Note: Descriptions are shown in the official language in which they were submitted.
STRI PP ING SOI,UTIO~ ANU PROCESS FOR STRIPPINC;
!;~OI~lP0~7NDS OF TITANIIJM FROM BASE ~IFTAI,S
Field of the Invention
This invention is a continuation-in-part of
U.S. Patent Application Serial No. 599,833 filed
October l9, 1990 and relates to an aqueous stripping
solution for selectively removing a titanium
compound, such as TiN or Ti~2, from a solid base
metal without chemically attacking the solid base
metal and to an accompanying process for stripping
compounds of titanium from base metals.
Background of I~vention
High performance components in aircraft
engine turbomachines such as compressor blades,
bearings and gears are typically coated with a
titanium metal compound such as TiN to improve their
wear charac~eristics and to provide erosion
protection. The engine parts are cast or otherwise
molded or machined ~rom superalloys, stainless
steels or alloy steels and represent ~ery e~pensive
precisio~ components. Removal of the coating from
the underlying base metal is necessary if a defect
is discovered in the coating and/or for restoring
worn comp~nents. It is essential to strip the
protective coating from the base metal wi~hout
suffering any detrimental attack to the underlying
base metal.
To selecti~ely strip a titanium compound
such as TiN from a solid base metal composed of a
superalloy, stainless steel or alloy steel without
chemically attacking the base metal is particularly
difficult when both the base metal and coating have
D-16302-l
~ ' - 2 - ~a7I9~ ~
a high corrosion resistance characteristic.
Stripping is e~en more difficult when the corrosion
resistance of the coating is equal to or greater
than the corrosion resistance of the substrate.
Although, stripping solutions containing
hydrogen pero~ide are known there is no known
aqueous based stripping solution using hydrogen
peroside which will permit the removal of a coating
of a titanium compound from a solid base metal
composed of a superalloy, stainless steel or alloy
steel without causing detrimental attack to the
underlying base metal. A chemical stripping
solution compri~ing hydrogen Rero~ide is described
in U.S. Patent Nos. 4,554,049, 4,410,396 and
4,545,918 respectively. The stripping solutions
disclosed in these patents are either unable to
strip compounds of titanium from base metals
composed of superalloys stainless steels and alloy
steels or will actively attack both the titanium
compound coating and the base metal.
SummarY of the Invention
The process of the present invention for
stripping a coating of a titanium compound from an -:
underlying base metal without suffering chemical
attack to the base metal comprises the steps of:
immersing the base metal and coating into
an aqueous solution ~ontaining a source of hydrogen
pero~ide, an alkali source of hydro~yl ions, and an
~cid, maintaining the solution temperature between
25~C and 85~C and ad~usting the molar ratio of the
components to cause the pH of the a~ueous solution
to be above a pH of 8. ,:
,
.-
: D-16302-1
. . .
.. . . . .
:. .'
.
' . '' ' ' ' ,,
, ' .; .' '
- 3 ~ 1 9
~ .
The stripping composition of the present
invention comprises an aqueous solution including an
alkali source of hydroxyl ions, a source of hydrogen
pero~ide and an acid with the constituents of the
solution in a concentration such that the pH o~ the
solution is above 8.
~rie~ Descriptlon of the ~rawin~s: :
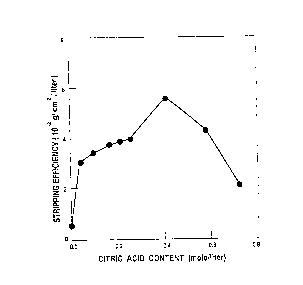
Figure 1 is a plot of stripping efficiency
versus the content of the preferred acid in mole per
liter for removing a TiN coating from an Inconel 718
base metal;
Figure 2 is a plot similar to that of Figure
1 showing stripping efficiency as a function of the
conkent of NH40H in mole per liter in the stripping
solution of the present invention;
Figure 3 is another plot similar to that of
Figure 1 of stripping efficiency ~s a function of the
content of hydrogen pero~ide in mole per liter in the
stripping solution of the present invention;
Figure 4 is a plot o~ the solution ~tripping
rate ~or stripping TiN ~rom an Inconel 718 coupon as
a function o~ the solution operating temperature; and
Figure 5 is a plot o~ the solution active
li~e of a preferred solution composition for removing
TiN from Inconel 718 base metal substrates and the
stripping efficiency as a ~unction o~ temperature.
~etailed ~escriptio~ of the Invention
~ ssentially any coating composition of a
titanium compound can be removed from any b~se metal
substrate by the process of the present invention
without detrimentally attacking the base metal. The
D-16302-1
_ 4 _ -2~ ~ 7 9 ~4
invention is particularly suited to the removal of
TiN or Ti~2 from a base metal composed of stainless
steels, superalloys or alloy steels.
The stripping solution of th~ present
invention comprises the following three components:
a source of hydrogen pero~ide, an alkaline source of
hydro~yl ions and a suitable acid in various
proportions to cause the pH of the solution to be
above 8 without corroding the substrate. The
stripping solution is prepared by first combining the
source of hydrogen pero~ide with water. The source
of hydrogen peroxide should be present in a minimum
concentration of .29 mole per liter and in a
preferred concentration range o~ between .29 to about
4.71 mole per liter (mole/L). Any source of hydrogen
pero~ide such as a perborate, as is well known to
those skilled in the art, may be used. Othes
compounds which readily dissociate into hydrogen
peroxide upon contact with water are also suitable.
The alkali source of hydro~yl ions (OH) is then added
to the solution. The hydro~yl ion is preferably
added in combination with ammonium ions through the
addition of ammonium hydro~ide (NH4OH). The source
of hydroxyl ions should be present in the stripping
~olution in a concentration of at least 0.29 mole/L
and prefeIably b~tween .29 mole/L and 3.23 mole~
An acid must also be present in the solution at a '
concentration of 0.026 mole/L a~d preferably between
0.026 mole/L and 0.76 mole/L. Any acid which will
not corrode the base metal may be used, preferably an
organic car~oxyl or carbo~yl-hyarogyl group acid such
as lactic acid, o~alic acid, tartaric acid, formic -
.
D-16302-1
~ , : - ,. . .
'. "' ' ' ' ' ' ' ' ~ ' '; '." ~'
: .: ,
, ' ,' '' ', , '
,:
.
- 5 ~
acid, propionic acid or citric acid. Alternatively,
a diluted inorganic acid such as, for example, acetic
acid, nitric acid, hydrochloric acid and sulfuric
acid may also be used provided it will not chemically
attack the base metal an~ is low enough in
concentration to maintain the solution pH above 8.
The pH of the stripping solution is critical
to the present invention and must be above pH 8 to be
effective. The preferred pH range is bet~7een pH 9-14
with a pH range of 10-12 being optimum. The pH of
the solution may be controlled by adjusting the
concentration of alkali, peroxide and organic acid
relative to one another provided ~ach is held to a
concentration within the preferred range.
Additionally, other alkali ions such as sodium or
potassium ions may be added to the stripping solution
,; ,~
by the addition of NaOH and~or KOH to establish the
desired mole concentratio~ and/or to adjust the pH of
the solution.
The ef~ectiveness of the stripping solution
of the present invention is det:ermined by the
ef~iciency in which the titanium compound coating is
removed from the substrate without suf~ering any
deleterious effect on the ~ase metal. A minimum
stripping efficiency of 1 ~ 10~2g~cm2/L and
preferably above 2 ~ 10~2g/~m2/~ is necessary for the
stripping solution to be acceptable for commercial
practice. The stripping efficiency is determined
based on total weight loss of the coating per unit
coating surface area for a given volume of stripping
solution over a time period until the solution is
considered inactive.
D-16302-1
,. . .
,:
- 6 - 2~719~
E~periments were conducted using numerous
aqueous compositions all containing various
proportions of hydrogen pero~ide, an acid and an
alkali source of hydro~yl ions. The following tables
I, II, III and IV identify the different solution
compositions all of which had no deleterious e~fect
on the base metal. All of the tests shown in the
Tables 1, II, III and lV were carried out by
i~mersing a TiN coated Inconel 71B* coupon (1.5 ~ 25
~ 50 mm) into the test stripping solution at between
60 and 85~ C~
Tabl e I
Effect of Citr;c Acid Content (H3C6H507~ on Stripping Efficiency
Stri ppi r~g
--Composi ti on Mol etE-_ Ef f i ci ency
Solution H20 Hzo2HH40H H3C6HS~7 pH ~10 2 g/c~2/L)
,
1 bal. 1.321.09 0 10 0.38
.
: - -
2 ba1. 1.321.09 O.û5 10 3.1 .
3 bal. 1.321.09 0.10 10 3.4
4 ~al. 1.321.0g 0.16 10 3.8
tal. 1.32 l.û9 0.21 lû 4.0
~ 6 bal. 1.32 l.û9 0.~6 10 4.1
- 7 bal. 1.321.09 û.42 9 5.7
8 bal. 1.321.09 0.59 9 4.4
9 bal. 1.321.09 û.73 ~.5 2.0
~ Inconel 718 is a registered trademark of the
Internatonal Nickel Corporation. .
D-16302-1 .
_ 7 ~ 9 ~ 4
Table I ~hould be read in conjunction with
Figure 1, which is based on the data of Table I,
showing the effect of citric acid on the stripping
ef~iciency of the solution. Citric acid is the
preferred acid component although any of the other
acids, as heretofore described, may be substituted
for citric acid at equivalent concentration or
equivalent pH levels to produce substantially
equivalent results. The stripping efficiency
increases monotonically with increasing concentration
of cit~ic acid provided the pH level is above 8.5.
The concentration of hydrogen pero~ide and the alkali
componen~ were held constant. It was determined from
e~perimentation that the presence of a minimum
concentration of acid was necessary to stabilize the
solution and to permit the stripping efficiency to
e~ceed the minimum level. The concentration ~f
citric acid should be above at least about 0.026
mole/L and pre~erably above O.O!j2 mole~L. The
m~imllm concentration of citric acid is appro~imately
0.76 mole/L, Upon exceeding thle maximum
concentration the pH of the solution drops to below
8.5 which reduces the stripping efficiency below the
effective minimum level.
D-16302-1
"
':
8 2 ~
'
.
Table II
, Effect oE NH4~H Content on Stripping Efficiency
: .
. StrippingComposition ~ole~L E~ficienc~
Solut~on ~2~ ~2~2 NH40H ~3C6~5~7 p~ (10 2 g/cm /L)
.. -
bal. 1.3Z 0 0.16 2 0.39
11 b~l. 1.32 0.37 0.16 10 300
' 4 bal. 1.32 1.09 0.16 10 3.8
. 12 bal. 1.32 1.46 O.lS 10 4.2
:~ 13 bal. 1.32 1.80 0.16 lO 4.0
,. . .
.; 14 bal. 1.32 2.51 0.16 ll 5.3
, ~ . .
~ 15 bal. 1.32 3.23 0.16 11 5.1
~ ,. .
Table II should be read in conjunction with
Figure 2 which is basea on the data of Table II ~and -
shows the effect of varying the concentration of
ammonium hydroxide (NH40H) in the stripping
solution. Ammonium hydroxide i.s the preferred alkali
source. The concentration level of citric acid and
pero~ide were held constant wh:;le adjusting the
concentration of NH40H. From Table II and Figure 2
it is apparent that the stripping solution does not
function effectively until the concentration of NH40H
is raised to a minimum level of about 0.29 molefL at
a pH of 8 or higher. The 1atte~ was confirmed by the
data show~ in Table IV as will be discussed in
greater detail later in the specification.
~;
, ' '
D-16302-l
,
', , , ., :. ' ;' ' . - " :" "'''' ' . , ~
,.. , , ' . . .
,, -' " ,. .. .
, , . , . ,:,~, ' '.: , ', ' . ' ',
_ 9 _
4 ~
Table III
Effect of H20z Content on Stripping Efficiency
Stripping
Composition Mole/LEfficienc~
Solution 1120 ~2~2 NH40~ ~3C6~507 P}~ ~10 2 glcm IL)
16 bal. 0.44 1.0~ 0.16 9 1.9
17 bal. 0.88 1.09 0.16 9 3.6
4 bal. 1.32 1.09 0.16 10 3.8
18 bal. 2.65 1.09 0.16 10 6.3
19 bal. 4.41 1.09 0.16 lO 6.9
bal. 2.65 2.17 0.16 11 6.2
Table III should be read in conjunction with
Figure 3 from which it is apparent that the stripping
ef~iciency directly increases with increasing
concentrations of hydrogen pero~ide up to about 2.94
mole/L at which concentration the stripping
ef~ici~ncy of the solution levels off. Accordingly,
although the hydrogen pero~ide concentration may be
further increased the ma~imum level should be about
9.71 mole/~ above which, ~or practical
considerations, ~here is a negati~e incentive to
further raise the hydrogen peroxide concentration.
The m;nimum concentration of hydrogen peroxide is
about ~.29 mole/~ and preferably abo~e 0.59 mole/~.
Typically the temperature of the solution
has an influence on the stripping rate and
efficiency. The reactivity o~ the solution increases
with increasing operating temperature and the
solution life decreases with increasing operation
':
~ 16302-l
:'
, ' :, ' , ~ ' , ' , .,
, , , : , ,; ' ~ . : ,:
lo- ~7~9~
temperature. Accordingly, to determine the optimum
solution temperature two test solutions were pxepared
using a different pero~ide to alkali molar ratio at a
constant acid concentration. The stripping rate was
evaluated as a function of the operating temperature
as shown in Figure 4. The composition of ~he two
test solutions were as follows:
Solution 12. 1.32 mole/L H202 + 1.46 mole/L
NH40H ~ 0.16 mole~L H3C6 H~07 balance water (marked
"0~ in Figure 4).
Solution 4. 1.32 mole/L H202 ~ 1.09 mole/L
NH~OH + 0.16 mole/L H3C6 H507 balance water (marked
~" in Figure 4).
The stripping rate is e~pressed in terms of
the total weight loss ~in grams) of the coating per
unit area (in cm2) per unit ~olume (in liters) per
unit time (in minutes). As shown in Figure 4 the
optimum stripping rate is realized at a solution
temperature e~ceeding 50~C and prefera~ly between
60~C and 85~C.
Although the optimum solution temperature is
above SO~C the solution may be. operated at a
temperature within a wide range e~tenaing from about
2S~C to about 9S~C as is evident from Figure 5 which
is a plot of the solution active life in minutes as
well as stripping efficiency against temperature. A
preferred solution of H20 ~ 1.32 mole/L H~O~ ~ 1.09
mole/L NH40H + 0.16 mole/L citric acid was used to
develop the plot. The solution active life was found
to decrease e~ponentially with increasing temperature
from about 1000 minutes at 25~C to about 2~ minutes
D-16302-1
,, ,
,. . . .. . . .. .. .
, . . . , "
, . .
, . . . .. . . . .
,, ,, : ~ : , : '
" , , , ,: . ,, , , :, :
.
.
'~ , '
11- 2~
at about 95~C. The stripping efficiency also
decreases rapidly with increasing temperature. At
higher operating temperatures of above about 85~C the
solution active life is simply too short for any
practical commercial use. Figure S should be
evaluated in conjunction with Figure 4 which
substantiates that the stripping rate is highest
above 50~C. Accordingly from both Figure 4 and 5 a
wide operating solution temperature of between 25~C
to 85~C is practical although the highest stripping
rate occurs above between 50~C and 85~C with 6D~C -
80OC being the preferred range for optimum stripping
with a reasonable solution active life.
The following Table IV is a compilation of
the data obtained using various alkali ammonium
compounds and NaOH at different pH levels for
comparison with the results of Table II on the effect
of stripping efficiency for the various test
solutions.
, . .
D-16302-1
"
. .. . .
''
'~
.: ,., .: , . .
: :,. ~ : . .
,, .,: - ,:, .
:
' , ~ . .
- , .
2 ~
.. , :
, ..; .,
~ o o ~ ~ ~ ~ Ul
N O -- ~ N O O O O O O
cD , ~
.~ ~ --~ ''
~~
~" ' O
~ Z
"_ ~~ ~ O O O O ¦ O o e~
~''. .. L~,
~, I I I O I I ~ I I I
o~ .
J N ~ ~ r; v~ 1--
.
' 0~ ~___ ______
~',. . .
R ~1
, r . . .
,,' ~ ~ . 'i
. .
, ~'' ' ',
.
- }3 -
From the above Table IV it is apparent that
a pH above 8 is necessary for the solution to provide
- an effective stripping efficiency and that ammonium
compounds other than NH40H do not produce effective
stripping efficiencies unless combined with NH4OH or
another source of hydro~yl ions such as-NaOH.
However, it is clear from all of the test data that
NH40H is the preferred alkali 60urce~ The ef~ective
concentration for the three critical components,
- viz., a source of hydrogen pero~ide, an alkali source
of hydro~yl ions and acid is 0.29 mole~L to ~.71
mole~L, 0.29 mole~L to 3.23 mole/L and 0.026 mole~L
to 0.76 moleJL, respectively. For the preferred
components H2O2; NH40H and citric acid the preferred
concentration is 0.59 mole/L to 4.71 mole/L, 0.~7
mole/L to 3.23 mole and 0.05 mole/L to 0.66 mole/L,
respectively.
~ Although the base metal in the test coupons
were all of Inconel 71~ other coupons including TiN
coated st~inless steels such as AISI440C and AISI
17-4 PH and alloy steels such as M50, M50NIL and
Pyrowear 53 were tested using the preferred ~tripping
solution. All demonstrated similar behavior to the
~ TiN coated Inconel 718 coupon~ with no deleterious
; . effect on the base metal.
~ he hydrogen pero~ide component in the
stripping solution of the present invention may be
generated in situ from any source of pero~ide which
: dissociates in water to form hydrogen pero~ide such
as a perborate, e.g. sodium perborate tetrahydrate
~NaBO3-9H2O) or any other know pero~ide compound
:: which will readily dissociate into hydrogen peroside
,
D-16302-1 ~
. ~.
,., . ,~
..:
- , ..
.:
:' , ' , '
,.. : . . . . .. . . . .
.. . . ..
.. .... .
~ 14 - ~7 ~
in the presence of water at atmospheric pressure and
within the operating temperatures of the present
invention. Ammonium pero~ydisulfate ((NH4)2S208) is
not a suitable source of hydxogen pero~ide for the :
present invention as is evident from the following
Table V despite the fact that ammonium
pero~ydisulfate is used to commercially produce
hydrogen pero~ide by hydrolysis at r~duced pressure
and ~levated temperature.
In accordance with the following Table Y TiN
coated Inconel.718 coupons ~1.5x25x50 mm) were
immersed into separate pero~ide containing solutions
with a specified pH of a~ove 8 and at temperatures of
between 60~C and 6S~C to evaluate the stripping
effectiveness of the solutions with the different
sources of pero~ide.
.
'
, ~,
;l i,
'~ ' " :,
,: .
D-16302~
,~'"
:
, .:
' . . , .'' . . ' - .' .. ': ,.. - ~
. : . .: - .
. ' ,: , ' ' ~ . ''' :' ,.
.. . . ~ .
' .
2~7~944
~ ~ O -- G~ O
. . o
~ o o o
o. o CO
. : .
o
~ '~ o ~ C~ I o
~'',: .
o o o
G
~ ,
' ILI
g~ ~ o I o u~ o o
c~
I O ~O I .
,: z . "
. '
: ;, ON .:
.
.,.' . :'
,~ . . . . . . .
, . :
''' .'' .
.'
,"' .
: '
,: . ' ' . ' . . , ,' , " ' ' . ' ', ' ~ , ' .
~' ' ' ; ' . ' . ,,' ., . '. ;, '',', ,
.. , . : :
2~194~
- 16 -
As is evident from the above table no ~-
stripping action was obser~ed in the solutions 34 and
35 containing ammonium peroxydisulfate and no weight
loss was found on the test coupons. The solutions 32
and 33 with sodium perborate tetrahydrate were
capable o~ stripping the TiN coating from an Inconel
718 substrate but at a reduced stripping efficiency.
This is in sharp contrast to the effect of an
otherwise identical stripping solution composition
containing hydrogen pero~ide.
Tables V and VI show the results of
corrosion on the base metal when the acid component
in the stripping solution contains the Cl- ion. In
solution No. 34 and 36, NH4Cl and CH30H were used
instead of an organic acid and in solutions No. 37-40
HCl was used. Both TiN coated Inconel 718 and 410
~- stainless steel coupons (1.5~25s50 mm in size) were
immersed into the solution No. 36 and only 410
stainless steel e~hibited corrosion attack due to the
presence of the Cl- ion from the NH4Cl solution. In
the tests in the following Table VI HCl was used as
the acid component to strip TiN from different
substrate materials at different concentration
levels. Accordingly, the chloride concentration
levels that cause pitting vary with the substrate
material composition. If an acid containing the
chloride ion is used in the stripping solution, the
con~entration of acid should be determined according
to the substrate material used.
,., .:
. . .
, :
D-16302-1
"
,
. ~, . . . .
., : ~ . . . , -- - ,
.,
.
. .
.. . . , . , . : .
,
,,
. . . . .
. .
- 17 - 2~71~44
TABLE VI
_ Composition (Hole/L)- Substrate
Solution HzO H2~2 HH40H tlC1 Mater;al Comments
37 Bal. 1.32 l.û9 O.lZ ~50 Steel P;tt;ng corrosiDn
attack
38 Bal. 1.32 l.û9 0.35 410 SS P;tt;ng corrosion
attack
:~ 39 Bal. 1.3Z 1.09 û.35 Inconel 718 No corrDsion
attack
Oal. 1.32 1.09 1.16 Inconel 718 Pitt;ng corrosion
~ttae.
~'' . .
~ .
" .
,, ' .
.
:'.
:
.' .
I)-163~2-l
. .
:, ' - " . . . ~ . . . .
.. . . .... . . .. ..
.. . . . . ..
.. , . ., . . ~
.' ' ' ' , ' ' ' ' '' ' . :' ~
~.
.
' ' ' ' .: : ~'