Note: Descriptions are shown in the official language in which they were submitted.
2 ~
HOECHST AKTIENGESELLSCHAFT HOE 89/F 360 DrO VP/PP
Description
Pyrimidine derivatives, process for their preparation,
agents containing them and their use as fungicides
The present invention relates to novel pyrimidine deriva-
tives, a process for their preparation, agents containing
them and their use as fungicides.
Pyrimidine derivatives are already known as active com-
ponents in fungicidal agents (cf. EP-A-270,362,
EP-A-259,139, EP-A 234,104). However, the action of these
compounds is not always satisfactory, in particular at
low application rates.
Novel pyrimidine derivatives have now been found which
have advantageous effects in the control of a wide
spectrum of phytopathogenic fungi, in particular at low
dosages.
The present in~ention therefore relates to the compounds
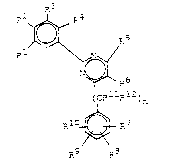
of the formula I, 2 ~ R5
(I)
(CRllRl2)n
R10 ~ R7
in which R9 R8
R1 is hydrogen, (Cl-C6)alkyl, (Cl-C4)alko~y-(Cl--C4)-
alkyl, (Cl-C4)alkylthio-(C1-C4)alkyl, (C2~-C~)alk-
enyl, (C2-C6)alkynyl, ( C3-C7 ) cycloalkyl, ( C3-C7 ) -
cycloalkyl(C1-C4)alkyl, it being poss ble for the
two last-mentioned radicals to be monosubstituted
2 ~J~ 3 ~ ~
-- 2 --
to trisubstituted in thP cycloalkyl moiety by
(Cl-C4)alkyl, or phenyl, phenoxy-(Cl-C4)alkyl,
phenylmercapto(cl-c4)alkyl~ phenyl-(C1-C4)aikyl or
phenoxy-phenoxy(cl-c4)alkyl~ it being possible for
the five last-mentioned radicals to be monosub-
stituted to trisubstituted in the phenyl moiety
by halogen, nitro, (C1-C4)alkyl, (C1-C4)alkoxy,
(C1-C4)alkylthio, (C1-C4)haloalkyl or (C1-C4~halo-
alkoxy,
R2, R~ and R4 are independently of one another hydrogen,
(C1-C6)alkyl or phenyl, it being pos~ible for the
phenyl radical to be monosubstituted to
trisubstituted by halogen, nitro, (C1-C4)alkyl,
(Cl-C4)alkoxy, (Cl-C4)alkylthio, (Cl-C4 ) haloalkyl,
or (Cl-C4)haloalkoxy,
R5 is hydrogen, (Cl-C6)alkyl, ( C3-C7 ) cycloalkyl,
( C3-C7 ) cycloalkyl-(C1-C4)alkyl, it being possible
for the two last-mentioned radicals to be mono-
substituted to trisubstituted in the cycloalkyl
moiety by (C1-C4)alkyl, ~Cl-C4)haloalkyl, (C1-C4)-
alkoxy, (C1-C4)alkylthio, (Cl-C4)alkoxy-(C1-C4)-
alkyl, (Cl-C4)alkylthio-(C1-C4)alkyl, halogen,
(C2-C6)alkenyl, (C2-C6)alkynyl, phenyl, phenoxy,
phenyl(C1-C4)alkyl, phenoxy-(C1-C4)alkyl, phenyl-
mercapto-(C1-C4)alkyl, phenylmercapto, phenyl-
(Cl-C4)alkoxy or phenyl-(Cl-C4)alkylthio, it being
possible for the eight last-mentioned radicals to
be monosubstituted to trisubstituted in the
phenyl moiety by halogen, nitro, cyano, (Cl-C4)-
alkyl, (C1-C4)alkoxy, (C1-C4)alkylthio, (C1-C4)halo-
alkyl or (C1-C4)haloalkoxy, or (C2-C4)alkenyloxy,
(C2-C4)alkynyloxy, (C1-C4)haloalkoxy r ( Cl-C4 ) alkoxy~
(Cl-C4)alkoxy or ~C1-C4)alkylthio-(C1-C4)alkylthio,
R6 is hydrogen, (Cl-C4)alkyl, (Cl-C4)alkoxy, (C2-C6)-
alkenyloxy, (C2-C6)alkynyloxy, (Cl-C4)alkylthio,
halogen or phenyl, it being possible for the
phenyl radical to be monosubstituted to trisubs-
tituted by halogen, nitro, cyano, (Cl-C4)alkyl,
(Cl-C4)alkoxy, (Cl~C4)alkylthio, (C1-C4)haloalkyl or
.,
-- 3 --
(Cl-C4)haloalkoxy,
R7, R6, R9 and R10 are independently of one anothe~ hyd-
rogen, halogen, nitro, cyano, (C1-C4)alkyl,
(C1-C4)alkoxy, (C1-C4)alkylthio, (C1-C4)haloalkyl or
(C1-C4)haloalkoxy,
Rll and Rl2 are independently of one another hydrogen or
(C1-C4)alkyl and
n is 1-3, and their acid addition salts.
The alkyl, alkenyl or alkynyl radicals here can be both
straight-chain and branched. Halogen is F, Cl, Br and I,
preferably F, Cl and Br. The prefix "halo" in the
description of a substituent here and in the following
means that this substituent can occur once or several
times with the samc or a different meaning. The prefix
"halo" comprises fluorine, chlorine, bromine or iodine,
in particular fluorine, chlorine or bromine. Examples of
haloalkyl which may be mentioned are: CF3, CF2CHF2, CF2CF3,
CC13~ CC12F~ CF2CF2CF3~ CF2CHFCF3 and (CF2)3CF3. Examples of
haloalkoxy are OCF3, OCF2CHF2 or OCF2CF2CF3.
Preferred compounds of the formula I are thos2 in which
R1 is hydrogen, (C1-C6)alkyl, phenyl, phenyl-(C1-C2)-
alkyl, phenoxy-phenoxy-(C1-C2)alkyl or phenoxy-
(C1-C2)alkyl, it being possible for the four last-
mentioned radicals to be monosubstituted to
trisubstituted in the phenyl moiety by halogen or
( Cl-C4 ) alkyl; or (C1-C3)alkoxy-( Cl-C2 ) alkyl,
R2 and R3 are independently of one another hydrogen,
(C1-C3)alkyl or phenyl, it being possible for the
phenyl radical to be monosubstituted to trisub-
stituted by halogen or (C1-C4)alkyl,
Rb is hydrogen,
Rs is hydrogen, (Cl-C6)alkyl, (C3-C3)cycloalkyl,
(C5-C6)cycloalkyl-(C1-C3)alkyl, phenyl, phenyl-
(C1-C2)alkylthio, phenyl-(C1-C2)alkyl, it being
possible for the three last-mentioned radicals to
be monosubstituted to trisubstituted in the
3 ~ ~
-- 4
phenyl moiety by halogen, (C1-C4)alkyl, (C1-~3)-
haloalkyl or (C1-C4)alkoxy, or (~1-C4)alkoxy,
(C1~C~,)alkylthio, (C2-C4)alkenyloxy, (c2-~4)alkynyl-
oxy, (C2-C3)alkenyl, (C2-C4)alkynyl or tC1-C4)alk-
oxy-(C1-C4)alkoxy,
R~ is hydrogen, (C1-C4)alkyl, halogen, phenyl or
(C1-C3)alkoxy,
R7, R~, R9 and R10 are independently of one another hyd-
rogen, (C1-~4)alkyl, (C1-C4)haloalkyl or (C1-C4)-
alkylthio,
R1l and R12 are hydrogen,
and n = 1, and their acid addition salts.
The following acids are suitable for the preparation of
the acid addition salts of the compounds of the formula
I:
hydrohalic acid such as hydrochloric acid or hydrobromic
acid, and in addition phosphoric acid, nitric acid,
sulfuric acid, mono- or bifunctional carboxylic acids and
hydroxycarboxylic acids such as acetic acid, maleic acid,
succinic acid, fumaric acid, tartaric acid, citric acid,
salicylic acid, sorbic acid or lactic acid, and sulfonic
acids such as p-toluenesulfonic acid or 1,5-naphthalene-
disulfonic acid. The acid addition salts of the compounds
of the formula I can be obtained in a simple manner by
customary salt formation methods, for example by dis-
solving a compound of the formula I in a suitable organic
solvent and adding the acid and are isolated in a known
manner, for example by filtering off, and, if desired,
purified by washing with an inert organic solvent.
The present invention also relates to a process for the
preparation of the compounds of the formula I.
The novel pyrimidine derivatives of the formula (I) can
be prepared by the following methods:
,
1) Pyrimidine derivatives of the formula I where R5 = H
can be obtained by reductive dehalogenation of
2 ~
-- 5 --
appropriate halopyrimidines of the formula I in
which R5 is halogen (Cl, Br, or I) and the remaining
substituents are as defined in formula I. The
dehalogenation can be carried out with hydrogen in
S the presence of catalysts (for example
palladium/carbon) in an inert solvent, for example
water, lower alcohol (such as methanol and ethanol),
ethyl acetate or toluene or mixtures thereof. The
addition of bases such as alkali metal hydroxides or
alkali metal carbonates or alkaline earth metal
hydroxides or alkaline earth metal carbonates is
advantageous. The reaction is carried out in the
range 15-60C under a pressure of 1 to 5 bar.
2) Pyrimidine derivatives of the formula I, in which R5
is (C1-C4)alkoxy, (C1-C4)alkylthio, phenoxy, phenyl-
mercapto,phenyl-(C1-C4)alkoxyorphenyl-(Cl-C4)alkyl
thio, it being possible for the 4 last-mentioned
radicals in the phenyl moiety to be monosubstituted
to trisubstituted by halogen, nitro, cyano,
(C1-C~)alkyl, (C1-C4)alkoxy, (C1-C4)alkylthio,
~cl-4)haloalkylor(cl-c4)haloalkoxy,or(c2-c4)alken
oxy, (C2-C4)alkynyloxy, (C1-C4)haloalkoxy, (C1-C4)alk
oxy-(C1-C4)alkoxy or (C1-C4)alkylthio-~C1-C4)alkylthio,
can be prepared by reaction of the appropriate
halopyrimidines of the formula (I) Rs = halogen with
an alkali metal compound of the formula where R5-Y
(II), in which R5 has the abovementioned meaning and
Y is an alkali metal. Examples of alkali metal are
sodium, potassium and lithium.
The reaction can be carried out between 0C and
130C in the course of 0.5 h to 72 h. The alkali
metal compound (II) can be employed in amounts of 1
to 2 mo] equivalents relative to 1 equivalent of the
halopyrimidine (I). The reaction is usually carried
out in the presence of a solvent.
In the cases in which an alkali metal compound R5-Y
-- 6 --
i5 employed in which Rs is (cl-c4)alkoxyl (C2-C4)-
alkenyloxy, (C2-C4)alkynyloxy, (C1-C4)haloalkoxy or
(C1-C4)alkoxy-(C1 C4 ) alkoxy,thecorrespondingalcohol
R50H or an ether (for example diethyl ether, dioxane
or tetrahydrofuran) or a mixture thereof is expedi-
ently used as the solvent. In the cases in which an
alkali metal compound R5Y is employed in which R5 is
(C1-C4)alkylthio, phenoxy, phenylmercapto, phenyl-
(C1-C4)alkoxy, phenyl-(C1-C4)alkylthio or (C1-C4)-
alkylthio-(C1-C4)alkylthio, an ether (for example
diethyl ether, dioxane or tetrahydrofuran), a
nitrile (for example acetonitrile), an aromatic
hydrocarbon (for example toluene or xylene) or a
mixture thereof i6 used as the solvent.
3) Pyrimidine derivatives of the formula (I), in which
R5 is (C1-C6)alkyl, ( C3-C7 ) cycloalkyl, ( C3-C7 ) cyclo-
alkyl-(C1-C4)alkyl, (C1-C4)alkoxy-(C1-C4)alkyl/ (cl-
C4 ) alkylthio-(C1-C4jalkyl or phenyl, it being pos-
sible for the last-mentioned radical ~o be monosub-
stituted to trisubstituted by (C1-C4)alkyl or (Cl-C4)-
alkoxy, can be obtained by reaction of appropriate
halopyrimidines of the formula (I) where R5= halogen
with Grignard compounds R5MgX (III), where R5 is
defined as indicated above and X is halogen (Cl, Br
or I), in the presence of nickel-phosphine complexes
such as, for example, 1,2-bis(diphenylphosphino)-
ethane-nickel(II) chloride or 1,3-bis(diphenyl-
phosphino)propane-nickel(II) chloride (cf. Chem.
Pharm. Bull. 16, 2160 (1978)). The reaction can be
carried out in the course of 2 - 48 h between 0C
and 80C or at the boiling point of the solvent. The
Grignard compound R5MgX (III) can be employed in
amounts of 1-2.5 mol equivalents relative to 1
equivalent of halopyrimidine (I). Suitable solvents
are ethers such as, for example, diethyl ether, THF,
- dioxane and dimethoxyethane.
The halopyrimidines I (R5 = halogen) can be obtained
-- 7
by reaction of the appropriate hydroxypyrimidines I
(R5 = 3H), in which R1-R4, R6-R12 and n are as defined
as in the general formula (I), with halogenating
agents. Halogenating agents which can be employed
are, for example, thionyl chloride, phosgene,
phosphorus oxychloride, phosphorus pentachloride,
phosphorus oxybromide or phosphorus tribromide. The
reactions can be carried out in a solvent, but also
without solvent.
The halogenating agent is employed in amounts of 1
to 4 equivalents relative to 1 equivalent of the
hydroxypyrimidine (I). The reactions can be carried
out in a temperature range from 25 to 160C. Sol-
vents employed are, for example, aromatic hydro-
carbons (for example benzene or toluene, inter alia)
or halogenated hydrocarbons (for example chloro-
benzene).
The hydroxypyrimidines (I) can be prepared by
condensation of the amidine derivatives (IV) with
~-oY~ocarboxylates (v)
p l~ P l ) n
R 9 R 8
R1 ~/ HX
~2
'' IV V
in which Rl-R4, R5-R12 and n are as defined in formula
I, X is halogen (for example chlorine, bromine or
iodine) and R13 is lower alkyl radicals such as
methyll ethyl and propyl.
The reactions are carried out in the temperature
2~3 s~
-- 8 --
range from 20 to 110C or at the boiling point of
the solvent within the course of 2 - 72 h. The ~-
oxocarboxylate V can be employed in amounts of 1 -
1.5 equivalents relative to 1 equivalent of amidine
derivative IV. The reaction is carried out in the
presence of a base and of a solvent. Bases employed
can be, for example, inorganic bases such as alkali
metal hydroxides and carbonates or organic bases
such as sodium alkoxides, trialkylamines and N,N-
dialkylanilines. Suitable solvents are lower alco-
hols (such as methanol and ethanol), cyclic ethers
(such as dioxane and THF), pyridine, N,N-dimethyl-
formamide, water or mixtures thereof.
The amidine derivatives IV and the ~-oxocarboxylates
V can be prepared by processes known per se (cf. J.
Org. Chem. 32, 1591 (1967) and Synthesis 1982, 451
and Organikum 1986, 516 et seq.).
The compounds of the formula I according to the invention
are distingulshed by an excellent fungicidal action.
Causative organisms of fungal disease which have already
penetrated into the plant tissue can be successfully
controlled in a curative manner. This is particularly
important and advantageous in those fungal diseases
which, after infection has occurred, can no longer be
controiled effectively with the otherwise customary
fungicides. The spectrum of action of the compounds
claimed inclut~les a plurality of various economically
important phytopathogenic fungi, such as, for example,
Piricularia oryzae, Venturia inaequalis, Cercospora
beticola, powdery mildew species, Fusarium species,
Plasmopora viticola, Pseudoperonospra cubensis [sic],
Leptosphaeria nodorum, Drechslera, various rust fungi and
Pseudocercosporella herpotrichoides. Benzimidazole- and
dicarboximide- sensitive and -resistant Boytritis cinerea
[sic] strains are particularly well controlled.
The compounds according to the invention are in addition
~ 3~ 3 ?~
_ g _
also suitable for use in industrial ~ields, for example
as wood preservatives, as preservatives in paints, in
cooling lu~ricants for metal processing or as preserva-
tives in drilling and cutting oils.
The invention al50 relates to agents which contain the
compounds of the formula I in addition to suitable
formulation auxiliaries.
The agents according to the invention in general contain
the active compounds of the formula I in amounts of 1 to
95% by weight.
They can be variously formulated, depending on how it is
prespecified by the biological and/or physicochemical
parameters. Suitable formulation possibilities are
therefore:
wettable powders (WP), emulsifiable concentrates (EC),
aqueous solutions (SC), emulsions, sprayable solutions,
dispersions having an oil or water base (SC), 8USpO--
emulsions (SC~, dusting agents (DP), seed dressings,
granules in the form of micro-, spray, absorption and
adsorption granules, watPr-dispersible granules (WG), ULV
formulations, microcapsules, waxes or baits.
These individual formulation types are known in principle
and are described, for example, in: Winnacker-Kuchler,
"Chemische Technologie" (Chemical Technology), Volume 7,
C-Hauser Verlag Munich, 4th Ed. 1986; van Falkenberg,
"Pesticides Formulations", Marcel Dekker N.Y., 2nd Ed.
1972-73; ~. Martens, "Spray Drying Handbook", 3rd Ed.
1979, G~ Goodwin Ltd. London.
The formulation auxiliaries required such as inert
materials, surfactants, solvents and other additives are
likewise known and are described, for example, in:
Watkins, "Handbook of Insecticide Dust Diluents and
Carriers", 2nd Ed., Darland Books, Caldwell N.J.; H.v.
Olphen, "Introduction to Clay Colloid Chemistry", 2nd
-- 10 --
Ed., J. Wiley & Sons, N.Y.; Marschen [sic], "Solvents
Guide", 2nd Ed., Interscience, N.Y. 1950; McCutcheon's,
"Detergents and Emulsifiers Annual", MC Publ. Corp.,
Ridgewood N.J.; Sisley and Wood, "Encyclopedia of Surface
Active Agents", Chem. Publ. Co. Inc., N.Y. 1964;
Schonfeldt, "Grenzflachenaktive Athylenoxidaddukte"
(Surface-active ethylene oxide adducts), Wi5s.
Verlagsgesell., Stuttgart 1976; Winnacker-Kuchler,
"Chemische Technologie" (Chemical Technology), Volume 7,
C. Hauser Verlag Munich, 4th Ed. 1986.
On the basis of these formulations, combinations with
other pesticidally active substances, fertilizers and/or
growth regulators can also be prepared, for example in
the form of a finished formulation or as a tank mix~
Wettable powders are preparations which can be uniformly
dispersed in water and, apart from the active compound
and a diluent or inert substance, additionally contain
wetting agents, for example polyoxyethylated alkyl-
phenols, polyoxyethylated fatty alcohols, alkyl- or
alkylphenolsulfonates and dispersing agents, for example
sodium ligninsulfonate, sodium 2,2~-dinaphthylmethane-
6,6~-disulfonate, sodium dibutylnaphthalenesulfonate or,
altexnatively, sodium oleylmethyltaurate. Emulsifiable
concentrates are prepared by dissolving the active
~5 compound in an organic solvent, for example butanol,
cyclohexanone, dimethylformamide, xylene or, alternative-
ly, higher-boiling aromatics or hydrocarbons with the
addition of one or more emulsifiers. Examples of emul-
sifiers which can be used are:
al}cylarylsulfonic acid calcium salts such as Ca dodecyl-
benzenesulfonate or nonionic emulsifiers such as fatty
acid polyglycol esters, alkylaryl polyglycol ethers,
fatty alcohol polyglycol ethers, propylene oxide-ethylene
oxide [lacuna] sorbitan fatty acid esters,
3S polyoxyethylene sorbitan fatty acid esters or
polyoxyethylene sor~itol esters.
- 11- 2~
Dusting agents are obtained by grinding the active
compound with finely divided solid substances, for
example talc, natural clays such as kaolin, bentonite,
poryphillite [ 5iC ] or diatomaceous earth. Granules can
either be prepared by spraying the active compound onto
adsorptlve granulated inert material or by applying
active compound concentrates by means of adhesives, for
example polyvinyl alcohol, sodium polyacrylate or,
alternatively, mineral oils, to the suxface of support
substances such as sand or kaolinites or of granulated
inert material. Suitable active compounds can also be
granulated in the customary manner for the preparation of
fertilizer granules - if desired mixed with fertilizers.
In wettable powders the active compound concentration is,
for example, about 10 to 90% by weight, the remainder to
100~ by weight is composed of customary formulation com-
ponents. In the case of emulsifiable concentrates, the
active compound concentration can be about 5 to 80% by
weight. Dust-like formulations contain mostly 5 to 20% by
weight of active compound, sprayable solutions about 2 to
20% by weight of active compound. In the case of
granules, the active compound content partially depends
on whether the active compound i5 liquid or solid and
which granulation auxiliaries, fillers etc. are used.
In addition, said active compound formulations optionally
contain the adhesives, wetting agents, dispersants,
emulsifiers, penetrants, solvents, fillers or support
substances customary in each case.
For application, the concen~rates present in commercial
form are optionally diluted in a customary manner, for
example by means of water in the case of wettable pow-
ders, emulsifiable concentrates, dispersions and some-
times even in the case of microgranulates. Dust-like and
granulated preparations, and sprayable solutions are
customarily not diluted further with other inert
substances before application.
3 2 S
- 12 -
The application rate required varies with the external
conditions such as temperature, humidity and the like. It
can vary within wide limits, for example between 0.005
and 10.0 kg/ha or more of active substance, but it is
preferably betwean 0.01 and 5 kg/ha.
The active compounds according to the invention in their
commerclal formulations can be applied either alone or in
combination with other fungicides knoT~n from the litera-
ture.
Examples of fungicides known from the literature which,
according to the invention, can be combined with the
compounds of the formula I are the following products:
imazalil, prochloraz, fenapanil, SSF 105, triflumizol,
~P 969, flutriafol, BAY-MEB 6401, propiconazole,
etaconazole, diclobutrazol, bitertanol, triadimefon,
triadimenol, fluotrimazole, tridemorph, dodemorph,
fenpropimorph, falimorph, S-32165, chlobenzthiazone,
parinol, buthiobate, fenpropidin, triforine, fenarimol,
nuarimol, triarimol, ethirimol, dimethirimol,
bupirimate, rabenzazole, tricyclazole, fluobenzimine,
pyroxyfur, NK-483, PP-389, pyroquilon, hymexazole,
fenitropan, UHF-8227, cymoxanil, dichlorunanid [sic],
captafnl, captan, folpet, tolylfluanid, chlorothalonil,
e-tridiazole, iprodione (formula II), procymidone,
vinclozolin, metomeclan, myclozolin, dichlozolinate,
fluorimide, drazoxolan, quinomethionate, nitrothal-
isopropyl~ dithianon, dinocap, binapacryl,
fentin acetate, fentin hydroxide, carboxin, oxycarboxin,
pyracarbolid, methfuroxam, fenfura [sic], furmecyclox,
benodanil, mebenil, mepronil, flutalanil, fuberidazole,
thiabendazole, carbendazim, benomyl, thiofante [sic],
thiofanate-methyl [sic], CGD-94340 E, IKF-1216,
mancozeb, maneb, zi.neb~ nabam, thiram, probineb,
prothiocarb, propamocarb, dodine, guazatine, dicloran,
quintozene, chloroneb, tecnazene, biphenyl, anilazine,
2-phenylphenol, copper compounds such as Cu oxychloride,
oxine Cu, Cu oxides, sulfur, fosetylaluminum [sic],
3 C~ 8
- 13 -
so~lum dodecylbenzenesulfonate,
sodium dodecyl sulfate,
sodium C13/C15 alcohol ether sulfonate,
sodium cetostearylphosphate ester,
dioctyl sodium sulfosuccinate,
sodium isopropylnaphthalenesulfonate,
sodium methylenebisnaphthalenesul~onate,
cetyltrimethylammonium chloride,
Salts of long-chain primary, secondary or tertiary
amines, alkylpropylenamines, laurylpyridinium bromide,
ethoxylated quaternized fatty amines, alkyldimethyl-
benzylammonium chloride and 1-hydroxyethyl-2-alkylimid-
aæoline.
The abovementioned combination components are known
active compounds, many of which are described in CH
[sic].R. Worthing, U. [sic] S.B. Walker, "The Pesticide
Manual", 7th Edition (1983), British Crop Protection
Council.
The active compounds according to the invention, in
particular those of the examples mentioned, can in
addition be present in their commercial formulations and
in the application forms prepared from these formulations
mixed with other active compounds, such as insecticides,
attractants, sterilants, acaricides, nematicides, fun-
gicides, growth-regulating substances or herbicides. The
insecticides include, for example, phosphoric acid
esters, carbamates, carboxylic acid esters, formamidines,
tin compounds, substances produced by microorganisms and
the like. Preferred mixture components are:
1. from the phosphoric acid e~ter group
azinphos-ethyl,azinphos-methyl,1-(4-chlorophenyl)
4-(O-ethyl, S-propyl)phosphoryl-oxypyrazole
(TIA-230), chlorpyrifos, coumaphos, demeton,
demeton-S-methyl, diazinon, dichlorvos, dimethoate,
ethoprophos, etrimfos, fenitrothion, fenthion,
2 ~
- 14 -
heptenophos, parathion, parathion-methyl~ phosalone,
pirimiphos-ethyl, pirimiphos-methyl, profenofos,
prothiofos, sulprofos, triazophos or trichlorophon.
2. from the carbamate group
aldicarb, bendiocarb, BPMC (2-(1-methylpropyl)-
phenylmethyl carbamate), butocarboxim,
butoxycarboxim, carbaryl, carbofuran, carhosulfan,
cloethocarb, isoprocarb, methomyl, oxamyl, primicarb
[ 5iC ], promecarb, propoxur or thiodicarb.
3. from the carboxylic acid ester group
allethrin, alphamethrin, bioallethrin,
bioresmethrin, eycloprothrin, cyfluthrin,
cyhalothrin, cypermethrin, deltamethrin, alpha-
cyano-3-phenyl-2-methylbenzyl 2,2-dimethyl-3-(2-
chloro-2-trifluoromethylvinyl)-cyclopropanecarboxy-
late (FMC 54800), fenpropathrin, fenfluthrin, fen-
valerate, flucythrinate, flumethrin, flu~alinate,
permethrin, resmethrin or tralomethrin.
4. from the formamidine group
amitraz or chlordimeform
5. from the tin compound group
azocyclotin, cyhexatin and fenbutatin oxide
6. Miscellaneous
abamektin, sacillus thuringiensis, bensultap,
binapacryl, bromopropylate, buprofecin, camphechlor,
cartap, chlorobenzialate [sic~, chlorfluazuron, 2-
(4-chlorophenyl)-4,5-diphenylthiophene (UBI-T 930),
chlofentezine, 2-naphthylmethyl cyclopropanecar-
boxylate (Ro 12-0470), cyromacin, DDT, dicofol, N-
(3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy~phenyl-
amino)carbonyl)-2,6-difluorobenzamide (XRD 473),
diflubenzuron, N-(2,3-dihydro-3-methyl-1,2-thiazol-
2-ylidene)-2,4-xylidine, dinobuton, dinocap,
endosulfan, fenoxycarb, fenthiocarb [sic],
- 15 ~ 2d ~
flubenzimine, flufenoxuron, gamma-HC~, hexythiazox,
hydramethylnon (AC 217 300), ivermectin, 2-nitro-
methyl-4,5-dihydro-6H-thiazine (SD 52618), 2-nitro-
methyl-3,4-dihydrothiazole (SD 35651), 2-nitro-
methylene-1,3-thiazinan-3-yl-carbamaldehyde [sic]
(WL 108 477), propargite, teflubenzuron, tetradifon,
tetrasul, thiocyclam, triflumaron, core polyhedrosis
and granulosis viruses.
The active compound content of the applicatlon forms
prepared from the commercial formulations can vary within
wide ranges, and the active compound concentration of the
application forms can be from 0.0001 up to 100% by weight
of active compound, preferably between 0.001 and 1% by
weight. Application is carried out in one of the custom-
ary ways suited to the application forms.
The following examples serve to illustrate the invention.
A. Formulation examples
a) A dusting agent is obtained by mixing 10 parts by
weight of active compound and 90 parts by weight of
talc as the inert substance and comminuting the
mixture in a hammer mill.
b) A wettable powder which is easily dispersible in
water is obtained by mixing 25 part~ by weight of
active compound, 65 parts by weight of kaolin-
containing quart~ as the inert substance, 10 parts
by weight of potassium ligninsulfonate and 1 part by
weight of sodium oleoylmethyltaurate as the wetting
agent and dispersant and grinding the mixture in a
pinned disk mill.
c) A dispersion concentrate which is easily dispersible
in water is obtained by mixing 40 parts by weight of
active compound with 7 parts by weight of a sul-
fosuccinic acid half~ester, 2 parts by weight of a
.
2~32~
- 16 -
sodium ligninsulfonate and 51 parts by weight of
water and grinding he mixture in a friction ball
mill to a finene~s of less than 5 microns.
d) An emulsifiable concentrate can be prepared from 15
parts by weight of active compound, 75 parts by
weight of cyclohexanone as the solvent and 10 parts
by weight of oxyethylated nonylphenol (10 E0) as the
emulsifier.
e) Granules can be prepared from 2 to 15 parts by
weight of active compound and an inert granulate
support material such as attapulgite, pumice
granules and/or quartz sand. Expediently, a suspen-
sion of the wettable powder from Example b3 having
a solids content of 30% is used and this is sprayed
onto the surface of attapulgite granules, dried and
mixed intimately. The weight content of the wettable
powder herP is about 5% and that of the inert
support material is about 95% of the finished
granules.
B. Chemical e~2mples
4-Benzyl-2-(6-methylpyTidin-2-yl)pyrimidine
(E~ample NoO 1)
0.2 g of 5% palladium/carbon is added to a solution of
1.48 g (0~005 mol) of 4-benzyl-2-(6-methylpyridin-2-yl)-
6-chloropyrimidine in 50 ml of ethanolO This mixture is
brought into contact with hydrogen under a pressure of
3 bar and at a temperature of 60C with vigorous stirring
for 2 h. The catalyst is then filtered off and the
filtrate is concentrated in vacuo. The residue iB taken
up .in water, and the solution is saturated with sodium
bicarbonate and extracted with CH2Cl2. The organic phase
is dried over Na2504 and concentrated. 1.25 g (g5.7%) of
a colorless oil are obtained.
~$~
17 -
~-Benzyl-2-(6-methylpyridin-2-yl)-6-metho~ypyrimidine
(Example No.: 2)
A sodiv~ methylate solution is prepared by dissol~ing
0~184 g (0.008 mol) of sodium in 40 ml of abs. methanol.
1.30 g (0.0044 mol) of 4-benæyl-2-(6-methylpyridin-2-yl)-
6-chloropyrimidine are added to this solution and the
mixture is boiled under reflux for 3 h. It is then
concentrated, water is added to the residue and the
mixture is extracted with methylene chloride. The organic
phase is dried using Na2SO4 and concentrated. 1.16 g
(90.5%) of a yellowish oil are obtained.
4-Benzyl-2-(6-methylpyridin-2-yl)-6-methylmercap~o-
pyrimidine
(Example No.o 3~
0.45 g (0.0065 mol) of sodium thiomethylate are added to
a solution of 1.3 g (0.0044 mol) of 4-benzyl-2-(6 methyl-
pyridin-2-yl)-6-chloropyrimidine in 50 ml of abs. aceto-
nitrile and the mixture is allGwed ~o boil under reflux
for 4 h. ~he solid is filtered off and the filtrate is
concentrated. The residue iB taken up in water and the
solution is extracted with methylene chloride. The
extract is dried over Na2SO4 and concentrated in vacuo,
3nd 1~13 g (B3.5%) of a yellowish oil are obtained.
The compounds of Table A can be prepared analogously to
these e~amples.
Abbreviations: Me = methyl
E~ = ethyl
Pr = propyl
Bu = butyl
2$~3'~
~ o
'-. ~.
t ~
~,
O t'~ N
N ~ N C~ N
a:~ N N
U~
~l 1 ~D
U~ ~ N
~ ~ . ~
J~
t-) N
~J O ~ O N O
O ~.) ~
~ N ~ N
~ .r ~ ~
N N N N N
~ _ _
~ a~ 3 .,,~
~11
~) t'~ N
O O U
N r-1
~ ~ N
~ U I U U U
. ~ ~ ~ ~ u~
$ ~ ~
-- 19 --
:n ~
._,j
~i
~ ~l
~) L'') Ul U') L~ ) L'~ U') L-') Lr) ' ')
V (.)
r~r~ N r~ r-
V ~ C.~ V S
~: S ~ ~
I
IJ~ Lr) O
I ~ ~ r,~
~ ) L~)
L~') L~ r~l S ~_)
Lr') r,~ 3 ~
C:~ ~ ) O O ~1~ 0 rJ~ ~_) S
r.~l
N
2 :~ 2
C
o
r~ r~ r~ ~ r~ r~ ~ ~ r~ r~ r~
:C 2 2 ~ ~C 2 2 2 2 ~
J O ~I N ) ~ Lr`) ~O
C o ~o t~
2 ~ 3 S7 8
-- 20 --
,,1
U '
~1
tn
~ ~ I
U ~
~1
~i
P~ U I
`D D `D`D `D D
Cj !V CJ t~ CJ C~ CJ
"-) L~7 Lrl _l .--1 _ _ _ ~ _
_ _~ rv C~ C~ r ) C,~ rV C
D
C~ d~ ~ ~ ~ C
~`~'`; N N N N N N N N
~- v v C~ ~_) C J C.) r C~
v r~.
r.7 Lr') r.7 Lr)
L'' C ( \~ C~ N 5'
~ I _ _ ~ _ _ _ _ = _ _
¢
r~
o
I C C~ C~ C C C ~.) C C~ !
r- r~ t~ O ~ t~l r7 ~ L~
~ , ~ ~1 ~I t~l N N t~ N t~ r "
C O
O Z ~
2 ~ 2 ~
-- 21 --
~1
~)
.,,
o
U~
~ ~1
C)
~ ~ ~ C~
C~ U U U U
O O U U U U
d~ d' N N tY~ ~') c~
O V U ~ U O U t ~_) U
`D ~ 5
~; ~ ~ X
X
.
Lr
~ ~) X O U~ 1 0
a~ x
E~ ~7 t7
X
X X
r~ co u o ~ ~ ~ ~
. N N (~ ~ N
O O
U Z ~ ~ N N
3i~
-- 22 --
O U
o o
h o L~
O
.. ..
~n h
,~ a.
~L, t,) E ~:
d' d'
L ) L') ~ ~ J L L') U J
~ r~
~`1
~ :C~
d'
.~
pL'`)L') I I
~D~O V
~ ~
L~ p ~ X ~ ~
~ X ~ x x ~ x :r: x
I¢~"
a) ~ ~ ~ X
q~ ~ ~ , ~ X~C X 3 ~ X ~
:1 ~ t~ t~ t~ t ~ ~ ~ ~ t~ t
~rl
t" ~L') ~D ~ 0 ~ ~ t~l t
~ æ ~, ~ ~ ~ ~ N ~ ~ t~l (~ ~
3 2 ~
-- 23 --
~ .
ra ~
U U
U~ ~
~ ra
~ ~` 7r ~7~D ~ d' _
rb v v"D ~ \1 ~N ~ ,~ v V
r~ ~ ~ ~ ~d~ ~~ I r~ v
';~ ~ d' r~j N N N N d' ~
r ~ N N ~ N N NN N N N
V V O V V V V Vr V r '
~D ~ v
:~
lll
1~ V O r ` , r ~ r
1~ T :C 1 ~ X
,rr)
E~ r,~J r~l ~r~r~ r~ ~ ~ r~ r ~ ~
O r~ ) t,) U ~V ~ V ~V
o
ra ~ T 2 .'~ ~: 2 ~ 2 ~
V r~ ~ rv 'v V rv
r~ d~ ~ o ~I N r~) ~
j: O ~1 ~ r-l ~1 ~ ~I N N N N r\l
O ~i5 N N N N N N `1 N r.\l r.~;
-- 24 --
r~
,-, ~? ~?
~? v ~?
u C~ o
.,~
_, t~
~? ~, ~?
~-i ~:
,~ ~ o ~
~,? ~ t~?
r~l J ~ . ..
U U _ t,~ t~l
(~?
~, rr~
.C r ~ ~ d'
~G ~ T
L? --
I , rJ
r~ t~
:1: t'?
O :r _ G v U X
J ~ t~?
t~ t~l t~ t ~
X 1: ~ _ _ = = ~ _
(_~ r ~ rJ ~J ~J
I
_
_, _ . _ _ ~ ~ _ :~ =
t~
_ .
_
O ~ ~
- _ ~ X -- ?d~ --
V ~ 'J I t~?
o 3:: s O ~n o o u,
t~? t'? t'l t~) t~ ~ r,~
f~ t~ ~
'v ~ ) U V V
t~ r?
o
o
t' t`- t~-l t'? t'l t~ tr~ t,~
X ~ ~
v ~v v V v v V ~ V
~rl U~
C: O ~ D r~ t~
t~ Z t~l t ~ r~ r~ t~ r. r~
?, `
.. . ..
r~
,~,,, _
~ I rJ C~ L'1
.. -L~ O O
~ _ r -~
3 ~ G
rr~l J I ~: O ~ r~,
u
,~ ~ ~ r.
I r-~
~i
.C
P~ U
Lr~ Lr~L~) L/~Lr~ L~l
~ !JrJ rJ `') , r_
_ _'_ _-- -- _ _
r~ or ~ rJr~ r~ '_
Lr~Lr~ r
~r~ r~
r~ r,
= _O C )~ ~)r~
L'~~
_ r~,
Lr~ U~ _ rJ
~' ~
r~rr)r.~) r.~ ) r.~~) r.~) r. ~ r~
1~ r,-~~ ~
~ (_~r~) U ;)~ ) r ) L) U
E~ r,~_ ~
W
O
o
._1 r,~ r,~) r.~) r, l ~ r,~) r,~
r~J r-l r~ .) U C~
o ~ r, \l ~ ~ Lr~ ~D
G~
~:: Z rr) r.-~ r,~ r,~
~J
3 --~
-~; r.\,
U~ ~
~ ', r\ .. ^,
~ 3
~ r~ .
N
,~
O N C~
7; ~ N
"~ rl~'-- L-O ~ N
.C .C .
~ ~ I X
L~ -- O U _ ~ L~ L') ' r,
(`'~ 5
~ ; j L')
~ N N N C- --
.~ -- ~
~ ~ X X _ X ~ ~
LJ') 11~) U') n L~ L'~ L~') L'--
2 N X X NXN N N N
E~ N
O ::~ X :1: X X 2 2 X
o
,.
X
2 2 X 1~ 2
. rl r~
~ 1 N (~ C~ Lr~ 3
C,) Z
.'.'J 8
-- 27 --
t ~ t~
U U ~ ) Cl h t I
G' L~ t~
r~ ,~ Z r~o r.~ r.\l Z r~
U L~ 1 U~ ~ t
.C _
4 r~ '
t~l t\~ t J t~
S~
U~
r
2: r.
U~ O ~ U ~
~; $ ~ O 0 th ~)?
X
~ u~ r u~ ul u~
_, t') ~D `O 'D `D ~ ~D `D
C!~ t~ U t~ U U U t~
E~ t,~,
~J
.r~
e ~ t~, t~. ~ u~ ~D t~
t ~Z ui ui Ll') ~ ui Lli ui
~ 3 3 ~ ~ J
-- 28 --
~,
, ," ~ o o
r.7 G~ r ~,
r.~ l~ ~ r.
N ~
_~j r ) ~r.~ r ) ~ r.~ O r~ ~ r~ O
r )~ . ~ r~
_~ U~ i~J ~ Ul .
lJ' '~
C) I Z 0N Z N (~ N r~l
r~ I I ~ ~ ~ r; ~ 1 r~
~u~ a UJ J ~ U~ ~J
C _I
~, r~
Ul _-:
"~
~ r ) ~ r ~
rJ N N N N N N
,.,
r~ ) rJ
~D
r ~ ~ N r~
U~ X
C~ U~ :~ O O O U~ O
;
d~
~ X
N ~ X ~
o
C
X
r~ ~ ~r rJ t~
.r~
~ r~ r~ N r.
C~ Z L~
~$~8~2~
~9
r
..
~n _ ~ r~
~ ,,~
.,~ _ ~
' ~ ~ O
,~~ ), .
~;~
~ ~ Lr~
~ ~)',~ \~ O 'D
.,~,rrJ Z f`~ ~,-,
n ~,
~ ,rr~ I . L'~
,Sr-l ~ U~
t~
~ `D
r~,) V r
_-) L~ --1Ir) U) L'l U
U U
'J r_ ~ f\)~'_) v ' ~ '
U r U r r~_) V V v
~) _ _ _ ~ _ _ _
T
V r ~ _
~ ~ r~
Lr~ Lr~fJ C) r ~
__ T , ~ O
~D ~D ~ ~ I
f~ U) ~ f~
L-` V ~D V V V V
f'~
_l
E-l N ~, ;C ~ C~r --
o
3 ~ r~ r~ ~ ~ r ~ r l r ~ r ~ r~
c~ u u c ~ ( ) v u rv v v
~ N r ) ~ Lr
,~ . r rSI rJ~
O Z
F~ a
-- 30 --
~n ~
. ~ o
N
._( ~
..
~ U .
._~ !a
~ ~ C:
U I ~ :~ ~
d' d' ~ ~) ~) 3
5: :C ~ d' d' ~ I I O
3 `S~ N N
t~ D 3
= -- U~ U
'~ 5: , , , , , ,
d' ~ ~ ~ N N
l I O I I I I I I , , ,
N N N N N N N N N N N N
~ ~ ~ X ~ ~
U O O U O ~_) O U O ( )
~D
r~ S ~ -- X
In
~ C~
_ ~ 5~ ~ ~ rl r
N d' X ~ ~ N 2 S:
~n C) ~ --I V C~
U~ O ~ O ~ t~ O 0 3~ 0
~,,
o
U
.~ r t~ r~ t~ r~ r~
,~ r~ r~ ~ r~ r~ r~ o r~ r~ U r~ r~
,~ `D r C~ cr o ~ ~ r~
~ .~ ~I N ~`J N N N N N N
~:: O .
o æ 3 `D `3 `D `D ~D `D `D ~D `3 ~ ~
2 ~
-- 31 --
o~ t;~ ~t
~o --,
:- r r t~
jJ J~
- ~ ~o o~ u~
u~ r
t~ . r
. ~ . ~ ~3 . r
t~ ~ co (n r~o
tO ~ d~
~ o t~
o . t~ ~ .
tn~) r ~r.~ ~o t~ r ~
_~ rJ ~ r~ Ut ~ r ~
~ ~ t;~ ~a o ~o ~ ~ r.~ a r~ ~
U~ r~ t r~
'~ r u~r u~r ~ E ~
~ ~ t~ t;~~ t o t~ ~ -- o t\~
t~ t~~ t~ t~ r t~
r d~ X r ~ 2: r r
E u~ ,~ E u~ , E u~
,~ ,C I
P~ ~
~o
,~
o _ ~ , ~ ~ ,AL
~o~o 3 ~ o
~, r ~r~_) J C )
r,~l ~r,~ ~ ~ "~ ,.; ,
= ''~
r~ r ) r~
Ln
:r:
,r
Lr') U U '-.) ~ ~
U~ O U~ ~ :I U
X 3 ~ X
C~
'~ X 3~ X X
~ Lr~
- ~ O uD
U ) U U ~ O U U
O ~ ~ r
Z ~ r r r r r r r
~ n3 ~ $
-- 32 --
I
._,
U~
I
u~ h
~ ~1
.c ,c ~r
~ o 3:
t_) L'`)
I Ll~ L~) X L~) _'`,
L" Lr~ L') T X `.D ' _
-- ~ ) T ~ ~O `D ~
~ ~ r~ N
L') Lrl~_) er ~ ,~ N ~
~ T -- T
I I (~ r_
.~1 r. i
X
er r~
t ) N N N
, ~ _
0 11 11 0
I J: _ N
~r
I
_ ~ N N N N .n_1
er ~ r~ ~ ~ T T ~ ~ ~_
Lrl ~ ) O U~ O ~;~ O
e~r
L~
I¢ ~ ~ X 3 r ~ 1 T
4~ ~ 1l1 t~ t~ t.) t)
~ t~ er '~
r. ~ ~ N ~') e~
O Z t~ 1~ 0 r o rD 0 c~ r,~ l r ~ r.
U
-- 33 --
U~
V~
_I a
o ~
. ,
~ U :~ X~
~ N
O ~ ~
I ~ C) ~r
~ ' I L)
IJ~ ~ O
I I I I
N rl ~ N N
:~-
~: r
r~ ~
~ X
o
Ll~ Lt)Ltl U~ Lr!
.~ ~~C , r ~ ~
~ ,i N N N N N
~ O ~ U
~: O -1 N
._~ . ~ ~
~ O
O æ a: co c~ CD a:
~ ~ b v ~ 2 8
- 34 -
C. Biological Examples
Example 1
Rice plants of the ~Ballila~ type about 5 weeks old were
treated with the concentration of the claimed compounds
indicated below after prespraying with 0.05% strength
gelatine solution. AEter the spray coating had dried on,
the plants were uniformly inoculated with a spore suspen-
sion of Piricularia oryzae and placed for 48 h in a
climatic chamber, which was kept dark, having a tempera-
ture of 25C and 100% relative atmospheric humidity. The
rice plants were then cultivated further in a greenhouse
having a temperature of 25C and 80~ relative atmospheric
humidity. Evaluation of attack was carried out after 5
days. The degree of attack was expressed in % of attacked
leaf surface in comparison to untreated infected controlplants. The results are collated in Table 1.
Table 1
Compound Leaf surface attacked with Piricularia
according ory~ae in % at mg of active compound/
to Example liter of spray liquor
500
1.4 0
1.1 0
1.2 0
1.3 0
2.9 0
2.10 0
3.1 0
6.5 0
6.3 0
-
untreated infected
plants 100
- 35 -
~xample 2
Barley plants were heavily inoculated in the 2-leaf stage
with barley mildew conidia (Erysiphe graminis hordei) and
cultivated further in a greenhouse at 20C and a relative
atmospheric humidity of about 50%. 1 day after inocula-
tion, the plants were wetted uniformly with the compounds
listed in Table 2 at the active compound concentration
indicated. After an incubation time of 7-9 days, the
plants were examined for attack by barley mildew. The
degree of attack is expressed in ~ of attacked leaf
surface, relative to untreated infected control plants
(= 100% attack). The result is summarized in Table 2.
Table 2
Compound Leaf surface attacked with barley
according mildew in % at mg of active compound/
to Example liter of spray liquor
500
1.1 0
1.2 0
1.3 0
2.9 O
6.3 0
25 untreated infected
plants 100
Example 3
Broad beans of the "Herz Freya" or "Frank's Ackerperle"
type about 14 days old were treated with aqueous
suspensions of the claimed compounds until dripping wet.
After the spray coating had dried on, the plants were
inoculated with a spore suspension (1.5 million
3 ~ ~
- 36 -
spores/ml) of Botrytis cinerea. The plants were
cultivated further in a climatic chamber at 20-22C and
about 99% relative atmospheric humidity. The infection of
the plants is manifested by the formation of black spots
on the leaves and stalks. Evaluation of the tests was
carried out 1 week after inoculation.
The degree of action of the test substances was assessed
as a percentage of the untreated infected control and is
reproduced in Table 3.
Table 3
Compound Leaf surfaces attacked with Botrytis
according cinerea in % at mg of active compound/
to Example liter of spray liquor
500
1.2 0
1.3 0
~ g O
2.10 0
3O16 0
6.3 0
6.7 0
untreated infected
plants 100
Example 4
Wheat plants of the "Jubilar" type were treated in the 2-
leaf stage with aqueous suspensions of the preparations
given in Table 4 until dripping wet.
After the spray coating had dried on, the plants were
inoculated with an aqueous pyknospore suspension of
Leptosphaeria nodorum and incubated at 100% relative
atmospheric humidity in a climatic chamber for several
2~3~
- 37 -
hours. Until expression of symptoms, the plants were
further cultivated in a greenhouse At about 90% relative
atmospheric humidity.
The degree of action is expressed as a percentage of the
untreated infected control and is reproduced in Table 4.
Table 4
Compound Leaf surfaces attacked with Leptosphaeria
according nodorum in % at mg of active compound/
to Example liter of spray liquor
500
. . .
1.4 0
1.1 0
1.2 0
1.3 0
7.1 0
7.2 0
7.4 0
5.1 0
2.9 0
2.10 o
3.16 0
3.17 0
3.1 0
6.18 0
6.5 0
6.3 0
untreated inf ected
plants 100
Example 5
Wheat of the "Jubilar" type was treated in the 2-leaf
stage with aqueous suspensions of the claimed compounds
until dripping wet. After the spray coating had dried on,
the plants were inoculated with an aqueous spore
~..
~,
- 38 -
suspension of Puccinia recondita. The plants were placed
dripping wet in a climatlc chamber at 20C and about 100~
relative atmospheric humidity fo. about 16 hours. The
infected plants were then cultivated further in a green-
house at a temperature of 22-25C and 50-70% relative
atmospheric humidity.
After an incubation time of about 2 weeks, the fungi
sporulates onto the entire leaf surface of the untreated
control plants such that an evaluation of attack of the
test plants can be carried out. The degree of attack was
expressed in ~ of attacked leaf surface in comparison to
untreated infected control plants and is reproduced in
Table 5.
Table 5
Compound Leaf surface attacked with Puccinia re-
according condita in % at mg of active compound/
to Example liter of spray liquor
500
1.1 0
1.2 0
6.3 0
1.3 0
25 untreated infected
plants lO0