Note: Descriptions are shown in the official language in which they were submitted.
WO 91tO9640 PC~/IJS90/07597
1- 2~72~
DI~ATATION BA~LOON C~THETER ~ND
MET~IOD OF MANtJF~CTU~E
BACKGROUND OF_THE INVENTION
1. Field of the Invention.
The present invention relates to the fiel~ --
angioplasty. In particular, the present in~en'io~
relates to a dilatation balloon catheter.
~. Descri~tion of the Prior Art.
Angioplasty has gained wide acceptance ir
recent years as an efficient and effective method ~o-
treating types of vascular diseases. In particula ,
angioplasty is widely used for opening stenoses in ~he
- coronary arteries, although it is also used for t~eat~ent
of stenoses in other parts of the vascular system.
The most widely used form of angioplasty makes
use of a dilatation catheter which has an inflatable
balloon at its distal end. Using fluoroscopy, the
physician guides the catheter through the vascular syste~
until the balloon is positioned across the stenosis. The
balloon is then inflated by supplying fluid under
pressure through an inflation lumen to the balloon. The
inflation of the balloon causes stretching of the artery
and prcssing of the lesion into the artery wall to re-
establish acceptable blood ~low through the ar'ery.
Two commonly used types o~ dilatation catheters
are referred to as "over-the-wire" and "non-over-the-
wire" cath~ters. An over-the-wire catheter is one i-
which a separate guide wire lumen is provided so that 2
guide wire can be used to establish a path across the
stenosis. The dilatation catheter can then be advance~
W091tO~lJ PCr/US90/07~97
~2- 2~72~
over the guide wire until the ballosn LS positioned
across the stenosis. A non-over-the-wire catheter acts
as its own guide wire, and thus there ls no need for a
separate guide wire lumen. A non-over-the-wire catheter
S can therefore achieve a smaller outer diameter f~r i s
main shaft since a guide wire lumen is not required.
There has been a continuing effort to reduce
the profile and shaft size of the dilatation catheter t~
allow the catheter to not only reach, but also cross, a
very tight stenosis. A successful dilatatiun catheter
must also be sufficiently flexible to pass through tight
curvatures, especially in the tortuous coronary arteries.
A further requirement of a success~ul dilatation catheter
is its "pushability." This involves the transmission of
longitudinal forces along the catheter from i~s proximal
end to its distal end allowing a physician to push the
catheter through the vascular system and across the
stenosis.
SU~ Y OF~THE INVENTION
The present invention is a non-over-the-wire
dilatation cathetar which has a low profile shaft, is
flexible, has good torque response and pushability, and
which also is relatively simple to manufacture.
One e~hodiment of the inventive catheter
includes a one-piece, flexible elongate tube having a
hollow proximal end formed by a tubular wall which
defin2s a lumen, and having a distal end. The distal end
of the tube has a tapered section, which has a diamete~
which necks down from a first diameter of the tube to a
second diameter smaller than the ~irst diameter. At a
W09l/~9~0 PCT/US90/07597
-3- 2072~8 ~
distal end of the tapered section, the lumen terminates
and a generally solid distal core is formed distally ~'
the tapered section. An inflatable balloon segment cir-
cumscribes the tube and has a proximal end bonded to ~.e
tube adjacent the tapered section and a distal end bon~le~
to the tube adjacent the solid section thereo~. Th.
interior of the balloon segment is in communication ~it~
the lumen via at least one apertur~ extending through the
tubular wall.
Preferably, a flexible coil spring tip extends
distally from the tube and balloon segment. The distal
end of the tube tapers to form a safety wire extending
within the coil spring and is attached to an end cap o~
the ccil spring. In a preferred embodiment, the tube is
formed from a stainless steel hypotube and coated wi~h
a lubricious material in the proximal unnecked segment.
In another embodiment, the tube is formed from
semi-rigid plastic tubing. In the distal end, the tube
has a tapered section which tapers from a first diameter
to a distal section of a smaller second diameter, formed
about a metal core wire. The distal ~ection has a
gener~lly solid cross-~ection. The metal core wire
tapers and extends out of the distal section to define
a safety wire, which extends within a coil spring, and
attaches to an end cap of the coil spring.
The inventive catheter is relatively simple to
manu~acture by a method including the formation of a
lumen from an elongated tube having a proximal section
and a distal section; the formation of a tapered portion
in the distal section of the tube wherein the cross-
WOgl/~9~0 PCT/US9~/0759~
2~72.~
sectional diameter of the tube tapers distall~ t~
terminate the lumen and form a qenerally solid dis.a'
core, either of metal or plastic, of plastic form~d about
a metal core wire; and mounting an inflatable balloo~ cn
the distal section of the tube, with a proximal end 5~
the balloon se~ment secured to the tube proximally of the
aperture and a distal end of the balloon segment secured
to the solid distal core.
Preferably, the cross-sectional diameter of th~
distal core wire is further reduced to define a distal
safety wire segment of the tube. In the case where tne
tube is semi-rigid plastic tubing, the metal core ~ire
is reduced in cross-sectional diameter to form a sarety
wire segment of the metal core wire. A coil spring is
secured to the distal core wire to extend distally fro~
the balloon about the distal safety wire segment. The
distal end of the distal safety wire segment is ~hen
secured to a distal end cap on the coil spring.
BRIEF DESCRIPTION OF THE INVENTION
FIG. 1 shows generally an angioplasty balloon
catheter in accordance with the present invention.
FIG. 2 is a detailed sectional view of the
distal portion of a first preferred embodiment the
angioplasty catheter.
FIG. 3 is a detailed sectional view of the
distal portion of a second preferred embodiment of the
angioplasty catheter.
DET~I~Æ~ ~ESCRIPTION O~ HE PREFERRED_~MBODIMENTS
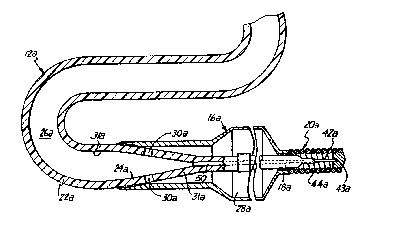
FIG. l shows generally an angioplasty balloon
catheter 10 in accordance with the present invention,
W~91/~9~0 PCT/US9~t~7~97
2 ~ 7 2 Ir3 ~ ~
includin~ a catheter tube 12, inflation manifold 14, and
an inflatable balloon 16. A distal section 18 of the
catheter 10 has a flexible spring tip 20. Catheter tube
12 is an elongated, flexible, one-piece tube, preferably
of stainless steel, or of semi-rigid plastic, coated on
its outer surface with a lubricious material, such as
silicon or Teflon. The lubricious coating allows the
catheter to move with ease through the vascuiature of the
body. Inflation manifold 14 is attached at the proximal
end of the tube 12 to provide fluid under pressure to
inflate the balloon segment 16. Inflatable balloon 16 is
preferably fabricated from a polymeric ma~erial such as
polyolefin copolymer.
In one preferred embodiment, anyioplasty balloon
catheter 10 has a length of approximately 135 centimeters
(53.15 inches) with catheter tube 12 having an outer
diameter between 0.020 and 0.040 inch (0.51 and 1.02
millimeters) in its proximal end and with the coil spring
tip 20 at the distal end of catheter lO having an outer
diameter between 0.012 and 0.016 inch (0.30 and 0.41
millimeters).
FIG. 2 shows in detailed sectional view the
balloon or distal portion of a first preferred embodlment
of the angioplasty balloon catheter 10 of the present
invention. In this embodiment, catheter tube 12 is a
stainless steel hypotube and has a hollow proximal section
22, of a first diameter, which tapers at elongated
tapered section 24 to terminate an inflation lumen 26
and form distal section 18 which has a second diameter
that is smaller than the first diameter. The tapered
section 24 of the tube 12 tapers from a tube (hollow
W~91/0~0 PCT/US90/07597
-6- 2~72~
proximal section 22) to elongated distal section 13,
which has a generally solid cross-section, as see~
FIG. 2. In a preferred embodiment, the tapering is uni-
form in tube diameter reduction, but a stepped or pro-
gressive reduction will also work. An inflatlon l~.an
26 is defined within the hollow proximal section 22 o~
the tube 12. The lumen 26 extends into the elon~ate~
tapered section 24 of the tube 12, and is in ~
communication with an interior 28 of the balloon 16 via
one or more apertures extending through the tube, as at
apertures 30 through tube wall 31 of the tapered section
24, as seen in FIG. 2.
The balloon 16 has a proximal section 32, an
inflatable section 33, and a distal section 34. The
proximal section 32 of the balloon 16 is connected to the
tapered section 24 at proximal adhesive joint 36, and t.~e
distal section 34 of the balloon 16 is connected to the
solid distal section 18 at a distal adheslve joint 38.
The balloon ends are preferably attached to their resp~c-
tive tube sections by an adhesive such as epoxy. Atleast one radiopaque marker band 40 circumscribes the
generally solid distal section 18 of the tube within
balloon 16. The proximal section 32 of the balloon
segment 16 may be formed from a separate flexible plastic
tube, such as polyethylene for increased flexibility,
having its proximal end bonded to the proximal tu~e
section 22 (proximally of apertures 30) and its distal
end bonded to the proximal end of balloon segment 33.
W09~ 0 PCT/US90/075~7
2~72~
Preferably, elongated solid dista-l section i~
of tube 12 further ~apers distally to form safety rhlr
~2. Safety wire 42 is attached by braze ~3 at a dist~:
end of a coil spring ~4 to form coil sprinq tip 20.
Flexible coil spring tip 20 serves to safely ~u1
catheter lO through the tortuous passages of the coron2ri
arteries. Coil sp.ring 44 is mounted on solid section l~.
at braze or solder joint 46 adjacent distal adhesive
joint 38. The coil spring tip 20 is bendable, and the
safety wire 42 can be bent to a position and wlll retai,~
the bend, thus facilitating the steerability of ~h~
ca~heter lO through a patien~'s vascular system. In use,
torque is applied to a proximal end of tube 12, and is
transmitted by (through) tube 12, tapered section 24,
distal section 18 to spring tip 20.
FIG. 3 shows a second preferred embodiment of
the angioplasty balloon catheter of the present inven-
tion. In this embodiment, a catheter tube 12a has a
hollow proximal section 22a, of a first diameter, which
tapers distally at elongated tapered section 24a to a
second smaller diameter. An elongated distal section
18a defines in part, the reduced diameter portion of tube
12a. In thi~ embodiment, catheter tube 12a is semi-rigid
plastic tubing, and distal section 18a of the semi-ri~id
plastic tube is ~ormed about a central metal core wire
50. Together, the plastic tubing and metal core wire ~0
comprise distal section 18a, which is essentially solid
in lateral cross-section. Balloon 16a is identical to
balloon 16 described with re~erence to the embodiment of
FIG. 2.
W0~ 9~0 PCT/US9~/07597
~8- 2~7~
An inflation lumen 26a is defined within the
hollow proximal section 22a of the tube 12a. rrhe lu~en
26a extends lnto the elongated tapered section 2~a of the
tube 12a and is terminated at a point ~here the tuDe
fits about the metal core wire 50 at distal sec'iorl ~a.
The lumen 26a is in fluid communication with an interio~
28a of the balloon 16a via one or more apertures extend-
ing through the tube, as at apertures 3Oa through tube
wall 31a of the tapered section 24a, as seen in FIG. 3.
Preferably, metal core wire 50 is reduced in
diameter as it extends di~tally and out of the distal
section 18a to form safety wire 42a. Safety wire 42a is
attached by braze 43a at distal end of a coil spring 44a
to form coil spring tip 20a. Again, flexible (and benda-
ble) coil spring tip 20a serves to safely guide the
catheter of the present invention through the tor.uous
passages of the coronary arteries.
The angioplasty balloon catheter of the present
invention is relatively simple to manufacture. The
method of manufacturing catheter includes the following
steps. A lumen is formed from an elongated tube having
a proximal section and a distal section. The tube can
be either metal or semi-rigid plastic tubing. A tapered
portion is formed iA the distal section of the tube. In
the tapered section, the cross-sectional diameter of the
tube is decreased distally. Preferably, the tapered sec-
tion tapers uniformly from a first diameter to a smaller
second diameter. In the instance where the tube is
metal, a distal portion of the reduced cross-sectional
diameter of tube forms an elongated, generally solid,
WO91~09~0 PCT/US90/075~7
_g _
2~72~
distal core, as seen in FIG. 2. Thus, a slngle-piece
metal inflation lumen and balloon core is create~ b~ a
~etal tube that is reduced distally to a solid core or
wire. In the instance where the tube is plastic, ~e
cross-sectional diameter of the tube is also decreased
distally in the tapered section and the tube ta3ers -^
a solid distal core section, or in a preferred embodi-
ment, the tube taper~ uniformly to a distal section where
the plastic tube is formed about a metal core wire as
seen in FIG. 3. Thus, an inflation lumen and balloon
core are created ~y a semi-rigid plastic tube which is
reduced distally to fit about a metal core wire.
In either arrangement, at least one apertu~e
through the taperPd section is then provided, and an
inflatable balloon segment is mounted on the distal
section of the tube. The proximal end of balloon segment
is secured to the tube proximally of the aperture, and
a distal end or balloon segment is secured to the solid
core. As mentioned above, a separate praximal or waist
segment of the balloon (extending generally over the
tapered section of the tube) may be provided. This
allows this proximal waist (i.e., segment 32 in FIG. 2)
to be formed from a material different from the balloon
segment, and which has enhanced flexibility characteris-
ti~s, thereby achieving improved trackability.
The tapered section of the tube can be formedby any suitable means such as rolling or compressive
loading of the tube. In this formation process, heating
and axial stretching of the tube may b~ performed as
well. The distal reduction in diameter of the tube thus
WO91J09~ PCT/~S90/0~597
- 10- ~ ~ 7 2 ~
increases flexibility of the tube in its distal environs,
which necessarily ~ust be the most ~lexible portion Oc
the catheter to permit its advancement throuqh the c~n-
voluted coronary arteries. Grinding techniques may also
be employed in combination with the manufacturing pr~-
cesses mentioned above to achieve the reduced dia~ete~
tube and to also achiev~ reduced wall thicknesses f~
those distal worked portions of the tube (the tapered
section 24 and distal section 18, as seen in FIG. ~).
Preferably, the generally solid distal core is
further formed to reduce its cross-sectional diamete~ to
a very small wire or ribbon core, thus defining a benda-
ble distal safety wire segment 42 of the tube 12, in the
case where tube 12 is metal (see FIG.2j. In the case
where the tube 12 is semi-rigid plastic tu~ing, the me~al
core wire 50 is further formed to reduce its crcss-
sectional diameter to a very small wire or core, thus
defining a distal safety wire segment 42a of the metal
core wire 50 (see FIG. 3). A coil spring is then secured
to the solid distal sectlon of the tube to extend distal-
ly from the balloon about the distal safety wire segment.
A distal end of the distal sa~ety wire segment is secured
to the coil spring, thus defining a distal tip on the
cathPter which is flexible and has no sharp edges. This
minimiz2s the possibility of inadvertently puncturing or
scraping the walls of the artery as the catheter is moved
therethrough.
In use, a physician first follows the typical
angioplasty procedura of positioning a guide catheter in
tha vascular system. The angioplasty balloon cathete~
W~9l/~0 PCT/US9~/07597
2 ~ 7 2 ,~ 7
of the present invention is then advanced distall~
through the guide catheter and into the stenosed coronar~,
artery. The phycician then further pushes the cathete~
to the point at ~hich the inflatable balloon thereon is
positioned across the stenosis. 3y using a d~fe injec;e~
.i.nto the coronary artery throu~h the guide catheter ,:hich
is detectable by fluoroscopy, the stenosis position is
detectable. The radiopaque marker on the catheter also
is detectable by fluoroscopy, thereby permitting the
physician to accurately position the balloon across the
stenosis for inflation. The flexible and steerable dis-
tal coil spring tip of the catheter aids in the safe and
effective movement of the catheter ~hrough the tortuous
passages of the coronary artery. Using an inflation
devicP (not shown) coupled to the inflation manifold 14,
the physiclan inflates the balloon with an inflation
medium (typically a 50/50 solution of saline and Renogra-
fin 76) through lumen 26 and apertures 30 of tube 12
(FIG. 2). The lnflatable section 33 of the balloon 16
expands and stretches the arterial wall, pressing the
lesion into the arterial wall and re~establlshin~
acceptable blood flow through the artery. The balloon
16 is then deflated via negative pressure applied from
the inflation manifold 14. The artery is again visual-
~5 ized using dye and angiography, and if the stenosis hasbeen dilated, the catheter is removed. If the stenos.s
has not been sufficiently dilated, the inflation proce-
dure may be repeated.
In conclusion, the present invention is an
33 improved angioplasty dilatation catheter. The one-piece
WO91/~9~o PCT/US90/07~7
-12- ~72~.?~
design for the proximal inflation ~ube.and distal balloon
core and catheter tip allows for ~ood torque transmission
from the proximal to distal ends and is relatively easy
to manufacture. The design wherein a semi-rigid plas ic
tube is formed about a distal metal core ~ire also allohs
for good torque transmission from the proximal to dis~al
ends and is relatively easy to manufacture. In the _~se
where the catheter tube is metal, the inventive catheter
does not require a separate guide wire or safety wire
since the one-piece tube itself tapers distally to form
the guide or safety wire. In the case where cathe~er
tube is semi-rigid plastic, the guide/safety wire i,
provided by the ~etal core wire onto which the plastic
tub~ is formed. These constructions provide a small
outer diameter for the catheter, thus lessening the
friction problems inherent in advancing an elongated tube
longitudinally, and aLso allowing less obstruction in the
guide catheter and coronary artery to the flow of dye.
~ lthough the present invention has been
described with reference to preferred embodiments,
workers skilled in the art will recognize that changes
may be made in form and detail without departing from the
spirit and scope of the invention.