Note: Descriptions are shown in the official language in which they were submitted.
-2-
The invention rela~es to a device for the continuous electrolytic
removal of metals from a solution cont3ining me~al ions in which an anude
and a ca~hode, each electrically connected to a power supply, are immersed;
the surface of the cathode takes the form of an endless flexible belt, runnin~
5 partially imrnersed in the solution, onto which metal is deposited and then
removed at a point outside the solution; and at least that portion of the
surface of the flexible belt onto which ~he metal is deposited is itself made
of metal, and a device for stripping the deposited metal is arran~ed outside
the solution and the belt is guided by return and/or drive rollers.
German patent DE-PS 36 40 020 describes an electrolytic cell for the
electrolytic deposition of metals from a liquid, in particular process water,
containin~ metal ions. The described cell possesses a large nurnber nf sheet
metal electrodes spaced apart in parallel arrangemen~ in a tank; the
cathodes are provided with openings and, depending on the distance from
15 the anode, are connected with the current source via connectin~ resistances
of various ma~nitude so that the same current density is applied to each
cathode.
In such a system, problems are caused by the relatively costly
process of rernoving the individual cathodes on whose surfac~s the metal is
20 deposited and also by the relatively labour-intensive removal of the
deposited material.
European patent application EP-OS 248 118 describes an elec~rolytic
device for the continuous production of metallic foil from a tank holdin~ a
solution containing metal ions. In this device the cathode partially immersed
25 in the solution is designed as a drum or a recirculating endless belt. In thearea where it is immersed in the solution the cathode is surrounded by an
anode arranged at a distance from it and provided with channels or openings
to permit the electrolyte to pass through. The metal deposited on the
cathode is separated from the cathode once the lat~er emerges frorn the
30 solution.
The cathQde possesses a metal surface, for example rnade of titanium
or tantalum, while the anode consists for example of titanium~ The solution
used is an acidic metal ian solution consistin~, for example, of copper
sulfate and sulfuric acid.
.
.
~ ,
~7~ 7
-3-
in this case, the continuous removal of metals by means of a movin~
cathode is problematical unless homogeneous, compact, self-supporting
me~al layers can be precipita~ed onto the ca~hode; in particular, i~ is difficult
to accomplish this in the case of slime-like metal structures or dendri~es, or
5 other types of undefinable metal s~ructures/ such as spherical s~ructures,
which when deposited do not form any homogeneous layers amon
themselves.
Furthermore, US Patent 2,099,873 describes an elec~rolytic deYice
for extracting flake material from an electrolytic bath fi~ted with a cathode
10 in the form of an endless recirculating flexible belt. The metal (chromium) is
cathodically deposited on~o the belt and is carried out of the electrolyte
solution to a separate flushin~ device where the precipitated metal
~ohromium) is separa~ed from the belt. The belt is guided by means ~f return
and drive rollers, and the drive roller around which the belt passes rotates
15 around a horizontal axis arranged above the ba~h; only a small part of the
laterai sur~ace of the ro!ler, which is lar~e in relation to the tank, is
immersed in the tank, and a large number of return rollers is arranged
outside the bath.
US Patent 2,748,071 describes a device for regeneratin~ iron chloride
20 in the copper etching process; here, again, an endless belt which consti~utes the cathode surface, is passed around return and drive rollers. Once it
emerges from the solution, the belt is directed to a water bath where the
copper is separated. The stripping device employed is a sur~icai scalpei and
the metal (copper~ is passed to another electrolytic tank. This patent does
25 not provide for the removal of metal in dried, powdered form.
US patent 2,964,453 also describes a regeneration process for a
copper etchin~ bath in which, again, a flexibie belt (platinum) is used and it
runs one after the other through an etching bath,-a rinsing bath and a
strippin~ bath. In the etching bath ~he bel~ is cathodically connected and in
30 the stripping b~th it is anodically connected. The patent makes no mention
of a rnechanical strippin~ device of the kind needed in practice for the
further processin~ of dried, precipitated material. US patent 2,986,568
describes an electrolytic process for the manufacture of or~anome~alliç
compounds which also makes use of an endless metal belt. In this casel it is
,? ~ ~
-4-
a problem to seal the hubs of the cathode roller and also to supply current
to the cathode roller in the electrolyte. Outside the bath, the endless belt
passes through a sta~ion fitted with a mechanicai stripping device in the
form of a knife.
Furthermore, German patent application DE-OS 40 35 328 describes
a device for the continuous slectrolytic removal of metals from a solution
containing metal ions by means of a cathode surfar,e in the form of a belt
running partially immersed in the solution and while in the solution it is
passed over a cathode roller; outside the solution, a stripping device and a
10 flushing devicc for the precipitated metal are arranged one after the other in
the direction of circulation of the belt. In a practical embodiment of the
invention, the cathode roller rotates about a horizontal axis and is connected
via a current take-off to the negative pole of a voltage sourGe.
When the axis of rotation of the ca~hode roller is horizontally oriented,
15 this creates problerns in sealin~ off both ends of the roller; furthermore,
increased transfer resistances may arise in the transfer of current to the
cathode roller due to the corrosion phenomena caused by the chemically
aggressive solution.
It is an object of the invention to extract metals precipita~ed in
20 inhomogeneous form from a solution containing metal ions; the aim is to
provide a substantially independently operating device so that costly, labour-
intensive procedures to remove ~he precipitated material can be dispensed
with, and furthermore no problerns are encountered with regard to the
sealing of the shaft or the hubs of the cathode roller. The current supply to
25 thc cathode roller should be located outside the electroiyte.
According to the invention, the axes of rotation of the return and
drive rollers are arran~ed at an an~le of between 20 and 70 relative to th
normal line perpendicular to the surface of the solution; a first roller, namelythe cathode roller, is irnmersed in the solution so that its lower hub and at
30 least part of its electrically conductin~ lateral surface are covered by the
solution, and at leas~ one second roller is arranged outside the solution as a
return roller; and the surface of the belt orien~ed towards the first roller is
electrically conducting.
2~ 7.
-5-
One major advantage of the device according to the invention is that,
on the one hand, a relatively large area of the iateral surface of the cathode
rolJer and thus the part of the belt encircling the cathode roller, are
immersed in the solution, while on the other hand no problerns are
5 encountered with the sealing of the lower hub which is immersed in ~he
solution in the tank.
In a preferred embodiment of the invention, a flushin~ device is
arranged after the stripping device, seen in the direction of circulation of ~hebelt, so that the belt is thoroughiy cleaned before it re-enters the solution.
Fur~hermore, the hub or shaf~ end of the first roller, which is used as
the cathode roller, projec~s from the solution and is connected with a
current take-off in order to make contact with the ne~ative pole of the
voltage source; it is advantageously possible in ~his way to immerse in the
solution practically the entire lateral surface and thus also the section of the15 fiexible belt encompassing the lateral surface in order to obtain a hi~h yield.
On the other hand, this method of making the electrical contact ~voids
corrosion problems and also the resultin~ increase in resistance in the
~ransmission of current to the cathode roller.
In a particularly advantageous embodiment, the cathode roller is
20 mounted in a bearin~ at just one end, namely its upper end; ~hat is to say,
oniy the section of shaft projecting from the ba~h solution, or only the hub
projecting from the solution, is used for mounting the first roller in a bearin~One major advanta~e of ~his particular embodirnent is that practically
no corrosion problems occur in the area of the bearings in which the roller i
25 mounted; in addition, because it is a~tached at one side only, the cathode
roller can be easily and quickly replaced.
In another preferred embodiment, after emerging from the bath
solution but before reachin~ the stripping device, the belt is passed by a
dryin~ device possessing radiant heatin~ coils. Below the strippin~ device,
30 ther~ is arranged ~ collec~ing container for the deposited electrolyte-
contairlin~ metal and this container is followed by a separatin~ clevice.
Ansther major advantage is ~hat the process can be easily op~imized
by adjustin~ the belt circulation speed and the current densi~y, so that thes
parameters can be op~imally adjusted in each case to the concentra~ion and
~ 7
-6-
temperature of the solution. A fur~her advan~age is to be seen in the fact
that the continuous output of product can be automated.
The invention will now be described in greater detail, with reference
to the drawin~s attached. In the drawings:
5 Fi~ure 1 shows a perspec~ive view of the device;
Figure 2 shows a similar device to that in Fi~ure 1, wherein the
electrode arrangement is housed in a cornpact frame;
Figure 3 shows a longitudinal section through the device; and
Figure 4 shows an embodiment which is substantially the same as that
in Fi~ure 1, wherein the first roller immersed in the solution is
lar~er in diameter than ~he second roller.
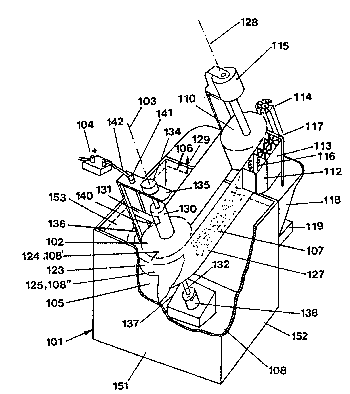
Accordin~ to Figure 1, the device accordin~ to the invention consists
of a tank 101 made of eiectrolyte-resistan~ materiai, such as elec~rolyte-
resistant plastic, containing the solution 108. In the tank is installed a first15 roller 102, acting as the cathode roller, which is elec~rically connected wi~h
the negative poie of a voltage source 1M and is mounted so that it rotates
about an axis 103 forming an angle a of approximately 45 be~ween itself
and a vertical line 129 runnin0 perpendicuiar or normal to the surface of the
bath solu~ion. On the sides of the cathode roller oriented towards the end
20 wall 1~1 and the side walls 152, 153 of the tank 101, the roller is
~ncompassed by a semicircular or U-shaped anode 105 made of expanded
or perforated metal and provided with an activation layer. This anode is
connected to the positive pole of the voltage source 104. The anode 105 is
shown in cutaway form in order to give a better view of the layout, and the
25 connection with the voltage source has also been omitted ~rom the drawing.
The surface level of the solution 108 in the tank is indicated by
refer~nce number 106. Both metallic surfaces of the flexible belt or band
107 runnin~ around the cathode roller have the same poten~ial as the
cathode roller 102, i.e. the belt is connected to the negative pole of the
30 power source throu~h thc upper hub 130 of the cathode roller and the
current collector 131.
In the area above the surface level of the electrolyte 108, the
mountin~ bra~ket 135 is fitted with a current coilector 131 which is
eiectrically connected to the electrically conducting upper hub 130 of the
-7-
first roller 102 by slip ring cont~cts. The electrical contaet of the current
collector 131 to the voltage source 104 is accomplished via wire 140 and
terminal 141 as well as wire 142.
The end of the upper hub 130 is mounted in a bearing 134 which is
5 attached ~o a bracket 135 that is depicted here only par~ially or in outaway
form. This braGke~ is attached by means of a iongitudin~l coiumn 136 to the
bearin~ mountin~ block 137 for the lower bearing 138. The bearin~
mountin~ block 137 and ~he bracket 135 are mechanically firmly attached
~o the tank 101 by joining elemen~s which are not shown in any further
10 detail here.
The lower hub 132 res~s in the lower bearing 138 which, because of
the wed~e-shaped desi0n of the bearing moun$in0 block 137, is oriented a~
an angle of 45 relative to the normal line 129 perpendicular to the surface
of the solution 108.
The flexible belt 107 runs round about half the periphery of the first
roller 102 which acts as the oathode roller, and ou~side the electrolyte
so!ution the belt 107 is guided past the various work stations such as the
drier 1~2, stripper or scraper 113 and flusher 114 by means of a second
roller 110 which acts ~s the drive roller. The roller 110 is powered by a
20 dia~rammatically depicted electric motor 115 (possibly driving throu~h a
g~ar system). The axis 128 of the seeond roller 110 runs parallel to the axis
103 o~ the cathode roller; ~he upper and lower bearings of the second roller
110 are mounted in a bracket 144 of substantially the same design as ~he
braeket and bearing assembly holding the ~irst roller. The shaft of roller 110
25 is sprin~-loaded so that the flexible belt 107 is always held under tension
and transported. In addition, it is possible to provide the ca~hode roller 102
vvith a cooling device in order to ~chieve a high current density on the belt
107 circulatin~ around ~he ca~hode.
Furtherrnore, it is possible to use a separatin~ device 123 consis~in0,
30 for example, of an ion-exchange membrane which divides the elec~roiyte
spaoe 143 of th~ tank 101 into a catholyte spac0 1124 on the side of the
separatin~ device 123 facing the first roller 102 ac~in~ as the cathode roller,
and into an anolyte space 125 on the other side of the separa~in~ device,
th~reby diviciing the solution 108 into a catholyte 108' and an anolyte
-8-
108''. t)f course, i~ is possible a~ any time, depending on the par~icular
application, to leave out the separating device and to create a common
electrolyte space 143 filled with electrolyte solution 108 or ~o use a
diaphra~m instead of an ion exchange membrane as the separatin~ device
5 123. The separating device 123, like the anode 105, is shown here in
cutaway f~rm for the sake of be~er overall viability of the desi~n.
The belt circulates at a speed of approximately 0.84 m/min at a
current density of 65()0 A/m2. It is possible to make ~he belt circula~e in the
range from 0.3 m/min ~o 1.44 rn/min, bu~ higher circulating speeds must be
10 compensated for by increasing the current density; the devices is desi~ned
for a maximum current density of 10,000 A/m.
At least 50% of the periphery of the cathode roller is immersed in the
electrolyte or catholyte, i.e. at least 50% of the peripheral surface area of
the roller is below the level 106 of the electrolyte. The temperature of the
15 electrolyte solution is in the range from 20 to 100C.
The tempera~ure in ~he drying device 112 is held below the limit
temperature of the belt in order to avoid diffusion between the precipitated
metal and the belt.
Any ~ases which are ~enerated can escape through the open upper
20 side of ~he tank 101. When the upper side of the tank is closed, additionai
openin~ are provided in the upper area of the tank in orcler to permi~ the
~ases ~enerated in the course of the process to escape or be drawn off.
These openill~s are not shown in the drawings.
When in operation, the flexible beit 107 leaves the flushing device
25 114 and is ~uided via the drive roller 110 back to cathode roller 1û2,
thereby becomin~ immersed in the solution 108. Electrical contact is always
made with the flexible belt 107 via the first roller 102, i.e. the cathode
roller. Durin~ the circulation of the belt, metal is precipitated from the
solution onto the irnmersed part oF the belt 107 which is in contact with
30 roller 102. A~ter passing through ~h~ solution 108, the belt leaves the bath
a~ain on the other side of the cathode roller 102. The material 127
consisting of precipitated m~tal and liquid particles of electrolyte is indicated
symbolically by dots on belt 107. The flexible belt 107 is then first passed
through the drier 112 whers the precipitated material is exposed to the heat
from radiant heating coils 116 and dried to such an ~xtent that it can be
passed on to the next work sta~ion, the stripping syst~m 113, where it is
mechanically removed from the bel~ by means of ro~ating brushes 117 and
scrapers. The electrolyte-containing metal which is stripped from the belt by
5 brushes and scrapers leaves the belt and falls into the funnel-shaped
collecting device 118; at this s~age, the metal consists essentially of a
con~lomerate of powder and cohesiYe fragments of deposi~ed material. In
order to improve the material flow, the collec~ing device may be fitted with
a vibrator. The electrolyte-containing metal is then separated from the
10 remaining particles of electrolyte by undergoing pressing and filterin~ in a
separating device 119, ~hus preventing the previcusly deposited metal from
dissolving once more. Next, the belt runs through the flushing device 114 in
which rotatin~ scraper brushes 120 remove all the remainin~ deposition
products from the belt 107 so that it can be circulated to the cathode roller
15 102 in the tank 101.
The arran~ement shown in Fig. 2 corresponds in function and
structure to the device described on the basis of Figure 1. The two rollers
102 and 110 are here rotatably mounted in a frame 154, and the first roller
102 serves as the cathode roller which is again connected with a current
2û collector 131 in orderto make electrical contact with the ne~ative pole of
the voltage source 104, while the opposed second roller 110 is connected
to a drive mechanism 1 15. A flexible belt 107 is held stretched between
the tw~ rollers by means of a tensioning spring. The belt acts as the
cathodic deposition surface and its rear surface makes electrical contact
25 with the roller 102 which serves as the cathode roller. The upper plate 15
of the frame 154 is drawn partially cut away in order to show the path of
the flexible belt 107. The dryin~ device 1 12, s~rippin~ device 1 13 and the
flushing device 114 are mechanically firmly attached to the frame 154, as
are also the funnel-shaped collection device 118 and the separatin~ device
30 1 19. The frame 154 rests in a position defined by spacer elements 157,
1158 on the floor of the tank 101, the upper plate 155 of ~he frame being
supported by the upper edge of the rear wall 159 of the tank. Compared
with the functionally identical device depicted in Figure 1, the device
according to Fi~ure 2 has the advantage of being more compact, also the
- 1 0-
electrode arrangement and individual components of the device, such as the
flexible belt or ~he electrical contac~ system are easier to replace, for which
purpose the frame 154 can be detached in a simple manner from the tank
101 .
In ~his embodiment as well, the firs-t roller 1û2 used as the cathode
roller may be mounted in bearings at one or both ends.
As shown in Figure 3, ~he first roller 1Q2, which is used as the
cathode roller, is fully immersed in the solution 108 so that the upper edge
of the cathode roller lies below the surface 106 of the liquid. The roller 102,
with its axis 103, is oriented a~ an an~le a of 45 rela~ive to the vertiçal line
129 perpendicularto the surface 106 of the liquid. The lower hub 132 of
roller 102 is mounted in bearing 138 and the upper hub in bearin~ 134, and
both bearings are joined with each other via braeket 135 which is in turn
firmly a~tached to bearing rnounting block 137. The 45 angle of inclina~ion
permits the tank 101 to be relatively small in volume. With the exception of
the inlet opening 121 and the outlet opening 122, also its upper side, ~he
tank is sealed off all round. The current is supplied to the cathode roller via
a current collector 131 which is depicted here in symbolic 70rm. After it
exits from the bath, the endless bel~ 107 runs above the upper ed~e of the
tank 101 toward~ the stripping devicc 113 which is equipped with rotating
brushes 117 to remove the electrolytically deposited metal frQm the flexible
belt 107. In order to achieve the most efficient possible method of
operation, the stripping device 1 13 is preceded by a dryin~ system 112
consisting of radiant heatin~ coils 116, symbolically depicted in ~he
drawing, which are used to dry the deposited material.
The electrolyte-containing metal which is removed at th~ stripper
station by means of rotatin~ brushes and scrapers leaves the belt and falls
into the funnel~shaped collecting device 118 with the adjoinin~ separating
device 119. At this stage the metal oonsists essentially of a eonglomerate
of powder and cohesi\/e ~ragments of deposited rnaterial. Next, the belt runs
throu~h the flushing device 114, which is also symbolically depicted, where
rotatin~ scraper brushes 120 remove all the remaining deposition produc~s
from the bel~ 107 so tha~ it can be returned ~o the tank 101. This is
acoomplished by means of a bloYver 146, symbolically depic~ed here, whieh
- 1 1 -
is attached ~o the bracket 144 and dir0cts a stream of gas throu~h a nozzle
146 onto the flushing device 114 for cleanin~ purposes. I lowever, instead
of the gas stream, it is also possible to use a s~ream of liquid. The bracket
system 144 is supported by means of longi~udinal columns 147, 148, as ~re
5 also the drier 112 and stripper 113, on a can~ilever bracket 149 rigidly
attached to the tank. The method of operation and the structure correspond
in all other respects with the device explained in Figure 1. Naturally, as
shown in Fi~ure 1, an ion-exchange membrane may also be provided
between the anode 105 and the first roller 102, which is used as the
10 cathode roller, so that an anolyte space and a catholyte space are formed.
The function of the embodiment shown in Fi~ure 4 corresponds essen~ially
to the device described in Figure 1, although the first roller 102 immersed in
the soiution has a larger diameter than the second roller 110' located
outside the solution. Because the distance between the two opposite sides
15 of the flexible belt 107 is reduced in the direction of the second roller 1 10',
more than 50% of the first roller 102 is encompassed by ~he belt 107, so
that a laryer contact surf~ce is achieved between the first roller 102 and the
belt 107. The distance between the two roller axes 103, 128 and the
diameter of the two roliers 102 and 110' is selected in such a way that up
20 to about 0.55 to 0.65 times, and in particular 0.6 times, the periphery of
the first roller 102 is encompassed by the flexible belt 107.
The use of a second roller having a smaller roller diameter ~han the
diameter of the first roller used as the cathode roller, has alraady been
described, and such a roller may also be used when the electrndes are
25 arranged in a cornpact frame as depicted in Figure 2.
The electrolyte solution used in tank 101 may, for example, be a zinc
sulfate solution while the precipitated metal consists essentially of 2inc. It
is, of course, also possible to use ~he device according to the invention for
the electrolytic deposition of o~her metals, for example heavy metals or
30 noble melals, from other alkaline or acid solu~ions containing metal ions