Note: Descriptions are shown in the official language in which they were submitted.
2~72~3~
-2- 133-0669/B
HETEROCYCLIC DION~S AS P~STICIDES AND
PLANT GROWT~ RRGULATORS
The present invention concerns substituted 3,5-dioxo-3,4,5,6-
tetrahydrooxazines as herbicides, processes and intermediates for thelr
preparation, compositions containing them and ~heir use as herbicides and
a~aricldes.
USP 4,695,673 describes a wide range of acylated 1,3-dicarbonyl
compounds and their use as herbicides but makes no rePerence to or
sugg~stion of the 3,5-dioxotetrahydrooxa~ine ring characterizing the
'.0 compounds of the present invention.
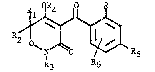
More particul~rly, the invention concerns compounds of formula I
~R5
wherein,
each of Rl, R2 and R3 is independently hydrogen, Cl8alkyl, carboxyl,
Cl0alkoxycarbonyl, phenyl or phenyl substituted by one to three
groups as R, or Rl and Rz together form a C3~alkylene bridge
R4 is hydrogen, C~aalkyl, Claalkylcarbonyl, Claalkoxycarbonyl,
C(O)NR7R~, Cl~alkylsulphonyl, P(O)-(OR~)2, R7P(O)-ORg, benzoyl or a
cation.
R is Cl8alkyl optionally substituted by 1 to 6 halogen atoms, Cl8-
alkoxy opt~onally substituted by 1 to 6 halogen atoms, Cl~alkyl-
carbonyl, Claalkoxycarbonyl, NR7R8, Ons(o)~Rlo~ NR7S02R8, halogen,
cyano or nitro.
R5 is Cl9alko~y substituted by 1 to 6 halogen atoms;
R~ is hydrogen or select~d from the maanings given for R;
each of R7 and R8 is independently hydrogen or Cl8alkyl;
R~ is Cl~alkyl;
Rlo is Cl~alkyl optionally substituted by 1 to 6 halogen atoms;
n is O or l;
n' is 0, 1 or 2;
providet that when Rl, R2 and R3 are methyl, R~ and Rff are hytrogen and
R is nitro, R~ is not difluoromethoxy.
In the above definltions, halogen ~ 3 con~eniently selected from
chloro, bromo and fluoro, Cl8alkyl moietie3, preferably have l to 4 carbon
atoms.
Each of Rl, R~ snd R3 is preferably hydrogen, Cl~alkyl especially
hydrogen or Cl3alkyl.
R conveniently si~ni~ies Cl~alkyl optionally substitut~d wlth
' '
.
~,~7213~
~3- 133-0659/B
halogen, -(O)n-S(O)n,-Cl4alkyl, halogen or nitro. It is preferably methyl,
CF3, Cl3alkylsulfenyl, Cl3alkylsulfonyloxy, chloro, broma or nit~o.
R5 is preferably fluoroalkoxy, more preferably OCF3.
R6 is preferably hydrogen, Cl4alXyl, Cl~alkoxy, bromo, chloro; it is
S more preferably hydrogen, methoxy or chloro.
R4 is con~eniently hydrogen, Cl4alkyl, C~3alkylcarbonyl, benzoyl,
Cl~alkylsulfonyl or a cation. It is preferably hydrogen, methyl, ethyl,
t-butylc~rbonyl, isobutylcarbonyl, benzoyl or methylsulfonyl. As a cation
R4 is preferably an alkali metal such as Na~, K~, Li~ or an } onium cation.
Examples of particularly preferred substituents are for Rl and R2 each
a) H, Cl4alkyl, phenyl or phenyl substituted by one to three groups
as R
b) H, Cl4alkyl phenyl or phenyl substituted by one to three
groups as R
c) H, Cl3alkyl
d) H, CH3
e) Cl3alkyl
R3 - a) Cl~alkyl
b) C~4alkyl
c) CH3, C2Hs
R4 H
R a) NO2, Cl, CF3
b) NO2, Cl
C ) NO2
Rs - OCF3
R6 H
Combinations of these substituent meanings are especially preferred.
A particularly preferret ~ingle compound is 2,6,6-trimethyl-4-(4-tri-
iluoromathoxy-2-nitrobenzyl)-2H-1,2-oxa7ine-3,5-(4H,6H)-d~one.
The compounds of the present inYention of formula I are new
substances which can be prepared by m~thods analogous to methods known in
the art, such as those described in European Patent Applicat~on EP 186,117
and references cited therein. ~ore particularly, they can be obtained by,
for example: rearranging an enol ester of formula (II)
~2 ~ II
wherein R~, R2, R3, R, R5 and R~ are a~ previously defined to give a co~pound
of formula I wherein R~ H.
Thi~ rearrangement i5 con~eniently ef~ected by reacting the compound
.
.
20r~2~34
-4- 133-0669/B
of formula II with a cyanide source and a moderate base.
For example, the reaction may 'oe carried out in the presence of a
catalytic amount of a source of cyanide anion and/or hydrogen cyanide,
together with 2 molar excess, with respect to the enol ester, of a moderate
base. The reaction is conveniently carried out in a solvent which is inert
under the reaction conditions, e~g. 1,2-dichloroethane, toluene, aceto-
nitrile, methylene chlorlde, ethyl acetate, dimethylforma~ide ~DMF) and
methyl isobutyl kecone (MIBK). In general, depending on the nature of the
reactants and tho cyanide source, the rearrangement may be conduc~ed at
temperatures up to about 800C. In som~ cases, for instanCQ when there is
a possible problem of excessive by-product formation, the temperatures
should be kept at about 400C maximum.
Preferred cyanlde sources are alkali metal cyanides such as sodium
and potassium cyanide; cyanohydrins of methyl alkyl ketones having from 1-4
carbon atoms in the al~yl groups, such as acetone or methyl isobutyl ketone
cyanohydrins; cyanohydrins of benzaldehyde or of C2-C~ aliphatic aldehydes
such as acetaldehyd~, proplonaldehyde, 8tC., cyanohydrins; zinc cyanide;
tri(lower alkyl) silyl cyanides, notably trimethyl silyl cyanide; and
hydrogen cyanide itself. Among cyanohydrins the preferred cyanide source
is acetone cyanohydrin. The cy~nide source is used in an amount up to
about 50 mole percent based on the enol ester. Generally about 1^10 mole
% of the cyanids source is preferred.
By the term "moderaee base" i9 meant a substance which acts as a base
yet whose strength or activity as a base lie~ between that of stron~ bases
such as hydroxides (which could cause hydrolysis of the enol ester) and
that of weak bases such as bicarbonates (whlch would not function
effectively). Moderate bases suitable for use in this reaction include
both organic bases such as tertiary amines and inorganic bases such as
alkali metal carbonates and phosphates. Suitable tertiary amines include
trialkylamines such as triethylamine, trialkanola~ines such as triathanol-
amine, and pyridine. Suitable lnorganic bases include potassium carbonate
and trisodium phosphate. The base is used in an amount of from about 1 to
about 4 moles per mole of enol ester, preferably about 1.3-2 moles per
mole.
When the cyanida source is an alkal~ metal yanide, part~cularly
potassium cyanide, a phase trAnsfer catalyst ~ay be included in the
reaction. Particularly suitable phas~ transfer catalyst3 are the Crown
ethers.
Compounds of formula I where R~ is other than hydro~en can be
prepared by reacting a compount o~ ~ormula I wherein R~ is hydrogen with
either
a) the group R40-OH and a catalyst, or
b) the group R40~Q and a moderate bsse, wheroin Q i5 8 halogen atom,
to give a compound of formula I where R~o i~ the de~ired substituent.
~72~3~
-5- 133-0669/B
The above reaction a) is carried out in the presence of a catalyst
such as concentra~ed sulfuric acid. The reaction is conveniently carried
out in a solvent which is also the reactant such as methanol, and at an
elevated temperature.
The above reaction b) is carried out in the presence of a moderate
base such as triethylamine or pyridine and oonveniently at RT or below.
The compounds of formula I may be recovered from the reaction mixture
in which they are formed by working up by es~ablished procedures.
The compounds of fo~ula II may be prep~red by reacting a compound
lO of formula III R
R2
with a compound of formula IV
Hal - C~Rs IV
R
This reaction is carried out in the presence of a base such as
triethylamine, potasslum carbona~e, pyridine, preferably triethylamine and
in an iner~ solvent such as tichloromethane, acetonitrile, toluene,
tetrahydrofuran, dimethylformamide. The reaction i~ conveniently carried
out at RT or below.
The remaining compounds of for~ula II may be prepared analogously.
The compounds of for~la III are new and also form part of the
invention.
They may be prepared by decarboxylating a compound of formula V
R2~ R20 v
~ .
R3
wherein R20 is alkoxy, especially ethoxy or me~hoxy and Rl, R2 and R3 are as
defined above. The reaction may be carried out at elsvated temperatur~s
e.g. 80 90~ and in an inert solvent such a~ e.g. wet dimethylsuloxlde.
The compounds of formula V may be prepared analogously to known
methods e.g. accordlng to the ollowing reaction scheme.
.
-
,
- 2072~3~
-6- 133-0669/B
R3NHOC(RlR2)COR20 + ClOCCHzCOR20
VI ~ VII
~ (a)
R3NOC(RlR2)cOR2o
COCH2cOR2o
VIII
~ (b)
V
Reaction (a) may be carrled out in an inert solvent such as dichloro-
methane and aqueous ether and in the presence of a base such as triethyl-
amine or sodium carbon~te at RT.
Rsaction (b) may be carried out in an inert solvent such as toluene
benzene or tetrahydrofuran in the presence of a base such as sodium
methoxide or sodium hydride.
The remaining starting materials and reagents employed in the process
described herein are either known or, insofar as they are not known, may
be produced in a manner analogou~ to the processes dsscribed herein or to
known processes [cf for compounds VI Kornowski et.al. Bull. Soc. Chim
: France 1966(2)683].
The compound 2-nitro-4-trifluoromethoxy-benzoic acid is new and also
forms par~ of the in~ention.
The compounds of this in~ention wherein R~ is H can have four
structural formulae b~cause of tautomerism as illustrated ~5 follows:
2 ~ = 2 ~ ~
N ~ O~N 5
I~
Ic R2 ~ = R2 ~
R3 R6 IJ o ~N OH 5
.
:
'.
20 ~2134
-7- 133-0669/B
It will of course be readily appr~ciated that whers R4 is other than
hydrogen such co~pounds may exis~ in forms Ib, Ic and Id or as mixtures of
these forms.
The novel compounds of formula I are useful for the control of weeds,
using pre- and/or post-emergent treatments. Compounds of formula I are
also useful as plant growth regulators (PGRs) and acaricides. T*e
compounds can be applied in the form of dusts, granules, solutions,
emulsions1 wettable powders, flowableg and suspension~. Application of a
compound of the present in~ention as herbicide~ ;s made according to
conventional procedure to the weeds or their locus using an herbicidally
effective amount of the compounds, usually from abou~ one-ten~h or les~ to
ten pounds per acre. Th application of a compound of the present
invantion to the nlocus~ of the weed includes application to the seeds, the
plant (weed) or parts of the plant, or the soil.
Applicatlon of a compound of the present invention as an acaricide
is made according to conventional procedure to the site of infestation
using an acaricidally effective amount of the compound, usually 100 g/ha
to 1 kg/ha.
The term "herbicide," as used herein, refers to an active ingredient
~0 which modifies the growth of plants becaus~ of phytotoxic or plant growth
regulatin~ properties so as to retard the growth of the plant or damage the
plant sufficiently to kill it.
Compounds of the present invention, when applied as either post or
pre-emer~ents, demonstrate high levels of herbicidal acti~ity on broadleaf,
~rass and sedge weeds. They also exhibit s~lectivity in wheat, corn,
cotton, some varieties of soybean and in some case~, ric~.
In the use of the compounds of formula I for combatting weeds and
acari, a compound of formula I, or mixtures thereo~, can conveniently be
employed as composltions in association with acceptable diluent(s) for
application to the weed, acarl or their loci. Such compositions also form
part of the present invention.
Methods of preparing suitable formulations which can be used with a
compound of the present invention are described in the lieer~ture along
with suitable liquid and solid carriers, such a~ in U.S. Patent 4,192,669
and 4,163,661, which are incorporated herein by reference. The optimum
usage of a compound of the present inven~ion is readily determinablP by one
of ordinary skill in the art using routine testing such a~ ~reenhouse
tes~ing and small plot testing.
Sui~able ~ormulations contain from 0.01 ~o 99% by weight o active
ingredient, from 0 to 20~ of ~ur~actant and fzom 1 to 99.99~ of qolld or
liquid diluent(s). Higher ratio~ of surfactant to active ingredient are
sometimes deairable and are achieved by incorporation into the formulation
or by tank mixlng. Application forms oP a composition generally contain
between 0.01 and 254 by weight of activs ingredient. Lower or higher
~ ' '
' ' '
~07~3~
-8- 133-0669/B
levels of active ingredient can, of course, be present depending on the
intended use, the physlcal properties of the compound and the mode of
application. Concen~rate forms of a compositlon intended to be diluted
before use generally contain between 2 and 90~, preferably between 5 and
81~ by weight of active ingredient.
Useful formula~ions of the compounds of formula I include dusts,
granules, suspension concentrates, wettable powders, flowables and the
like. They are obtained by conventional manner, e.g. by mixing a compound
of formula I with the diluent(s) and optionally with other ingredients.
Alternatively, the compounds of formula I may be used in
micro-encapsulated form.
The compounds of for~ula I can be combined with a cycLodextrin to
make a cyclodextrin inclusi~n complex for apylication to the weed, acari
or their loci.
Agriculturally acceptable additives may be employed in the herbicidal
compositions ~o i~prove the perform~nce of the actlve ingredient and to
reduce foaming, ca~ing and corrosion, for example.
"Surfactant" as used herein means an agriculturally acceptable
material which imparts emulsifiability, spreading, wetting, dlspersibility
or other surface-modifying properties. Examples of surfactants are sodium
lignin sulfonate and lauryl sulfate.
"Diluent" as used herein means a liquid or solid agriculturally
acceptable material used to dilute a concentratsd material to a usable or
desirable strength. For dusts or granule~ it can be e.g. talc, kaolin or
diatomaceous earth, for liquid concentrate for~s for example a hydrocarbon
such as xylene or an alcohol such as isopropanol, and for liquid
application forms e.g. water or diesel oil.
The compositions of this inventio~ can also compris~ other compounds
having biological activity, e.g, compounds having similar or complementary
acaricidal or herbicidal activity for broadsyectrum weed control or
compounds having an~idotal, fungicidal, insecticidal or insect attractant
activity.
The following examples are provided to illustrate the practice of ths
present invention. Temperature is giYen in degrees Centigrade. RT means
~5 room temperature. Parts and percen~a~es are by weigh~.
2~7~3~
-9- 133-0669/B
E~AMpT.F 1
Preparation of 2,6,6-trimethyl-4-(2-nitro-4-trifluoromethoxybenzoyl)-
2H-1,2-oxazine-3,5(4H, 6H)-dione (Formula I Rl~R2-R3~CH3; R"-R~ H;
R~NOz; R5 OCF3: Compound No. 1)
71.4 g of 2,6,6-trimethyl-5-(2-nitro-4-trifluoromethoxybenzoyloxy)-
6H-1,2-oxazine-3-one is dissolved in 300 ml of acetonitrile and 49 ml of
triethylamine. To this solu~ion, cooled in a water bath, is add0d 3.2 ml
of ace~one cyanohydrin. The resul~in~ solution is stirred at r.t., under
nitrogen, overnight. The reaction mixture ls concentrated to a syrup an~
the syrup is portioned between 400 ml of water and 150 ml of dlchloro
methoxy. The combined extracts are washed with 2N HCl, brine, dri0d and
evaporated to dryness to give oily residue. The crude product is then
crystsllized from ethanol to yield 2,6,6-trimethyl-4-(2-nitro-4-trirlu~ro-
methoxybenzoyl)-2H-1,2-oxazine-3,5(4H, 6H)-dione, m.p. 73-75.5.
Proceeding analogously to Exa~ple 1 the following compounds of
formula I are obtainPd.
~ABLE A
Cpd Rl R2 ~3 R4 R Rs R6 m.~.
1 CH3 CH3 CH3 H NO2 4-OCF3 H 73-75.5
2 CH3 CH3 CH3 ~ Cl 4-OCHF2 H 95-970
E~AMPLE 2
Preparation of 2-nitro-4-trifluoromethoxybenzonitrile
To a stirred solution of 64.30 g of 2-nitro-4-trifluoromethoxyaniline
(Zhur. Obshchei. Khim 31, 915-24 (1961)) in 72 ml of concPntrated
hydrochloric acid and 190 ml of water, cooled to 0 i9 added dropwise over
3S min. a solution of 20.0 g of sodium nitrite in 75 ml of water. After
stirrlng at 0C for an addition~l 45 min., the diazonium salt solution is
added dropwise, in portions, over 75 min. to a ~tirred solution of 82.69
g o~ potassium cyande and 46.62 g of cupric sulfate in 400 ml of water at
60-65C. The gray-greenish mixture is stirred and heated at 60-65C for
an additional 30 min and then allowed to cool to r.t. The mixture is
filtered through celite and the precipita~e is washed with dichloromethane
(3 x 200 ml). The dichloromethane washings ~re used to sxtract the aqueous
solutlon. The combined sx~racts are washed with water, br~ne, dried and
evaporated to dryness to give an oily resldu~ which i~ chro~atographed on
silica gel to give 31.44 g of 2-nitro-4-trifluoromethoxybenzonitrile.
.
!
2~P~2~13~
-10- 133-0669/B
E~AMPLE 3
Preparation of 2-nitro-4-trifluoromethoxy benzoic acid
A stirred solution of 40.96 g of 2-nitro-4-trlfluorobenzonitrilz in
90 ml of concentrated sulfuric acid and 125 ml of water is refluxed for 45
hrs. The reaction mixture is cooled and dlluted with 200 ml of cold water.
The aqueous supernatant is decanted from th0 brown cryust~lline gu~. The
crystalline gum is dissolved in ether and extracted with 0.5 N sodium
hydroxide solution. The combined basic aqueous solution is cooled and
acidified with concentrated hydrochloric acid and then extracted wlth
ether. The combined extracts are dried and evaporated to dryness to give
crystalline 2-nitro-4-trifluoromethoxybenzoic acid m.p. 119C.
E~AMPLE 4
Preparationof2,6,6-trimethyl-5-(2-nitro-4-trifluoromethoxybenzoyl-
oxy)-6H-1,2-oxazine 3-one (Formula II Rl-R2-R3-CH3; R4-R6-~H; R-N02;
R5-OCF3
A stirred solution of 43.98 g of 2-nitro-4-trifluoromethoxybenzoic
acid in 100 ml of thionyl chloride is refluxed for 2 hrs and then
concentrated to dryness to give oily 2-nitro-4-tr~fluoromethoxybenzoyl
chloride. To a stirred solution of 27.53 g of 2,6,6-trimethyl-2H-1,2-
oxazine-3,5(4H, 6H)-dione and 35 ml (25/mm) of triethylamine in 290 ml of
dichloromethane, cooled in an ice bath, under nitrogen, is added dropwise
a solution of 2-nitro-4-trifluoromethoxybenzoyl chloride in 100 ml of di-
chloromethane. After s~irring at 0C at r.t. for 2 hrs., the reaction
mixture is diluted with 150 ml of dichloromethane and the solution is
washed with water, brine, dried and evaporated to dryness to give 2,6,6-
2,6,6-trimethyl-5-(2-nitro-4-trifluoromethyoxybenzoyloxy)-6H-1,2-oxazine-3-
one.