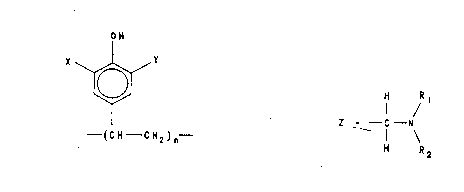Note: Descriptions are shown in the official language in which they were submitted.
` WO91/10756 PCI'/US91/00202
- 2~722~8
~UR}~ACE-TRE~ATMBN~ M13T~OD FOR TIN-PI.aq~:D DRAWN AND IRONED CAN~
TECHNICAL FIELD
The present invention relates to a novel surface
I treatment method for tin plated DI cans, i.e., cans formed
by the drawing and ironing of tin plated steel sheet. The
method imparts excellent corrosion resistance and paint ad-
hesivity to the surface of the can prior to its being
painted or printed, and also imparts the excellent slide-
ability (low frictional resistance) that is required for
smooth transport of the can by automatic conveying equip-
ment, particularly modern high speed conveying equipment.
BACKGROUND ART
The invention of Japanese Patent Application Laid Open
[Kokai or Unexamined] Number 1-100281 [100,281/89] is an
example of a surface treatment liquid for tin plated DI
cans. This teaching of the prior art employs a film form-
ing liquid for the treatment of metal surfaces. This solu-
tion has a pH of 2 to 6 and contains 1 to 50 gram per liter
("g/L") of phosphate, 0.2 to 20.0 g/L of oxyacid ions, 0.01
to 5.0 g/L of tin ions, and 0.01 to 5.0 g/L of condensed
- 20 phosphate. Treatment with this conversion treatment solu-
tion afforded a highly corrosion resistant phosphate film
on the surface of tin-plated DI cans.
However, in recent years tin-plated DI cans have been
produced using low levels of t:in plating in response to
economic considerations, and this has required that its
surface treatment provide far more corrosion resistance
than before. Moreover, when treatment is conducted by
prior methods, in some cases the gloss of the base metal
is degraded due to etching of the base metal. Accordingly,
there is a demand for a surface treatment which does not
damage the external appearance by reducing thP gloss.
Treatment methods intended to provide corrosion re-
sistance and adhesivity through the use of water soluble
resin are exemplified by the in~ention in Japan~se Patent
Application Laid Open Number 1-172406 [172,406/89]. This
invention provided as an example of the prior art comprises
. ~
, ~
, .:, . . .: ,. .. . ..
~: ,. , .. ~ .
':
' ~: , ' , ' . ' ' ," , :
".' , ' . ': :
WO91~10756 PCT/~S91~0~2
` 20722~8 2
a method in which the metal surface is treated with a solu-
~ tion which contains an effective derivative of a polyhydric
`- phenol compound. However, the disclosed method does not
- generate a satisfactorily stable corrosion resistance.
In addition, the metal can manufacturing process often
suffers from a problem with transfer or transport: the
slideability of the outer surface of the can during convey-
or transport of the can may be poor due to a high friction
coefficient of the outer surface, so that the can may be
tipped over sideways. Can transport to the printer in the
most modern high speed can lines is a particular problem in
this regard. Accordingly, there is demand in the can manu-
facturing industry for a reduction in the static friction
coefficient of the outer surface of cans, which at the same
time does not cause any adverse effects on the adhesion of
~ any paint or lacquer subsequently coated on the can. ~he
; invention of Japanese Patent Application Laid Open Number
64-85292 [85,292/89] comprises a method for improving this
slideability. The reference teaches a surface treatment
composition for metal cans which contains water-soluble
organic material selected from phosphate esters, alcohols,
monobasic, and polybasic fatty acids, fatty acid deriva-
~` ti~es, and mixtures of the foregoing. While the disclosed
method does in fact generate an increase in the slideabil-
; 25 ity, it does not improve the corrosion resistance or paint
adhesion.
United States Patent 4,517,028 teaches in general
terms treatment of metals with aminated derivatives of
poly{vinyl phenols}. This reference, however, makes no
specific reference to treating tin plate or DI cans.
DESCRIPTION OF THE INVENTION
Problem to Be Solved bY the Invention
The principal goal of the invention is to provide a
~ single treatment for DI cans that will result in increased
- 35 corrosion resistance, good adhesion to subsequently applied
paint or ~imilar organic coatings, and a low coefficient of
friction on the outside can surface, for efficient process-
,:
', .
.
:
.' , ' ' ' , , . ' .. .
~, , , ':
W091/10756 PCT/US9~/00202
3 ~ .,
ing in automated can processing lines using high speed con-
veyors and printers.
Summarv of the Invention
t It was discovered that a film with excellent corrosion
resistance, paint adhesion, and slideability could be
formed on a DI can surface by controlling the conditions of
~' treating the surface as follows:
(1) A liquid treating composition is prepared by dissolv-
ing in water an oligomer haYing a chemical composition
specified by the general formula:
OH
x ~"Y
--(CH--CH2 ) n
wherein n is a number with a value between lO and 30
and each of X and Y independently represents hydrogen
. 20 or a group Z, wherein Z has a chemical composition
; conforming to the general formula:
H R1
., I /
Z - --C--N
I
:- 25 H R2
wherein each of Rl and R2 is an alkyl or hydroxyalkyl
group having ~rom l to 5 carbon atoms, except that at
.i least 25 ~ of the total of all the X and Y groups in
the oligomer are Z rather than hydrogen.
(2) The pH of the surface treatment solution containing
. the oligomer described in item (l) is adjusted to a
value between 4 and 6 by the addition of orthophos-
~ phoric acid and/or condensed phosphoric acid.
35 (3) The sur~ace treatment liquid as prepared in step (2)
is heated to a temperature of at least 40 but prefer-
ably to not more than 60 degrees Centigrade and the
, . . .
.. . .
: ' . :
., . .. . - ~ . ~ ~ , :
': ', ., ': , . . . . , , ' ':
. .
' :, , , ' ' ~ . , ' : : , ' ':
, . : . . ,
.
. . . . .
: . . . . .
'' .. ' ' ' :. ,
: '' ' ' : ' ':
.
WO91/10756 PCT/U~9~ 2~2
` 2~722~8 ~
ably to not more than 60 degrees Centigrade and the
heated surface treatment liquid is then sprayed on the
cleaned surface of tin plated DI can for a time o~ at
least 5 and preferably not more than 60 seconds.
(4) The aforesaid spray treatment is followed by thermal
drying or by a water rinse and then thermal dryingO
Pre~erably, there is no water rinse before drying aft-
er contact of the surface of the DI can with the heated
surface treatment liquid as specified above. If there is
water rinsing b~fore drying, it is preferred that at least
the la~t such water rinse be with deionized or other puri-
fied water substantially free from dissolved solids. If
there is no rinsing with water before drying, it is nor-
mally preferred to let the sprayed cans drain under the
influence of gravity, and/or to remove some of the liquid
from the can surface by mechanical means such as an air
flow, rollers under slight pressure, or the like, to avoid
the presence of excessive amounts of the surface treatment
liquid on the surface during drying.
Details of Preferred Embodiments of the Invention
The value of n in the general formula given above for
the oligo~er dissolved in the surface treatment liquid is
10 to 30. At values of n below 10, little or no improve-
ment in corrosion resistance will be observed on DI tin
plated cans. A value of 31 or more for n results in a
- poorly stable aqueous solution which cannot readily be used
in practical applications.
In the general Pormula for group Z, Rl and R2 repre-
; sent alkyl or hydroxyalkyl groups having 1 to 5 carbon
atoms. When they contain six or more carbons, the stabil-
, . .. .
ity of the aqueous solution is reduced. The introduction
xatio for the group Z should be 25 to 100 mole % referred
to the total number of X and Y groups in the oligomer. The
water solubility of the oligomer may not be adequate when
over 75 ~ of the total of X and Y groups present are hydro- -
; gen.
The oligomer solids content in the treatment liquid
. ~ .
. - .
' , ': ': "' ' ' ' ' ' . ~, ". ' :.' ,: '
', ' " ' ' '
: ', ' ' , , . ': '
., ' .' ~ ,, .
.. . .
~ ~ WO9l/~0756 PCT/US91/~2~2
~ 5 i 20722~
preferably is from 0.1 to 0.5 ~ by weight ("w/o") of the
total liquid. Below 0.1 wJo, it is very dlfficult to form
a stable film on a DI tin can surface. On the other hand~
th~ treatment solution is costly above 0.5 w/o, with little
or no additional technical benefit.
- The pH of the treatment solution should be adjusted to
4 to 6 through the use of orthophosphoric acid and/or a
condensed phosphoric acid such as pyrophosphoric acidO
Substantial etching of the can surface occurs at a pH below
4 and impairs film formation. At a pH above 6, the solu-
tion has a short life because the oligomer tends to pre-
cipitate and sediment. The pH can normally be adjusted
into the range of 4 to 6 by the addition of 0.05 to 0.3 w/o
orthophosphoric acid or 0.03 to 0.2 w/o pyrophosphoric acid
- 15 referred to the total surface treatment liquid. Other con-
densed phosphoric acids and mixtures of condensed acids or
of condensed and orthophosphoric acids can also be used.
In addition, the treatment liquid should be heated to
at least 40 degrees Centigrade during use. The treatment
liguid is poorly reactive below 40 degrees Centigrade, and
this works against the formation of a highly corrosion re-
sistant film. On the other hand, little or no benefit due
to heating is observed when the liquid is heated to above
- 60 degrees Centigrade, and unnecessary heating is expen-
; 25 sive.
~he spraying time should be at least 5 seconds. Only
an inadeguate reaction is obtained at less than 5 seconds,
and a strongly corrosion resi~tant ~ilm i8 not developed.
On the other hand, treatment times in excess of 60 seconds
do not afford any increase in performance and increase the
expense.
The surface treatment method of the present invention
is described below through several illustrative examples of
particularly preferred embodiments of the invention, and
-its usefulness will be demonstrated by comparison with com-
parison examples. The examples are not to be regarded as
limiting the in~ention, except in so far as noted ~in the
.,
,, ,, : , .
., , - ', :
, ... ,. ': , ' ,
: .:
.
WO91/l0756 ` ` ~- PC~/U~ 2~2
207 22~8 6
claims.
General CQnditiQns ~or Examples
A small sprayer was used for the degreasing and sur
face treatment of the cans. This small sprayer was de- Y 1
signed to giv~ spray conditions identical to those encount-
ered in spray treatment with the can washers which are cur-
rently in use in the can manufacturing industry.
The corrosion resistance of a treated can was evaluat-
ed through the iron exposure value ("IEV"), which was mea-
sured according to the directions in United States Patent
4,332,646. The corrosion resistance is better at lower IEV
values.
The paint adhesiveness was evaluated as follows: an
epoxy-urea can paint was coated to a film thickne~s of 5 to
7 micrometers (microns) on the surface of the treated can,
which was subsequently baked for 4 minutes at 215 degrees
Centigrade; the can was then cut into a 5 x 150 millim ter
("mm") strip, onto which was hot-pressed polyamide film in
order to afford a test specimen; and this was then peeled
in a 180 peel test to give the peel strengkh. Higher
peel strength values correspond to a better adhesiveness.
The slideability of treated cans was evaluated by
measurement of the coefficient of static friction of the
`~ outer surface of the can. Values of this coefficient of
static friction of less than or egual to 0.9 are preferred, `
while values within the range of 0.7 to 0.8 are particular-
ly preferred.
The oligomer used in all the examples below according
to the invention had the averaye general formula:
0~ ~ H2CH2H ~;'
X ~ ,~ \C H
--(CH--CH2)n
wherein n had an average value of 20 and X represented
' ~ :
,,, ., , , , , ~,
':
..
WO~1~10756 PCT/U~ 2~2
` ~722~8
hydrogen. This oligomer was synthesized as follows: lO0
grams ("g") of CellosolveTM solvent (the monoethyl ether of
ethylene glycol) was introduced into a l liter reaction
flask equipped with a condenser, nitrogen inlet tube, over-
head stirrer, and thermometer, and 60 g of poly{4-vinyl
phenol} with an average molecular weight of 2,500 was added
and dissolved; 40 grams of 2-methylamino ethanol and lO0 g
of deionized water were added, and the contents of the
flask were heated to 50 degrees Centigrade; 40 g of 37%
formaldehyde solution in water was added o~er l hour, fol-
lowed by stirring at S0 degrees Centigrade for 2 hours and
by further heating to 80 degrees Centigrade and stirring
for an additional 3 hours at that temperature; the reaction
product was cooled, 15 g of 85 % orthophosphoric acid was
added, and 700 g of deionized water was also added. After
reaction with these added ingredients, the oligomer was
precipitated by the addition of 10% sodium hydroxide
solution until the pH reached 8 to 9. The precipitated
oligomer was then filtered off, washed with water, and
dried to afford the oligomer used.
Exam~le l
Tin plated steel sheet was drawn and ironed to afford
tin plated DI cans, which were spray-rinsed with a hot 1%
aqueous solution of a weakly alkaline degreaser (FINE
~ 25 CLEANERTH 4361A from Nihon Parkerizing Company, Limited,
!-''. Tokyo) and then rinsed with water~ Cans were then sprayed
for 40 seconds with surface treatment liquid 1 (described
below), heated to 50 degrees Centigrade, followed by a wash
with tap water, then a lO second spray with deionized water
- ~ 30 (with a specific resistance of at least 3,000,000 ohm-cm),
then drying for 3 minutes in a hot air dryer at 180 degrees
Centigrade. Surface-treatment liquid l had the following
composition:
oligomer solids 0.2 weight %
75% orthophosphoric acid O.l weight ~
water 99.7 weight %
.' ,,
. .
.
. .
,. : '' ' ~ . ' ~ .
,
,
'' . ' .' ' :, ,
.
WO91/1~756 PCT/USg~ 2~2
~0722~ 8 ~ ~
~ .
- Tin plated DI cans werr cleaned as in Example 1~ then
spray treated for 40 seconds with surface treatment liquid
2, heated to 50 degrees Centigrade. This was followed by
a water wash and drying as in ~xample 1. The composit1on
of surface treatment liquid 2 was:
oligomer solids 0.2 weight %
50% pyrophosphoric acid 0.1 weight %
; water 99.7 weight %
pH 5.5
The oligomer used was the same as in Example 1.
Example 3
Tin plated DI cans were cleaned as in Example 1, then
- spray treated for 10 seconds with the above described
surface treatment liquid 1 (cf. Example 1), which had been
heated to 50 degrees Centigrade. This was followed by a
~ water wash and drying as in Example 1.
.. . .
`~ Example 4
Tin plated DI can was cleaned as in Example 1, then
spray treated for 40 seconds with the above described sur-
face treatment liquid 1 (cf. Example 1), which had been
heated to 50 degrees Centigrade. This was followed by
~ draining, without water rinsing, and then drying in a hot
- air dryer at 180 degrees Centigrade for 3 minutes.
Comparison ExamPl~ I
~in plated DI cans were cleaned as in Example 1, -
spray treated for 40 seconds with comparison surface treat-
ment liquid 1, heated to 50 degrees Centigrade, then washed
with water and dri~d as in Example 1. Comparison surface
- 30 treatment liquid l had the following composition:
oligomer solids 0.2 weight %
75% orthophosphoric acid 1.5 weight %
water 98.3 weight %
pH 2.0
The oligomer used was the same as in Example 1.
W091~10756 ` `PCT/US9liO~
9 `` ` 2~22~
Comparison Exam~le 2
Tin plated DI cans were cleaned as in Example ll
spray treated for 2 seconds with the ahov~ described sur-
face treatment liquid 1 ~cfo Example l), which had been
heated to 50 degrees Centigrade, then washed with water and
dried as in Example l.
- Com~arison Exam~le 3
Tin plated DI cans were cleaned as in Example l, then
spray treated for 40 seconds with the Comparison surface
treatment liquid 2, heated to 50 degrees Centigrade, then
washed with water and dried as in Example l. The composi-
tion of Comparison surface treatment liquid 2 was:
oligomer solids 0.2 weight %
- 70% orthophosphori¢ acid O.l weight %
15 water 99.7 weight %
pH 5-5
The oligomer used for Comparison surface treatment
liquid 2 was not the same as that used for the Examples and
the preceding Comparison examples, bu~ instead had the ap-
: 20 proximate formula:
. o~
2 5 X ~ , C H ~ 5 0 5 H
--(CH--CHz ) n
wherein n has an average value of 20 and X represents
~ hydrogen. This oligomer was synthesized as follows: lOO
30 g of poly~4-vinylphenol) (average molecular weight = 2,500)
was charged to a 1 liter reaction flask eguipped with a
condenser, nitrogen inlet tuba, overhead stirrer, and
th~rmometer, and it was then dissolved by the addition of
500 g o~ 1,4-dioxane. This solution was maintained at
approximately lO degrees Centigrade, and 80 g of liquid
sulfur trioxide (S03) was added over l hour. This was
. : :.. .. :
WO91/10756 PcT/u~9~ 2~2
20722~8 lO
followed by heating to 80 degrees Centigrade and reaction
for 4 hours with stirring. Neutralization with 10% sodium
hydroxide solution and removal of the solvent by distilla-
tion afforded the oligomer used above.
Table 1 reports the results of the Examples and Com-
parison Examples, which confirm an excellent corrosion re
sistancel adhesiveness, and slideability for the conditions
; according to the present invention and superiority over all
the Comparison Examples. Thus, treatment o~ DI tin cans
according to the present invention provides an excellent
corrosion resistance and paint adhesion to the surface of
~ tin plated cans and also imparts the excellent slideability
that is required for a smooth conveyor transport of the
cans.
-- 15
Table 1
TEST RESULTS OF THE EXAMPLES AND COMPARISON EXAMPLES
,~ . .
IEV Peel Strength, Coefficient
-:; Ka Force/5 mm Width of Friction
.. ~ .
Example 1 100 2.0 0.8
Example 2 100 2.0 0.8
Example 3 100 2.0 0.8
Example 4 40 2.0 0.7
; Comparison 350 1.5 1.0
Example 1
: Comparison 550 1.7 1.0
~xample ~
: Comparison 700 1.5 1.0
Example 3
,
. .