Note: Descriptions are shown in the official language in which they were submitted.
WO 91/09138 PCI`/US9~ 560
, . ,
- 2~7~9
KlT FOR USE IN DIAGNOSING MAI~DIES
CROSS~ ERENCE TO REI~Th'D APPLICATIONS
This application is a continuation-in-part of my co-pending application9 Sen No.363,854, filed June 9, 1989, which was a continuation of my earlier application, Ser. No.
902,313, filed August 28, 1986, now abandoned, which, in turn, was a continuation~
part of my still earlier application, Ser. No. 547,767, which has now issued as U.S. Pat.
No. 4,614,722, on September 30, 1986.
Additionally, this application is related to my earlier application, Ser. No.
913,940, now issued as U.S. Pat. No. 4,788,155, on November 29, 1988.
BAC~GROUND OF TE{E INVENIION
The present invention relates to a kit for use in diagnosing the presence or
absence of maladies in a subject, and, more particularly, to a kit for use in performing
the test described in my prior patents.
','
In my referenced prior patents, and my referenced co-pending application (the
disclosures of which are incorporated herein by reference), I describe a novel method
of diagnosing maladies in a subject, by means of comparing the numbers and size
distributions of the subject's blood cells before and after exposure to a substance having
a known relationship to the malady for which the subject is being tested.
For ease of description, I refer to my inventive test as being directed to the
diagnosis of a presence or absence of a malady. It is intended, however, that that
, .
:`'
.
. ,............... , ~ , ~
: ~ , - . -, . ,:
~:
,, . . ; . :
.
WO 91/09138 PCr/US90/07560
f.,~
2~71~09 2
~errn be interpreted in the broadest sense. The test may be used to ascertain the
cause of a known malady. For example, a patient who is known to suffer from
migraine headaches, may be said to have had a diagnosis already performed. However,
my test may be used to ascertain the cause of the migraine, such as a food sensitivity.
S In this sense, then, the test may be used to diagnose a migraine as being a migraine
caused by an allergic reaction to a food or a chemical ingested by the patient, as
opposed to being a migraine resulting from some other cause.
It is considered urmecessary, in light of the lengthy description of the tests
themselves contained in my earlier patents, to describe herein the steps of the test in
10 detail. It is sufficient to summarize that the patented tests describe a methodology
for the reliable and objective determination of the presence or absence of a malady
in a subject.
Broadly spealcing, this methodology includes the steps of:
(a) drawing a sample of the subject's blood;
(b) separating the sample into a plurality of separate volumes, each havmg
approximately equal distributions of blood cells therein;
(c) exposing at least one (test) volume of the blood to a substance h~ng
a predetermined relationship to the malady;
~` (d) measuring the number and/or size distribution of blood cells in a control
`~ 20 volume of the subject's blood;
(e) permitting the blood in the test volume to react with the substance;
(f) measuring the number and/or size distribution of blood cells in the test
volume after reaction has been given the opportunity to occur; and
(g) comparing the control and test volume measurements to determine
25 thereby if the subject has the malady for which he or she is being tested.
Again, this is a brief description of the patented test, and reference is once
more made to the disclosures of the referenced patents for detai]s thereof.
wo 91/09138 Pcr/us9o/o756o
3 2071~9
To date, however, there has been no effective and conveluent apparatus for
performing the tests.
As the test has been performed heretofore, the user thereof was compelled to
collect a multitude of receptacles, into each of which he would introduce a selected
5 test substance (except, of course, for the receptacles used for the control volurnes).
The test substance would be purchased separately, from a different vendor, and
different types of test substances would have to be collected from different sources.
For example, food extracts could be purchased from certain sources, while
monoclonal antibodies, used for testing for certain types of cancers, would have to be
10 collected from a different source.
Furthermore, while it was known that control samples were needed, it was not
known how many samples were optirnal for given applications, and so it was uncertain
how many control samples should be acquired.
There is therefore a need for a single source of the tools needed to perform
15 the described test, in its various forrns. ~ -
QBJECIS AND SUMMARY OF T~IE INVENIION
Accordingly, it is an object of the invention to provide a kit for use inperforming a test for diagnosing maladies which overcomes the drawbacks of the prior
- art.
'
It is a further object of the invention to provide a kit which contains at leastone test receptacle and at least one control receptacle for use in performing a diagnosis
for a malady.
., . ~ . ~
.;
.
.
wo 91 /os1 38 Pcr/us9oto7s6o
2~7~909 ~, '
It is a still further object of the invention to prov~de a kit for use in diagnosing
maladies which includes a number of control samples for performing a broader range
of tests for different maladies.
Brie9y stated, there is provided a kit for use in diagnosing maladies that includes
5 at least one test receptacle, having a test substance disposed therein. Each test
receptacle has a different test substance, or combination of test substances, therein.
The kit further includes at least one control receptacle. The nurnber of controlreceptacles preferably relates to the number of tests to be performed, namely one
control for every ten tests to be done, with a minimum of five. Preferably, the kit
10 further includes a Iysing agent for Iysing blood cells which are not used as part of the
test to be performed, and a blood diluent for dilutirlg the volume of blood to be
measured. The kit may also include at least one empty receptacle for use in cleaning
the equipment used to perform the test, and a carrier to hold the various components
of the kit together for ease of transportation.
,;:
In accordance with these and other objects of the invention, there is provided
a kit for use in diagnosing the presence or absence of a malady in a subject, the kit
comprising: a firse, test, receptacle; a test substance disposed within the first receptacle9
the test substance having a predetermincd relationship with the malady; and a second,
control, receptacle; whereby separate volumes of the subject's blood may be placed into
20 the first and second receptacles, for measuring the degree of reaction between the test
substance arld the blood of the subject.
According eo a feature of the invention, there is further provided a kit for usein diagnosing the presence or absence of maladies in a subject, the kit comprising: a
first plurality of first, test, receptacles; a second plurality of test substances, at least
25 one of the test substances being disposed in each of the first plurality of the first
receptacles, each of the plura]ity of test substances having a predetermined relationship
with at least one malady; and a second, control, receptacle; whereby separate volumes
of the subject's blood may be placed into each of the plurality of the first receptacles
~ . .
:, .
.,~ .
.;~ ' . ' .
'::
WO 91/09138 PCr/US90~1i756Q
~- !
s ` 207~909
and the second receptacle, for measuring the degree of reaction between each of the
test substances and the blood of the subject.
According to a still further feature of the invention, there is still further provided
a kit ~or use in diagnosing the presence or absence of maladies in a subject, the ldt
S comprising: a first plurality of first, test, receptacles; a second plurality of test
substances, at least one of the test substances being disposed in each of the first
plurality of the first receptacles, each of the plurality of test substances having a
predetermined relationship with at least one malady; and at least five control
receptacles; a third, cleaning, receptacle, for cleaning equipment used in perfolming
10 the diagnoses; a blood diluent for diluting the blood of the subject during the diagnoses;
and a Iysing agent, for Iysing blood cells in the blood of the subject; wherein the kit
includes at least one control receptacle for each ten of the first receptacles; whereby
separate volumes of the subject's blood may be placed into each of the plurality of the
first receptac}es and the control receptacles, for measuring the degree of reacJdon
15 between each of the test substances and the blood of the subject.
The above, and other objects, features and advantages of the present inven~ion
will become apparent from the following description read in conjunction wi~h ~e
accompanying drawings, in which li~e reference numerals designate the same elements.
.
; BRIEF DESCRIPTION OF T~IE DRAWINGS
Fig. 1 is a perspective showing the various elements of the inventive kit.
:
Fig. 2 is an e~loded perspective showing a single receptacle of the kit of Fig.
1.
Fig. 3 is a perspective of a secondary embodiment of the inventive kit.
.
.~' ' ` '
. .
,
.
- .
WO 9l/09138 PCr/US9~7S6~
~7~U~3 6
DETAILED DESCRIPrION OF TlIE PREFERRhl) EMBODIMENT
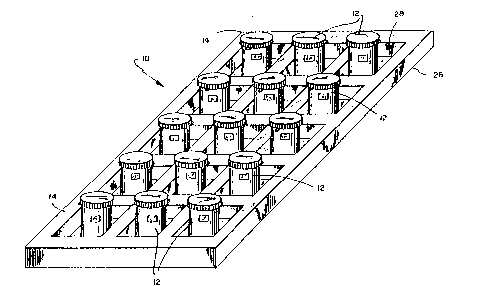
Referring now to Fig. 1, there is shown, generally at 10, a kit for use ~n
diagnosing maladies in a-subject, in accordance with the invention.
In its simplest form, kit 10 includes a plurality of test receptacles 12~ and a
S second plurality of control receptacles 14. Test receptac]es 12 and control receptacles
14 differ not at all, except in their respective contents. They are each sealed
receptacles made of any sturdy and durable material, such as polystyrene. ~
exemplary receptacle is shown in Fig. 2, generally at 16.
.
Receptacle 16 includes a generally cylindrical body 18, and a lid 20. When
10 receptacle 16 is closed, it will be secure against outside contaminants. This will
preserve the integrity of receptacle 16. Receptacle 16 also preferably includes a label
22, for identifying contents 24 thereof, i.e. whether it is a test receptacle (such as
pictured, where the test substance is a food extract for corn), or a control receptacle,
in which case contents 24 will be neutral.
- . j
Returning now to Fig. 1, kit 10 further preferably includes a carrier 26, havinga plurality of apertures 2~ for retaining test receptacles 12 and control receptacles 14.
Carrier 26 may be made of any suitable material for holding test receptacles
12 and control receptacles 14, and its mode of manufacture w~l be appreciated bythose of ordinary skill in the art.
The difference between test receptacle 12 and control receptacles 14 is solely
in their respective contents (24 in Fig. 2).
Into each test receptacle 12, a suitable test substance will be placed as its
contents (see 24 in Fig. 2). The nature of contents 24 will depend on the type of
.
WO 91/0sl38 PCr/US90/07560
2~7~09
malady tO be diagnosed with that par~icular test receptacle. As described in thereferenced patents, a test may be made for a particular malady in different ways, for
example, by addmg a suspected reaction-provoking substance (e.g. a suspected food
allergen, or a suspected chemical to which the subject is perhaps sensitive) or an
S antibody specific to that malady (e.g. a monoclonal antibody specific to a cancer for
which the subject is tested). For a more detailed discussion of the nature of the
reaction which is to be measured by the test, reference is made to U.S. Pat. No.4,778,155, col. 9, line 27- col. 10, line 5. It will also be appreciated that ~he
predetermined relationship may be that a ttest substance may cause a reaction, and
10 the test may be performed, then, to determine if the test substance in fact has caused
the suspected reaction.
The manner in which any particular substance is to be placed into a test
receptacle 12 will depend on the nature of the substance. For example, where thetest substance is to be a food extract, or a mold, to test for an allergic reaction to the
15 food, very little extract (on the order of a few milligrams) is needed to perform the test
effective~y. This amount of material is difficult to handle, and so it is preferred that
the test substance be carried on a carrier medium. This preference is compounded, m
that the extract should be in a stable state, i.e. non-reactive with the emirorime~9
particularly with respect to moisture and/or air.
A stable test substance may be fabricated by preparing a solution containing
the extract and a suspension medium, such as a rnixture of equal parts glycerine and
water. rhe preferred solution would have one part extract to nine parts suspension
medium by weight. Once the solution is mixed, appraq~imately 10 microliters of the
- solution is delivered to the carrier, which in this embodiment is preferabb a nylon
disk.
. .
The disk is then rapidly frozen. At that point, the liquid portion of the solution,
in the form ~f ice, is removed by sublimation, under a high vacuurn, at a low
temperature, appx. 50 degrees below zero Celsius.
~ .
,.
:. . ' ' . ' :
wo 9l/0~138 Pcr/~s~oi~756~ 1
2 0 7 ~ 9 8
It is here noted that this method of manufacture is specific to the case of foodextracts In the case of other types of test substances, the general procedure-would
be the same, but the precise proportions of test substance to suspension medium may
differ. That would be a matter of some minor experimentation, well within the scope
5 of one of ordinary skill.
It is here also explicitly noted that the test substance need not be a ~re
substance. For example, it may be desired to test for reactions caused by a
combination of medications in a patient, and so the test substance could be a
combination of the two (or more) medicines.
It will also be appreciated that this is but one method of preparing test
receptacles 12. It would be possible to carry test substances on any suitable, neutral7
medium, such as a plastic pellet, or even on the floor of the receptacle itself, if the
receptacle is so formed as to permit such manufacture. Those of ordinary skill in the
art will appreciate that the mode of manufacture of test receptacles 12 and control
15 receptacles 14 will be strictly a matter of design choice, within the purview of such an
ordinarily slcilled person. It will be further appreciated that the precise details of ~e
shape and construction of test and control receptacles 12 and 14, respectively, will
depend on the intended application. The receptacles must conform to the intendeduse.
This, too, is a minor design choice, which those of ordinary sldll will be able to
deterrnine for their intended use.
An important factor in the manufacture of kit 10 is that the contents of each
test receptacle 12 and control receptacle 14 should be identical, but for the inclusion
of the test substance in the test receptacle. In other words, even though the test
; 25 substance is ca~ried on a carrier medium which is believed to be neutral with respect
to the subject's blood, that carrier medium should also be placed in control receptacles
., ' , : ,
.
. . -
wo 9~/09138 pcrtuss~ 56~
9 -` ` - 2~7~9~9
14 as well, in case there is any reaction between that medium and the subject's blood.
Additionally, it assists in avoiding any potential problem in measurement, by ensuring
that the volume of material in each type of receptacle is identical.
As to the relative number of test and control receptacles 12 and 14, respectively~
5 it is preferred that at least five control samples be used. In practice, different tests are
performed sequentially, not in parallel. This means that each measurement is
performed at a slightly different time. Where a number of tests are to be perfonned,
possibly over one hundred in some instances, the difference in time between the first
measurement and the final measurement could be as much as an hour or more. If the
10 control specimen is analyzed first, it may have different characteristics than if it were
measured last, since, even in a non-reactive situation, a small percentage of blood cells
of some patients, in certain instances may deteriorate over time.
Furthermore, it is now preferred that the comparisons be made on the basis of
correlation to a standard deviation from the expected results, and the use of an average
15 of several control samplings renders a more accurate result.
These reasons for false readings may be avoided by use of several eorl~rol
samples. For example, if control samples are prepared at different intervals, and the
test samples are compared with the contrd sample nearest in time, then more accurate
readings are possible. Furthermore, since the test results are comparative, an average
20 of several control readings may provide a better average of test results, leading to more
accurate readings.
~ After experimentation, it has been determined that, in the currently preferred
- testing protocol, at least one control measurement should be taken for every ten tests
performed, with a minimum of five control samples. This may differ when, over time,
2S different equipment becomes available vith either greater accuracy, or greater speed,
- so that deterioration becomes less of a factor.
, ...... ` ' . ., ' ' ' .. ':~''' . '
..
. . - . . - ~:
. . .
.
. . . ~ .
wo 9l/09138 Pcr/uS9û~7~
20719~ lO ``
The above description details one preferred embodiment of the invention,
name]y where the inventive kit includes only test receptacles and control receptacles,
carried in a carner. In some applications, however, it may be also preferred to have
other items be made a part of kit 10.
S Figure 3 illustrates a supplemental kit, shown generally at 30, which illustrates
the additional items which may be a part of the inventive kit.
i
It may be preferred that supplemental kit 30 be af~xed to kit 10, and it may
be so manufactured. Here, for simplicity of illustration, it is shown separately.
Supplemental kit 30 includes a set of blank receptacles 32, for use in cleaning
10 equipment used to perforrn the diagnoses, a lysing agent 34 and a blood diluent 36
all carried in a carrier 38, having apertures 40.
Blank receptacles 32 may be used for cleaning the equipment used for
performing the diagnosis. As will be readily appreciated, the performance of any teslt
involving medical diagnoses must be done carefully, and so the equipment used mus~
15 be regularly msintained. Blank receptacles 32 may be used for carrying- detergen~s ~o
` place them in the equipment for cleaning, and then carrying the detergents away after
cleaning.
As noted, in some applications it is necessary to Iyse certain blood cells so that
measurements may be made. In these applications, lysing agent 34 would be useful.
20 It is preferred that the active lysing agent (for example, Saponin, from Sigma Chemical
Co.) be present in a one-per cent solution in bacteriostatic water, in an amountsufficient to perform the tests to be made. Precise measurements may be made in
accordance with the protocols set forth in my U.S. patents referenced above.
.
': .
'.' ' .: : .
... . . .
.,: .
wo 91/09138 PCI/US9Oi!~7S~a~
2~7~
11
It will also be noted that the type of lysing agent will depend on the type of
blood cell to be Iysed, since different measurements may be taken of different types
of cel~s, i.e. red blood cells, white blood cells or platelets.
Similarly, since a blood diluent is used in most applications, it is also pre~erred
S that supplemental kit 30 include blood diluent 36, which is preferably a substance which
is non-reactive with human blood, and is pH balanced to the acid level of human blood
(7.4). Suitable solutions would be a mixture of buffered normal saline, 0.9% sodium
chloride buffered to (appx.) pH 7.4 with sodium bicarbonate. The total volume ofblood diluent 36 provided would depend on the number of tests (and controls) tG be
10 performed, and so will depend on the particular application contemplated for any kit
I0 and supplemental kit 30.
The collection of parts described above will permit the efficient and accurate
performance of my patented test, by providing, for the first time, all of the components
needed to perform the test.
I5 Having descn~ed preferred embodiments of the invention with reference to the
accompanying drawings, it is to be understood that the invention is not limited ~o klawe
precise embodiments, and that various changes and modifications may be effected
therein by one skilled in the art without departing from the scope or spirit of the
invention as defined in the appended claims.
;'. ~ ' ~ , . . : .