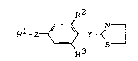Note: Descriptions are shown in the official language in which they were submitted.
207~21 7
The invention relates to 2-(4-substituted phenylhydra-
zino)-2-thiazolines and 2-(4-substituted phenylazo)-
2-thiazolines, some of which are new, to processes for
their preparation, and to their use for combating ecto-
parasites.
It has already been disclosed that 2-phenylhydrazino-
2-thiazolines and 2-phenylhydra~o-2-thiazolines, each of
which is substituted in the benzene ring, have ectopara-
sitic action (DE-OS (German Published Specification)
3,133,918; US Patent 4,046,753).
The present invention relates to
1. The use of thiazolines of the general formula (I)
R2
Rl-Z ~ r--~S ~ (I)
in which
Y represents -NH-N~- or -N=N- and
Z repr~sents a direct bond, O, S, SO or SO2, and
Rl represents halogen-substituted alkyl, alkyl-
sulphonyl, or represents optionally substituted
Le A 28 434 - 1 -
phenyl, and 2 ~' 7 ~ ~ ~ 7
R2 and R3 represent identical or different radicals
from the series comprising hydrogen, halogen,
halogen-substituted alkyl, CN or NO2,
for combating ectoparasites.
2. Thiazolines of the general formula (I)
R2
R1 z ~ y~
R3
in which
Y represents -NH-NH- or -N=N- and
10Z represents a direct bond, O, S, SO or SO2, and
Rl represents halogen-substituted alkyl, alkyl-
sulphonyl, or reprssents optionally substituted
pher.yl, and
R2 and R3 represent identical or different radicals
15from the series comprising hydrogen, halogen,
halogen-substituted alkyl, CN or NO2.
3. Processes for the preparation of the thiazolines of
the formula I in which Y represents the group
Le A 28 434 - 2 -
. _ . . . . . _ . . _
-NH-NH-, characterised in that 2 ~ 7 2 2 i 7
a) phenylhydrazines of the formula (II)
R2
R 1 _ z~NH -NH2 ( I I )
in which
R~, R2, R3 and Z have the meaning given under 2.
are reacted with thiazolines of the formula
(III)
~N
S~S - Ra
in which
Ra denotes an optionally substituted alkyl
radical,
in the presence of strong acids or
b) thiosemicarbazides of the formula (IV)
Le A 28 434 - 3 -
2~7221 7
R2
. ~ H~ Nh-C~2-CH2-X (IV)~
R3
in which
R1, R2, R3 and Z have the abovementioned
meaning,
X denotes a hydroxyl group, an alkyl-
sulphonyloxy or arylsulphonyloxy group,or
a halogen atom,
are cyclised, if appropriate in the presence of
a strong acid, or
c) isothiosemicarbazides of the formula (V)
RZ
Rl_z ~ NH~NH~6~5CH2CHZNHZ (V)
R3 NH
in which
R1l R2, R3 and Z have the abovementioned
meaning,
are cyclised, if appropriate in the presence of
a strong acid, or
Le A 28 434 - 4 -
2~72217
d~ in the event that Y represents the azo group
-N=N-,
compounds of the formula (I) in which Y represents
a hydrazino group and Rl, R2, R3 and Z have the
abovementiohed meaning, are dehydrogenated with the
aid of oxidants.
In formula (I),
Y preferably represents a direct bond, O, S, SO or SO2,
R1 preferably represents 1-9 halogeno-Cl~-alkyl,
C14-alkylsulphonyl, or represents optionally sub-
stituted phenyl,
R2 and R3 preferably r~present hydrogen, chlorine, fluo-
rine, bromine, 1-5-halogeno-C14-alkyl, CN or NO2.
In formula (I),
Y particularly preferably represents a direct bond~ 0,
S, SO or SO2,
Rl particularly preferably represents 1-7-halogeno-
Cl3-alkyl, methylsulphonyl, or represents optionally
substituted phenyl,
R2 and R3 particularly preferably represent hydrogen,
Le A 28 434 - 5 -
20722~l 7
chlorine, fluorine, bromine, 1-5-halogeno-C1z-alkyl,
CN or NO2.
Y represents a direct bond, O, S, SO, SO2,
R~ represents CF3, CF2Cl, C~Cl2, CF2E~r, CCl3, CHF2,
CF3CH~, CF3CF2, CHFCi-CF2, CHF2CF2, CH2FCF2, CF3CHF,
CF3CHCl, CF3CCi2, CFCl2-CF2, CF2Cl-CF2, CF3CFCl,
(CHF)2CH, (CF3~2CH, CH2Cl-CH-CH2F, CH3-CH-CH2F or
optionally substituted phenyl,
R2 and R3 represent hydrogen, fluorine, chlorine, bromine,
CN, ~02 or CF3.
Substituents of the optionally substituted radicals which
may be mentioned are:
one or more identical or different radicals from the
group comprising Cl-C4-alkyl, in particular methyl or
ethyl, C1-C4-alkoxy, in particular methoxy, ethoxy,
methylenedioxy or ethylenedioxy, each of which is option-
ally substituted by fluorine or chlorine, Cl-C~-halogeno-
alkoxy, in particular trifluoromethoxy, fluorochloro-
ethoxy, C1-C~-alkylthio, in particular methylthio, Cl-C4-
halogenoalkylthio, in particular trifluoromethylthio,fluorochloromethylthio, Cl-C4-alkylsulphonyl, in par-
ticular methylsulphonyl, Cl-C4-halogenoalkylsulphonyl, in
particular trifluoromethylsulphonyl, Cl-C4-halogenoalkyl,
in particular trifluoromethyl, halogen, in particular
fluorine or chlorine, NO2 , CN, amino, ClC4-alkyl- or
Le A 28 434 - 6 -
~7221 7
dialkylamino, C1-C4-halogenoalkylamino, acylamino, in
particular acetylamino, or phenyl, phenylthio or phenoxy,
each of which is optionally substituted by one of the
abovementioned radicals.
The following compounds of the formula (I) may be men-
tioned individually:
2-[(4-trifluoromethylphenyl)hydrazino~-2-thiazolines
2-~((2,4-bis-trifluoromethyl)phenyl)hydrazino]-2-thia-
zoline
2[(2-trifluoromethyl-4-methanesulphonylphenyl)hydrazino]-
2-thiazoline
2-[(2-trifluoromethyl-4-trifluoromethanesulphonylphenyl)-
hydrazino]-2-thiazoline
2-[(4-(1,1,2-trifluoro-2-chloroethyl)sulphonylphenyl)-
hydrazino]-2-thiazoline
2-[(2-trifluoromethyl-4-phenylsulphonylphenyl)hydrazino]-
2-thiazoline
2-[(2,6-dichloro-4-trifluoromethanesulphonylphenyl)-
hydrazino]-2-thiazoline
2-[(2-chloro-6-fluoro-4-trifluoromethylphenyl)hydrazino]-
2-thiazoline
2-[(2-chloro-4-trifluoromethanesulphonylphenyl)-
hydrazino]-2-thiazoline
2-[(4-trifluoromethoxyphenyl)hydrazino]-2-thiazoline
2-[(2-chloro-4-trifluoromethylphenyl)hydrazino]-2-thiazo-
line
2-[(4-trifluoromethanesulphonylphenyl)hydrazino]-2-thia-
zoline
Le A 28 434 - 7 -
2~22~ 7
2-[(2-chloro-3-fluoro-4-trifluoromethyl)hydrazino]-
2-thiazoline
2-[(2-chloro-4-trifluoromethanesulphonylphenyl)-
hydrazine]-2-thiazoline
2-[(2,6-dichloro-4-trifluoromethylphenyl)hydrazino]-
2-thiazoline
2-[(4-trifluoromethylthiophenyl)hydrazinoj-2-thiazoline
2-[(4-difluoromethylthiophenyl)hydrazino]-2-thiazoline
2-~(4-difluoromethanesulphonylphenyl)hydrazino]-2-thiazo-
line
2-[(4-(2,2,2-trifluoroethoxy)phenyl)hydrazino]-2-thiazo-
line
2-[(4-difluoromethoxyphenyl)hydrazino]-2-thiazoline
2-[(4-difluorochloromethoxyphenyl)hydrazino]-2-thiazoline
2-[(4-(1,3-difluoro-2-propoxy)phenyl~hydrazino]-2-thiazo-
line
2-[(4-(1-fluoro-2-propoxy)phenyl)hydrazino]-2-thiazoline
2-[(4-(2,2,2-trifluoroethylthio)phenyl)hydrazino]-2-thia-
zoline
2-[(4-pentafluoroethoxyphenyl)hydrazino]-2-thiazoline
2-[(4-(2-fluoroethoxy)phenyl)hydrazino]-2-thiazoline
2-~(4-(1,1,2-trifluoro-2-chloroethylthio)phenyl)-
hydrazino~-2-thiazoline
2-[(4-(1,1,2-trifluoro-2,2-dichloroethoxy)phenyl)-
hydrazino]-2-thiazoline
2-[(4-(1,1,2-trifluoroethoxy)phenyl)hydrazino]-2-thiazo-
line
2-[(4-(perfluoro-2-propyl)phenyl)hydrazino]-2-thiazoline
2-[(4-difluorobromomethyl)phenyl)hydrazino]-2-thiazoline
2-[(1-trifluoromethylphenyl)azo3-2-thiazoline
Le A 28 434 - 8 -
2 ~ 7 ~ 7
2-[((2,4-bistrifluoromethyl)phenyl)azo]-2-thiazoline
2-[(2-trifluoromethyl-4-methanesulphonylphenyl)azo]-
2-thiazoline
2-(2-trifluoromethyl-4-trifluoromethanesulphonylphenyl)-
azo]-2-thiazoline
2-[(4-(1,1,2-trifluoro-2-chloroethyl3sulphonylphenyl~-
azo]-2-thiazoline
2-[(2-trifluoromethyl-4-phenylsulphonylphenyl)azo]-
2-thiazoline
1~ 2-[(2,6-dichloro-4-trifluoromethanesulphonylphenyl)azo]-
2-thiazoline
2-[(2-chloro-6-fluoro-4-trifluoromethylphenyl)azo]-
2-thiazoline
2-[(2-chloro-4-trifluoromethanesulphonylphenyl)azo]-
2-thia~oline
2-tl4-trifluoromethoxyphenyl)-azo~-2-thiazoline
2-[(2-chloro-4-trifluoromethylphenyl)azo]-2-thiazoline
2-[(4-trifluoromethanesulphonylphenyl)azo] 2-thiazoline
~ 2-[(2-chloro-3-fluoro-4-trifluoromethyl)azo]-2-thiazoline
2-[(2-chloro-4-trifluoromethanesulphonylphenyl)azo]-
2-thiazoline
2-~(2,6-dichloro-4-trifluoromethylphenyl)azo]-2-thiazo-
line
2-[(4-trifluoromethylthiophenyl)azo3-2-thiazoline
2-tt4-difluoromethylthiophenyl)azo]-2-thiazoline
2-[(4-difluoromethanesulphonylphenyl)azo]-2-thiazoline
2-[(4-(2,2,2-trifluoroethoxy)phenyl)azo]-2-thiazoline
2-[(4-difluoromethoxyphenyl)azo]-2-thiazoline
2-[(4-difluorochloromethoxyphenyl)azo]-2-thiazoline
2-[(4-(1,3-difluoro-2-propoxy)phenyl)azo]-2-thiazoline
Le A 28 434 - 9 -
2 ~ 7 2 2 ~ ~
2-[(4-(1-fluoro-2-propoxy)phenyl)azo]-2-thiazoline
2-[(4-(2,2,2-trifluoroethylthio)phenyl)azo]-2-thiazoline-
4-methanesulphonyl
[(4-pentafluoroethoxyphenyl)azo]-~-thiazoline
2-[(4-(2-fluoroethoxy)phenyl)azo]-2-thiazoline
2-[(4-(1,1,2-trifluoro-2-chloroethylthio)phenyl)azo]-
2-thiazoline
2-[(4-(1,1,2-trifluoro-2,2-dichloroethoxy)phenyl)-azo]-
2-thiazoline
2-[(4-(1,1,2-trifluoroethoxy)phenyl)azo]-2-thiazoline
2-[(4-(perfluoro-2-propyl)phenyl)azo]-2-thiazoline
2-[(4-difluorobromomethyl)phenyl)azo]-2-thiazoline
The compounds of the formula (I) are prepared for example
by process 3a), in which 2-trifluoromethyl-4-methane-
sulphonylphenylhydrazine is reacted with 2-methyl-
mercapto-2-thiazoline, as shown by the equation
CF3
CH3025~--NH NH2 t ~ .
SMe
CF3
CH3502 ~ NH-NH--~
The compounds of the general formula (II) and (III~ are
reacted in the presence of diluents at temperatures
Le A 28 434 - 10 -
~ Q 7 ~ 2 ~ ~i
between 20 and 200C, preferably between 40 and 160C,
most advantageously at the reflux temperature of the
diluent employed.
Suitable diluents are all inert organic solvents. These
include, in particular, aliphatic and aromatic,
optionally halogenated hydrocar~ons, such as pentane,
hexane, heptane, cyclohexane, petroleum ether, benzine
ligroine, benzene, toluene, methylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, chlorobenzene
and o-dichlorobenzene, furthermore ethers such as diethyl
ether and dibutyl ether, glycol dimethyl ether and
diglycol dimethyl ether, tetrahydrofuran and dioxane,
furthermore ketones such as acetone, methyl ethyl ketone,
methyl isopropyl ketone and methyl isobutyl ketone,
moreover esters, such as methyl acetate and ethyl
acetate, furthermore nitriles such as, for example,
acetonitrile and propionitrile, benzonitrile, glutaro-
nitrile, in addition amides such as, for example,
dimethylformamide, dimethyl acetamide and N-methyl-
pyrrolidone, and also dimethyl sulphoxide, tetramethylenesulphone and hexamethylphosphoric triamide and alcohols
such as methanol, ethanol, 2-propanol, 3-propanol as well
as mixtures thereof, also aqueous mixtures thereof.
The compounds o~ the formulae (II) and (III) are employed
in equimolar amounts, a small excess of one or the other
component does not provide any essential advantages.
Working-up is carried out in a manner known per se, for
Le A 28 434 - 11 -
2 ~3 7 ~ 7
example by treating the reaction mixture with water,
separating the organic phase, removing the solvent and
purifying the product by chromatography or recrystallisa-
tion. In individual cases, the crystalline product which
has precipitated directly after cooling of the reaction
mixture can be filtered off and then, if appropriate,
purified.
The compounds of the formula (III) are known (see, for
example, Tetrahedron Lett. 1971, 4359).
The compounds of the formula (I) can furthermore be
prepared for example by cyclising 4-(2-bromoethyl)-
1-(2-trifluoromethyl-4-phenylsulphonyl)-thiosemicarbazide
in accordance with process 3b:
CF3
~SC2~NH-NH-C-NH-CH2-CH2-E~r
CF3
~3SOz~NH-NH--< ~
The compounds of the general formula (IV) are cyclised in
lS the presence of diluents at temperatures of between 20
and 180C, preferably between 30 and 160C, and most
advantageously at the reflux temperature of the diluent
Le A 28 434 - 12 -
2a~22~'~
employed.
Suitable diluents are all inert organic solvents. These
include, in particular, aliphatic and aromatic,
optionally halogenated hydrocarbons such as pentane,
hexane, heptane, cyclohexane, petroleum ether, benzine,
ligroine, ~enzene, toluene, methylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, chlorobenzene
and o-dichlorobenzene, furthermore ethers such as diethyl
ether and dibutyl ether, glycol dimethyl ether and
diglycol dimethyl ether, tetrahydrofuran and dioxane,
furthermore ketones such as acetone, methyl ethyl ketone,
methyl isopropyl ketone and methyl isobutyl ketone,
moreover esters such as methyl acetate and ethyl acetate,
furthermore nitriles such as, for example, acetonitrile
ancl propionitrile, benzonitrile, glutaronitrile, in
addition amides such as, for example, dimethylformamide,
dimethylacetamide and N-methyl-pyrrolidone, and also
dimethyl sulphoxide, tetramethylene sulphone and hexa-
methylphosphoric triamide and alcohols such as methanol,
ethanol, 2-propanol, 3-propanol, as well as mixtures
thereof, also aqueous mixtures thereof.
Working-up is carried out in a manner known per se. As a
rule, the compounds precipitate after cooling of the
reaction mixture as crystals in the form of their salts,
salt~ being formed with those acids which originate
during the reaction. When X represents chlorine, bromine
or arylsulphonyloxy, these acids are, in particular,
hydrochloric acid, hydrobromic acid or arylsulphonic
Le A 28 434 - 13 -
2~72~ ~ ~
acid. Liberation takes place by dissolving the salts in
water, if appropriate under hot conditions, and subse-
quently treating this solution with bases.
The following may be mentioned as bases: alkali metal
alcoholates, alkaline earth metal alcoholates and
tertiary amines. The following bases may be mentioned as
being particularly preferred: triethylamine, pyridine,
picolines, trimethylamine, N-methylmorpholine, N~ethyl-
pyrrolidine, diazabicyclo(4,3,0)-uncecene (DBU),
1,4-diaza-bicyclo-2,2,2-octane (DABCO), diazabicyclo-
(3,2,0)nonene (DBN).
The product which has precipitated under cold conditions
is subsequently filtered off with suction, dried and
purified by chromatography or by recrystallisation,
following known methods.
Some of the compounds of the general formula (IV) are
new. They are obtained in a manner known per se, by
reacting isothiocyanates of the formula (VI)
X-CH2-C~2-NCS (VI),
in which X has the abovementioned meaning
with substituted phenylhydrazines of the general formula
(II).
Isothiocyanates of the formula (VI~ are known (see, for
Le A 28 434 - 14 -
2~72~ ~ 7
example, Chem. Lett. 1989, 965).
Phenylhydrazines of the formula (II) are known or can be
prepared by known processes (Houben-Weyl Vol. X/2 (1967),
177 et seq.).
Compounds of the formula (IV) can be prepared, for
example, following processes as are described in Chem.
Lett. 1989, 965 or Can. J. Chem. 49 (1971), 971.
The compounds of the formula (I) can furthermore~ be
prepared by cyclising an isothiosemicarbazide in accor-
dance with the equation
CF~
CF~SO2 ~ H-NH- 6 - s CH2 - CH2NH2
NH
CF3
CF3S02~NH-NH~ ~
for example following process 3c.
To this end, compounds of the formula (V) are reacted in
the presence of diluents between 20 and 200C, pre-
ferably between 40 and 160C, most advantageously at the
reflux temperature of the solvent employed. The solvents
Le A 28 434 - 15 -
2~72217
are mentioned further below in connection with the
particularly preferred embodiment.
Working-up is carried out in a manner known per se, for
example by treating the reaction mixture with water,
separating the organic phase, removing the solvent and
purifying the crude product obtained by chromatography or
recrystallisation. In the event that the product precipi-
tates directly after cooling of the reaction batch, it is
filtered off and, if appropriate, subsequently purified
by chromatography or crystallisation.
In the event that the compounds of the formula (V) are
employed in the form of a salt, for example the hydro-
bromide or hydrochloride, the end products are initially
also obtained as the hydrobromide or hydrochloride. In
lS these cases, the end product is liberated following the
procedure described under process 3b.
The compounds of the general formula (V) are known or can
be prepared by processes known per se (see, for example,
Houben-Weyl IX (1955), 912-913). This preparation is
carried out, for example, by reacting the thiosemicar-
bazides of the general formula (VII) with, for example,
2-bromoethylamine or 2-chloroethylamine.
Le A 2B 434 16 -
2~72~
The thio~emicarbazides of the formula (VII)
R2
Rl-~NH-NH-C~ tVlI),
~( 3 NH2
R
in which Rl, R2, R3 and Z have the abovementioned meaning
are prepared by reacting phenylhydrazines of the formula
(II) with thiocyanic acid which has preferably been
prepared in situ (see, for example,` Indian J. Chem. 233
(1983), 1243). The phenylhydrazines (II) are prepared by
the process cited under process 3b.
In its preferred embodiment, process 3c is carried out as
a single-step process. For example, the 2-trifluoro-
methyl-4-trifluoromethanesulphonylphenylthiosemicarbazide
is reacted with 2-bromoethylamine to give the thiazoline
according to the equation:
CF35O2 ~ NH-NH-C~ ~ Br-CHzCHz-NH2 Hsr
NH2
( CH3 ) 2CH~H ~CF3 N
CF3S02 <~ )--NH-NH~
Ref lux `--' S
The compounds of the general formula (VII) are reacted
out in the presence of an inert organic solvent at
temperatures from 20 to 220C, preferably at 40 to
200C, particularly preferably at the specific reflux
temperature of the solvent or solvent mixture employed.
Le A 28 434 - 17 -
2~72217
Suitable diluents are all inert organic solvents. These
include, in particular, aliphatic and aromatic,
optionally halogenated hydrocarbons such as pentane,
hexane, heptane, cyclohexane, petroleum ether, benzine,
ligroine, benzene, toluene, methylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, chlorobenzene
and o-dichlorobenzene, furthermore ethers such as diethyl
ether and dibutyl ether, glycol dimethyl ether and
diglycol dimethyl ether, tetrahydrofuran and dioxane,
furthermore ketones such as acetone, methyl ethyl ketone,
methyl isopropyl ketone and methyl isobutyl ketone,
moreover esters such as methyl acetate and ethyl acetate,
furthermore nitriles such as, for example, acetonitrile
and propionitrile, benzonitrile, glutaronitrile, in
addition amines such as, for example, dimethylformamide,
dimethylacetamide and N-methyl-pyrrolidone, and also
dimethyl sulphoxide, tetramethylene sulphone and hexa-
methylphosphoric triamide and alcohols such as methanol,
ethanol, 2-propanol, 3-propanol, 4-butanol and 5-pentanol
as well as mixtures thereof, also aqueous mixtures
thereof.
Working-up is carried out in a manner known per se. As a
rule, the compounds are obtained after cooling of the
reaction mixture as crystals and are purified after
filtration, for example by chromatography or crystallisa-
tion. If bromoethylamine or chloroethylamine are employed
in the form of their hydrobromides or hydrochlorides, the
end products are also initially obtained in the form of
Le A 28 434 - 18 -
2~7?,~ L7
the hydrobromides or hydrochlorides. To liberate the end
products, the salts are dissolved in water, if appro-
priate under hot conditions, and this solution is sub-
sequently treated with bases.
The following may be mentioned as bases: alkali metal
alcoholates, alkaline earth metal alcoholates and
tertiary amines. The following bases may be mentioned as
being particularly preferred: triethylamine, pyridine,
picolines, trimethylamine, N-methylmorpholine, N-ethyl-
pyrrolidine, diazabicyclo(4,3,0)-uncecene (DBU),
1,4-diaza-bicyclo-2,2,2-octane (DABCO), diazabicyclo-
(3,2,0)nonene (DBN).
The product which has precipitated under cold conditions
is subsequently filtered off with suction, dried and
further purified, for example by chromatography or by
recrystallisation, following known methods.
The compounds of the general formula (VII) and, for
example, bromoethylamine or chloroethylamine/ or their
hydrohalides are employed in equimolar amounts, a small
excess of one or the other component does not provide any
essential advantages.
Compounds of the general formula (I) in which Y denotes
an -N=N- (Azo) group are obtained by reacting 2-[(2-tri-
fluoromethyl-4-trifluoromethanesulphonylphenyl)-
hydrazino]-2-thiazoline with an oxidant such as, for
example, hydrogen peroxide, to give 2-[(2-trifluoro-
Le A 28 434 - 19 -
2~7~2~7
methyl-4-trifluoromethanesulphonyl phenyl)azo]-2-thiazo-
line, for example in accordance with process 3d, fol-
lowing the equation
CF3 N toxida~ion] CF? N
C F 3 5 2--<~NH - NH~ ~ N = N~
S CF3-502 5
The reaction is carried out for example by dehydrogena-
S ting compounds of the general fonmula (I) where Y is-NH-NH-, using an oxidant, if appropriate in an inert
organic solvent.
Examples of suitable oxidants are metal oxides such as
silver oxide, lead oxide, mercury, selenium oxide as well
as atmospheric oxygen, iron~III) chloride, lead tetra-
cetate, mercuxy acetate, sodium dichromate, copper
sulphate,potassium hexacyanoferrate, nitrous acid, dilute
or fuming nitric acid, sodium persulphate, sodium hyypo-
- chloride, permanganates, elemental sulphur, sodium
perborate, hydrogen peroxide and chromic acid.
Suitable diluents are all inert organic solvents. These
include, in particular, aliphatic and aromatic,
optionally halogenated hydrocarbons such as pentane
hexane, heptane, cyclohexane, petroleum ether, benzine,
ligroine, benzene, toluene, methylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, chlorobenzene
Le A 28 434 - 20 -
2~722~ ~
and o-dichlorobenzene, furthermore ethers such as diethyl
ether, and dibutyl ether, glycol dimethyl ether and
diglycol dimethyl ether, tetrahydrofuran and dioxane,
furthermore ketones such as acetone, methyl ethyl ketone,
methyl isopropyl ketone and methyl isobutyl ketone,
moreover esters, such as methyl acetate and ethyl
acetate, furthermore nitriles such as, for example,
acetonitrile and propionitrile, benzonitrile, glutaro-
nitrile, in addition alcohols, such as methanol, ethanol,
2-propanol, 3-propanol, butanol and pentanol, and mix-
tures thereof, also aqueous mixtures thereof. The pH of
the reactions can be varied within wide limits. For
example, can be carried out in acid medium such as, for
example, glacial acetic acid, but also in basic medium
such as, for example, aqueous sodium hydroxide solution.
The reaction temperatures are between -30 and +180C,
preferably between 0 and 110C. The oxidation methods
are reviewed in Houben-Weyl, Vol. X/3 (1965), pages
371-380. Working-up is carried out in a manner known per
se, for example, aqueous/by extraction or separation of
the reaction products after neutralisation by extraction
or filtration. The product can be purified further for
example by chromatography or neutralisation.
The active substances are suitable for combating animal
pests such as arthropods, preferably insects and arach-
nids, which occur in animal keeping and animal breeding
in domestic animals and productive livestock, as well as
zoo animals, laboratory anLmals, experimental animals and
Le A 28 434 - 21 -
2~722~7
pets, while having a favourable toxicity to warm-blooded
species. In this context, they are active against all or
individual development stages of the pests and against
resistant and normally-sensitive pest species.
Combating the animal pests is intended to prevent disease
and the transmission thereof, deaths, and reduced perfor-
mance (for example in the production of meat, milk, wool,
hides, eggs), so that the use of the active compounds
allows more economical and simpler animal keeping, or is
only made possible in certain areas.
The pests include:
From the order o~ the Anoplura, for example Haematopinus
spp., Linognathus spp., Solenopotes spp., Pediculus spp.,
Pthirus spp.;
from the order of the Mallophaga, for example Trimenopon
spp., Menopon spp., Eomenacanthus spp., Menacanthus spp.,
Trichodectes spp., Felicola spp., Damalinea spp.,
Bovicola spp;
from the order of the Diptera, for example Chrysops spp.,
Tabanus spp., Musca spp., Hydrotaea spp., Muscina spp.,
Haematobosca spp., Haematobia spp., Stomoxys spp., Fannia
spp., Glossina spp., Lucilia spp., Calliphora spp.,
Auchmeromyia spp., Cordylobia spp., Cochliomyia spp.,
Chrysomyia spp., Sarcophaga spp., Wohlfartia spp.,
Gasterophilus spp., Oesteromyia spp., Oedemagena spp.,
Hypoderma spp., Oestrus spp., Rhinoestrus spp.,
Melophagus spp., Hippobosca spp..
Le A 28 434 - 22 -
2Q72~1 ~
From the order of the Siphonaptera, for example
Ctenocephalides spp., Echidnophaga spp., Ceratophyllus
spp..
From the order of the Metastigmata, for example Hyalomma
5 sPP- r Rhipicephalus spp., Boophilus spp., Amblyomma spp.,
Haemophysalis spp., Dermacentor spp., Ixodes spp., Argas
spp., Ornithodorus spp., Otobius spp.;
from the order of the Mesastigmata, for example
Dermanyssus spp., Ornithonyssus spp., Pneumonyssus spp..
From the order of the Prostigmata, for example
Cheyletiella spp., Psorergates spp., Myobia spp., Demodex
spp., Neotrombicula spp.;
from the order of the Astigmata, for example Acarus spp.,
Myocoptes spp., Psoroptes spp., Chorioptes spp., Otodec-
15 tes spp., Sarcoptes spp., Notoedres spp., Knemidocoptesspp., Neoknemidocoptes spp. Lytodites spp., Laminosioptes
spp..
The domestic animals and productive livestock include
mammals such as, for example, cattle, sheep, goats,
horsesl pigs, dogs, cats, camels, water buffaloes,
donkeys, rabbits, fall~w deer, reindeer, pett-bearing
animals such as, for example, minks, chinchilla and
raccoon, and birds such as, for example, chickens,
turkeys, pheasants, geese and ducks.
25 Laboratory and experimental animals include, for example,
mic~, rats, guinea pigs, golden hamsters, dogs and cats.
Le A 28 434 ~ 23 ~
, _ . . . _
20722~
The pets include, for example, dogs and cats.
The administration can be carried out both prophylacti-
cally and therapeutically.
The active compounds are administered directly or in the
form of suitable preparations enterally, parenterally,
dermally, nasally, by environment treatment, or with the
aid of active-compound-containing shaped articles such
as, for example, strips, plates, tapes, collars, ear
tags, limb bands, markin~ devices.
Enteral administration of the active compounds is effec-
ted, for example, orally in the form of powders, tablets,
capsules, pastes, boli, drinks or granules, or solutions,
suspensions or emulsions which can be administered
orally, medicated feed or drinking water. Dermal adminis-
tration is effected, for example, in the form of dipping,spraying or pouring-on and spotting-on and powdering.
Parenteral administration is effected, for example, in
the form of injection tfor example intramuscularly,
subcutaneously or intravenously) or by implants.
Particular emphasis is given to the preparations for
dermal administration. These include solutions, suspen-
sion concentrates and emulsion concentrates, and also
microemulsions which are diluted with water prior to
administration, or pour- and spot-on formulations,
powders and dusts, aerosols and active-compound-con-
Le A 28 434 - 24 -
20722~7
taining shaped articles as well as dust bags or back
rubber.
The surface-active substances include:
emulsifiers and wetting agents such as anionic surfac-
tants, for example alkylsulphonates, alkyl sulphates,arylsulphonates, sodium lauryl sulphates, fatty alcohol
ether sulphates, monoethanolamine salt o~ mono/dialkyl-
polyglycol ether orthophosphate, calcium alkylaryl-
sulphonate;
cationic surfactants, for example cetyltrimethylammonium
chloride;
ampholytic surfactants, for example disodium N-lauryl
beta-iminodipropionate or lecithin;
non-ionic surfactants, for example polyoxyethylated
castor oil, polyoxyethylated sorbitan monooleate, poly-
oxyethylated sor~itan monostearate, glycerol mono-
stearate, polyoxyethylene stearate, alkylphenol poly-
glycol ethers, polyoxyethylated sorbitan monopalmitate,
polyoxyethylene lauryl ether, polyoxyethylene oleyl
ether, polyoxyethylene mannitan monolaurate, alkyl
polyglycol ether, oleyl polyglycol ether, dodecyl poly-
glycol ether, ethoxylated nonylphenol or isooctylphenol-
polyethoxyethanol.
The preparations can furthermore contain:
adhesion promoters~ for example carboxymethylcellulose,
methylcellulose and other cellulose and starch deriva-
tives, polyacrylates, alginates, gelatin, gum arabic,
polyvinyl pyrrolidone, polyvinyl alcohol, methyl vinyl
Le A_28 434 - 25 -
2072217
ether/maleic anhydride copolymers, polyethylene glycols,
paraffins, oils, waxes, hydrogenated castor oil,
lecithins and synthetic phospholipids.
The preparations can contain colorants such as inorganic
pigments, for example iron oxide, titanium oxide, Prus-
sian Blue, and organic dyestuffs such as alizarin, azo
and metal phthalocyanin dyestuffs.
The preparations can contain spreading agents, for
example silicone oils having various viscosities, fatty
acid esters such as ethyl stearate, di-n-butyl adipate,
hexyl laurate, dipropylene glycol pelargonate, esters of
a branched fatty acid of medium chain length with satura-
- ted fatty alcohols Cl6-Cl8, isopropyl myristate, isopropyl
palmitate, caprylic/caproic esters of saturated fatty
alcohols of chain length Cl2-C~8, isopropyl stearate, oleyl
oleate, decyl oleate, ethyl oleate, ethyl lactate, waxy
fatty acid esters, dibutyl phthalate, diisopropyl
adipate, ester mixtures relating to the latter, and
others;
triglycerides such as caprylic/caproic triglyceride,
triglyceride mixtures with vegetable fatty acids of chain
length C8-Cl~ or other specifically selected natural fatty
acids, partial glyceride mixtures of saturated or unsatu-
rated, optionally also hydroxyl-containing fatty acids,
monodiglycerides of the C~/C10-fatty acids and others;
fatty alcohols such as isotridecyl alcohol, 2-octyl-
dodecanol, cetylstearyl alcohol or oleyl alcohol.
Le A 28 434 - 26 -
20722~ 7
To produce solid preparations, the active compound is
mixed with suitahle excipients, if appropriate with the
addition of adjuvants, and the mixture is formulated as
desired.
Excipients which may be mentioned are all physiologically
acceptable solid inert substances. Substances which can
be used are inorganic and organic substances. Inorganic
substances are optionally crushed and fractioned, for
example synthetic and natural ground rocks such as
kaolins, talc, chalk, quartz, diatomaceous earth, sodium
chloride, carbonates such as calcium carbonate, hydrogen
carbonates, aluminas, silicas, clays, precipitated or
colloidal silicon dioxide, or phosphates.
Examples of organic substances are sugars, cellulose,
foodstuffs and feedstuffs such as dried milk, animal
meals, cereal meals, crushed cereals, starches or saw-
dust.
Adjuvants are preservatives, ~ntioxidants, colorants
which have already been mentioned further above.
Other suitable adjuvants are lubricants and glidinq
~gents such as, for exa~ple, magnesium stearate, stearic
acid, talc, bentonite, sukstances which promote disinte-
gration such as starc~ or crosslinked polyvinyl pyrroli-
done, binders such as, for example, starch, gelatin or
linear polyvinylpyrrolidone as well as dry binding agents
such as microcrystalline cellulose.
Le A 28 434 - 27 -
2~722~
The active compounds, in the form of their abovementioned
solid or liquid formulations, can also exist in encapsu-
lated form.
The active compounds can also be administered in the form
of an aerosol. To this end, the active compound in a
suitable formulation is distributed finely under pres-
sure.
.
It can also be advantageous to administer the active
compounds in formulations which release the active
compound in a delayed manner. Examp~es which may be
mentioned are active-compound-containing shaped articles
such as plates, tapes, strips, collars, eartags, tail
tags, limb bands, halters or marking devices. Other
formulations which may be mentioned are active-compound-
containing implants and boli.
The active compounds can also be administered togetherwith the feed and/or the drinking water.
,,
~he active compounds can exist in the formulations on
their own or as a mixture with other active compounds or
synergists.
Formulations which are administered directly contain
between 10-7 and 5 per cent by weight, preferably between
10-4 and 1 per cent by weight, of active compound.
Le A 2fl 434 - 28 -
,
.
2 1 7
Formulations which are only administered after further
dilution contain 1 - 95 per cent by weight, preferably
5 - 90 per cent by weight, of active compound.
Example A
5 In-vivo mini dip test with ticks
Test animals:
Boophilus microplus (all parasitising devPlopment
stages/larvae, metalarvae, nymphs, metanymphs, adults) of
Boophilus microplus (SP-resistant Parkhurst strain).
Solvent:
35 parts by weight of ethylene glycol monomethyl ether
35 parts by weight of nonylphenol polyglycol ether
~osts:
Before the beginning of the test, the cattle are shorn in
such a manner that hairy areas of 8 x 8 cm in size remain
on the back and on the sides. The distance of the areas
to each other is approx. 20 cm, and they are arranged so
that they are offset diagonally.
Testing procedure:
Cattle are infected 12 times at 2-day intervals with
Le A 28 434 - 29 -
2~7221'~
batches of 2000-4000 starved Boophilus microplus larvae
aged 14-28 days. The female adults which develop from day
21 - 23 after the infection are counted as a measure for
the infection pressure in each of the areas. On day 24,
the individual areas are wetted thoroughly (2 minutes)
with 200 ml of a dilution of the formulated test sub-
stance by placing the mini dip apparatus on the areas.
To produce a suitable formulation, three parts by weight
of active compound are mixed with seven parts of the
abovementioned solvent/emulsifier mixture, and the
resulting emulsion concentrate is diluted with water~to
the specific concentration desired. After the treated
areas have dried, gauze bags are attached around the
areas with the aid of a dermatologically acceptable
adhesive in such a way that they surround the areas
completely. The bags are made of gauze and are cylindri-
cal in shape, ~ 12 cm, and have attached to them a 3 cm
wide base ring. This bag is closed by means of rubber
bands.
Testinq criteria:
From day 24 - 45 after the infestation, the female adults
which develop on the animal in the individual bags are
counted. The reduction in the number of developi~g female
adults with fertile egg clusters on the treated areas in
comparison with untreated control areas is used as a
criterion for the effectiveness. The effectiveness is
expressed as a percentage. Here denotes that 211 ticks
hav~ been killed, 0~ denotes that no ticks have been
Le A 28 434 - 30 -
2~72217
killed.
Active compound Active compound Cure effectiveness
Ex. No.concentrationin % Boophilus
in ppm of a.i. microplus in the
mini dip
1 300 100
100 100
100
Example B
Scab mite test
Test animals: Psoroptes ovis (larvae, nymphs, adults)
Solvent: 35 parts by weight of ethylene glycol mono-
methyl ether
35 parts by weight of nonylphenolpolyglycol
ether
To produce a suitable formulation, three parts by weight
of active compound are mixed with seven parts of the
abovementioned solvent/emulsifier mixture, and the
resulting emulsion concentrate is diluted with water to
the specific concentration desired.
1 ml of this active compound preparation is pipetted into
PP blister films of suitable size. Approx. 25 mites are
Le A 28 434 - 31 -
2 Q 7 2 ~ 1 ~
then transferred into the active compound preparation.
The effectiveness of the active compound preparation is
determined after 24 hours. The effectiveness is expressed
as a percentage. 100~ here denotes that all mites have
been killed; 0% denotes that no mites have been killed.
Active compound Active compound Cure effectiveness
Ex. No. concentration in % Psoroptes ovis
in ppm of a.l.
2 10 100
1 100
Examples
a) General procedure for the preparation of the com-
pounds of the general formula (I) (Y = NH-NH)
according to process c) in its specific embodiment
RZ
Rl_Z~NH-NH-C~ t Br-CH2CH2-N~2 ~ HBr
NH2
R
R 1 - Z~NH - NH~
R3
Le A 28 434 - 32 -
2Q722~ 7
0.15 mol of 1-(4-substituted phenyl)-3-thiosemi-
carbazide and 0.15 mol of 2-bromoethylamine hydro-
bromide are refluxed for 12 hours in 800 ml of
isopropanol. After cooling, the mixture is stirred
with saturated sodium hydrogen carbonate solution
and extracted by shaking with methylene chloride,
and the volatile components are stripped off in
vacuo. The residue is recrystallised from petroleum
ether.
The compounds of the general formula (I) where
Y = -NH-NH-, which are listed below in Table 1, are
obtained according to this process.
Table 1
R 1 - z~NH - NH~ ~ ( I )
EX . No . Z Rl R2/R3M . p . [ C ]
- CF3 H 170
Z - CF3 2 - CF314 0
3 52 CH3 2-CF3 147
4 52 CF3 2 - CF39 4
S02 CF2CHFCl H 120
6 50Z {~ Z-CF3 61
Le A 28 434 - 33 -
20722~7
Table 1 (Continuation)
Ex. No. Z Rl RZ/R3 M.p. tC]
7 52 CF3 2,6-Cl2
8 ~ CF3 2-Cl-6-F 144
9 52 CF3 Z-C1
0 CF3 H l90
11 - CF3 2-C1 150
12 52 CF3 H
1~ - CF3 2-Cl-3-F
14 52 CF3 2-C1
~ CF3 2,6-C12 01
16 S CF3 H
l7 5 CHF2 H
18 S02 CHF2 H
19 0 CF3CH2 H
C~2H H
21 0 CFzCl H
CH2F`
22 0 CH H
CH2F
CH2F`
23 0CH H
CH3
24 CF3CH2 H
oCF3CF2 H
Le A 28 434 - 34 -
2~'722~
Table 1 (Continuation)
Ex. No. Z Rl R2/R3 M.p. [C]
2~ 0 CH2FCH2 H
27 S CHFClCF2 H
Z8 0 CFC 1 2CF2 H
2~ 0 CFH2CH2 H
CF3~
- CF H
CF3
31 - CF2Br H
b) General procedure for the preparation of the com-
pounds of the general formula (I~ where Y is -N=N-
by oxidation
~) with ~22
A mixture of 0.05 mol of 2-(4-substituted phenyl-
hydrazino)-2-thiazoline in 120 ml of methylene
chloride and 100 ml of 2-normal sodium hydroxide
solution is treated with 7 ml of 30~ strength H2O2
and refluxed for 18 hours. 2 ml portions of 30%
s~rength H2O2 are then added after 8 hours and then
a further 6 hours. The organic phase is su~sequently
separated off, the aqueous phase is extracted with
methylene chloride, the combined organic phases are
concentrated in vacuo, and the residue is recrystal-
lised from hexane/ether.
- Le A 28 434 - 35 -
2~7~2~ ~
~) with silver oxide
1.7 g of silver oxide are added to a solution of
0O02 mol of 2-(4-substituted phenyl)-3-thiosemi-
carbazide in 200 ml of ethyl acetate. The mixture is
subsequently stirred for 18 hours at room tempera-
ture, the liquid is filtered off and concentrated in
vacuo, and the crystalline residue i9 stirred with
petroleum ether.
The compounds of the general formula (I) where Y is
-N=N-, which are listed in Table 2 below, are
obtained according to these processes ~) and/or ~).
Table 2
R~
R1_z ~ N=N~
Exo No. Z Rl R2~3 M.p [C]
32 ~ CF3 H
33 - CF~ 2-CF~
34 52 CH3 2-CF3
S02 CF~ 2-CF3
36 S02 CF2CHFCl H
37 S02 ~ 2-CF3
Le A 28 434 - 36 -
2!~7~,2~ 7
Table 2 ( Continuation
Ex. No. Z Rl RZ/R3 M.p. [C]
-
38 SO2 CF3 ~,6-C12
39 - CF3 2-CI-6-F
52 CF3 2-Cl
41 CF3 H 100
42 ~ CF3 Z-Cl 130
4 3 SO2 CF3 H
44 - CF3 2-CI-3-F
SO2 CF3 2-Cl
4~ - CF3 2,6-~:12
4 7 S CF3 H
~ ~ S CHF2 H
4 9 52 C~F2 H
5 0 O CF ~CH2 H
51 0 CF2H H
5 2 O CF2C 1 H
CH2F`
53 O CH H
CH2F
CH2F`
54 O CH H
CH3
5 5 S CF3CH2 H
5 6 o CF3CF2 H
Le A 28 434 - 37 -
2~7~2~7
Table 2 ( Continuation
Ex. No. Z R1 R2/R3 M.p. t C]
57 0 CH2FCH2 H
5 8 S CHFC l CF2 H
5 9 0 CFC l 2CF2 H
6 0 o CFH2CH2 H
CF3~
61 - CF H
CF3
6 Z - CF2Br H
Le A 28 434 - 38 -