cent by weight of the composition, the first carbon
black comprising particles having average size in the range from 35 to 300 nm
and the second carbon black comprising particles having average size in the
range from 15 to 25 nm.
It is preferred that the composition be composed of from 40 to 80 per
cent by weight of polymer and from 20 to 60 per cent by weight of the
mixture of carbon blacks. Most preferred is a composition comprising 52 to
72 per cent by weight of polymer and 28 to 48 per cent by weight of the
mixture.
Preferably the first and second carbon blacks are both of the kind
commonly called "furnace" blacks, produced by the well-known furnace process
rather than by other known methods such as the channel, or thermal, process.
Any polymer may be used in producing the conductive polymer
composition of the invention. It is preferred, however, that the polymer
utilized be thermoplastic, such as polyethylene, ethylene vinyl acetate,
polypropylene, polyamide, polyethylene terephthalate or polyethersulphone.
Surprisingly, the use of a mixture of first and second carbon blacks as
described has been found to produce conductive polymer compositions with PTC
characteristics having electrical properties which are substantially more
reliable than prior compositions, as evidenced by repeatability of these
characteristics over many cycles of heating and cooling above and below the
critical temperature. Moreover, the compositions have critical temperatures
which ere significantly less variable than thoae of prior art compoaitiona and
exhibit a sharper change in the rate of change of resistance with temperature
around the critical temperature than is noted for normal PTC compositions
utilizing one carbon black.
Conductive polymer compositions as described may be formed into
electrical elements to exploit the electrical properties mentioned. Far
example, the elements may be resistors, heaters or sensors or the like.
_.
- 4 -
The conductive polymer compositions of the invention may also be
utilized in the preparation of conductive ink formulations. This is achieved
by mixing any suitable transient carriers with the conductive polymer
compositions of the invention along with any conventional additive.
The conductive polymer compositions of the invention are readily
prepared by mechanical methods. The polymer or polymers and the carbon
blacks may be admixed on a conventional mixing machine of the type normally
used for mixing rubber or plastics such as a mill roll, an extruder or a
Banbury mixer. The mixture produced by the machine may be formed by
moulding or extrusion into electrical elements as mentioned or it may be
formed into pellets which may be used subsequently in extrusion or moulding
equipment.
The critical temperature in a conductive polymer having PTC
characteristics depends to some extent upon the size and shape of the polymer
element and the manner in which current connections are made. If the current
connections are on one surface of a polymer element, the current is mainly
conducted through a thin surface film, commonly called "planar" conduction,
which may produce higher critical temperatures than if the connections are
made at opposite ends of a polymer element. In the latter case the
conduction, commonly called "bulk" conduction, is more or less uniformly
through the body of the element. For polymer elements of a uniform size and
shape produced from compositions as described which exhibit PTC
characteristics, it has been found that the critical temperatures depend
largely upon the particular polymer or polymers which are present.
The invention will be understood more readily by reference to the
following examples. There are many other forms of the invention, as will be
obvious to one skilled in the art once the invention has been fully disclosed.
It will accordingly be recognized that these examples are only given for the
purpose of illustration and are not to be construed as limiting the scope of
the invention in any way.
The following testing procedures are used in the determination of the
properties of the carbon blacks utilized in the preparation of the conductive
~Q6~~21
- 5 -
polymer compositions of the invention and in the determination of the physical
properties of the novel conductive polymer compositions
B.E.T. Surface Area - ASTM D 3037.
Particle Size - ASTM D 3849.
DBP Absorption - ASTM D 2414.
Surface Resistance - DIN 53482.
In preparing the formulations of the following examples which are
illustrative of the invention, the carbon blacks shown in the following
Table 1 have been utilized. The blacks shown in Table 1 are typical of the
carbon blacks which may be utilized in the invention.
TAB L E 1 SUITABLETYPES OF CONDUCTIVECARBON BLACK
CARBON SURFACE PARTICLE DBP PROCESS
BLACK AREA SIZE ABSORPTION OF
m~/g nm cc/100g PREPARATION
REF. (ASTM D (ASTM D 2414
3037 ) >
(ASTM
D 3849
)
1 140 20 116 Furnace
2 42 41 120 Furnace
3 30 60 64 Furnace
4 230 15 65 Furnace
5 25 75 64 Furnace
6 7* 148 41 Thermal
7 220 16 105 Furnace
* Determined by D 1510.
ASTM
EXAMPLES 1 - 6
EFFECT OF POLYMER COMPOSTTION ON CRITICAL TEMPERATURE
Examples 1 to 6 comprised mixtures as shown in the following Table 2.
._
- 6 -
TAFSLE 2
EXAMPLE Nl)MBERS : 1 2 3 4 5 6
Ingredients Percentages by
Weight
First Conductive Carbon Black (Ref.20 20 20 20 20 20
1>
Second Conductive Carbon Black 20 20 20 20 20 20
<Ref. 2) ~
EVA copolymer 1 <18/. Vinyl Acetate)60
Polyethylene 60
Polypropylene 60
Polyamide 6,6 60
Polyethylene terephthalate 60
Polyethersulphone 60
Critical temperature <'C> 80 90 132 160 275 350
Critical Temperature is the temperature at which the Positive Temperature
Coefficient (PTC? Factor equals 10. The PTC Factor at any temperature is the
surface resistance at that temperature divided by the surface resistance at
'C.
Table 2 shows that different polymers, when used with the same carbon
blacks to produce conductive polymer compositions according to the invention,
produce different critical temperatures.
20 EXAMPLES 7 - 12
EFFECT OF CARBON BLACK CONCENTRATION
ON PTC CHARACTERISTICS
Examples 7 to 12 comprised mixtures shown in the following
as Table 3.
TABLE 3
EXAMPLE NUMBERS : 7 8 9 10 11 12
Ingredients Percentages by Weight
First Conductive Carbon Black <Ref. 22 20 18 18 17 16
1)
Second Conductive Carbon Black <Ref. 22 20 18 18 17 16
2)
EVA copolymer 1 tl8x Vinyl Acetate) 56 60 64 64 66 68
Critical temperature ('C) 81 80 64 61 58 55
Table 3 shows that changing t he proportions of the carbonblack
components can affect the critical temperature of conductive lymer
po
compositions according to the invention,but the effects are less than
severe
_,_
those produced by changing the polymer base, as shown in Table 2. The
difference in critical temperature between Examples 9 and 10 is typical of
differences obtained due to variations in dispersion during manufacture and in
measurement accuracy.
EXAMPLES 13 to 1S
EFFECT OF CARBON PARTICLESIZEON CHARACTERISTICS
BLACK PTC
Examples 13 to comprisedmixtures the followingTable
19 as shown 4.
in
Their PTC Factors areshown
at various temperatures in
Table
5.
TABLE 4
10EXAMPLE NUMBERS 13 14 15 16 17 18 19
Ingredients Percentages by Weight
CARBON BLACK PARTICLE
REFERENCE SIZE
1 20 20 20 20
152 41 20
3 60 20
4 15 20 20
5 75 20 20
6 148 20 20 20
207 16 20 20
EVA Copolymer 60 60 60 60 60 60 60
1
TABLE 5
TEMPERATURES('C)
30 40 50 60 70 80 90 100
25 EXAMPLE NUMBER PTC FACTOR
13 1.4 1.8 2.5 3.6 6.8 16 188 318
14 1.5 1.8 2.7 4.8 12 41 171 518
1.3 1.7 2.1 2.7 4.0 6.0 8.2
16 1.6 2.7 4.0 10.5 25 31 3330 3882
30 17 1.3 1.6 2.0 2.4 3.1 6.1 4.8 '-
18 1.4 1.7 2.4 3.3 5.4 12.2 22
19 1.3 1.7 2.2 3.2 4.7 9.7 12.6
Table 5 shows how the PTC characteristics of the compositions can be
_ 8 _
varied over a very wide range by changing the particle sizes of the carbon
blacks used.
Other electrical properties of compositions according to the invention
are illustrated by the accompanying drawings in which
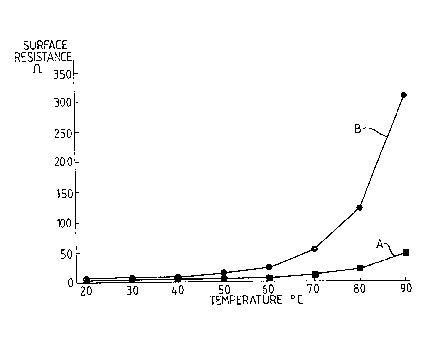
Figure 1 shows graphs of surface resistance against temperature for
identically shaped specimens formed from a conventional
conductive polymer composition (i.e. a composition utilizing a
single carbon black) and for a conductive polymer composition
according to the invention,
Figure 2 is a series of graphs of positive temperature coefficient
factor against temperature for identical specimens of the
compositions identified in Table 3 as Examples 7 to 12,
Figure 3 shows graphs of resistance against time for identically
shaped specimens of the composition identified in Table 3 as
Example 8, the specimens being maintained at two
temperatures, and
Figure 4 is a graph of stability of resistance over a number of
switching cycles of 6 minutes conducting at 15 volts and 18
minutes not conducting for a specimen of another composition,
comprising ethylene vinyl acetate 52x and carbon blacks
reference 1 and reference 2, as described in Table 1, each
24x, by weight, the specimen being maintained at 23'C.
Graph A in Figure 1 compares the surface resistance with temperature
for a specimen formed from a conventional conductive polymer composition
comprising 60 percent by weight of EVA copolymer 1 mixed with 40 percent by
weight of conductive carbon black reference i (see Table 1). Graph B shows
the same comparison for an identically shaped specimen formed from the
composition identified in Table 2 as Example 1, comprising the same polymer
with 20 percent by weight of each of conductive carbon blacks references i
and 2. It can be seen that the resistance of Example 1 increases much more f.
rapidly with increasing temperature than that of the conventional conductive
polymer composition.
20~7'~~1
_ g _
Figure 2 shows six graphs, of positive temperature coefficient factor
against temperature in degrees Celsius, for the compositions identified in
Table :i as Examples 7 to 12.
Figure 3 shows two graphs, of resistance in ohms against time in
minutes, of two specimens of the composition Example 8 maintained at
temperatures of 65 and 70'C respectively. Both samples were first annealed
for a period of about twenty minutes, after which the resistance became
substantially constant. This shows the possibility of control by temperature
of devices manufactured from conductive polymer compositions according to the
invention.
In a subsequent extended test, in which each specimen, after the initial
annealing period, was monitored through 100 cycles of 6 minutes conducting at
volts and 18 minutes recovery, the resistance of the specimen stored at
65'C increased from its initial resistance of 40 ohms to 50 ohms and the
15 resistance of the specimen stored at 70'C decreased from its initial
resistance of 100 ohms to 90 ohms.
Figure 4 shows how the resistance of a specimen of the composition
mentioned above remained substantially constant throughout over 250 cycles of
conduction and recovery. The graph shows measured resistance as a percentage
of the original resistance of the specimen before annealing, plotted against
the number of cycles of conduction and recovery. The specimen was first
annealed at 23'C whilst conducting until the initial increase in resistance
appeared to cease, as indicated by the initial steep part of the graph. It
was then switched off for the first recovery period, after which the cycles
were repeated as mentioned. There was little further change in resistance.