Note: Descriptions are shown in the official language in which they were submitted.
2~7~7~
HYDRONETALURGICAL PRODUCTION OF ZINC_OXIDE FROM
ROASTED ZINC CONCENTRAT~S
FIELD_OF TEE INVRNTION
Thi3 invention relates to the production of high
quality zinc oxide from roasted zinc sulphide
concentrates.
~ACRGROUND OF T~E INVEN~ION
~ y "roastQd zinc sulphide concentrates" is meant
the product obtalned by roasting a zinc sulphide
bearing concentrate. The term zinc calcine i8 al80
u~ed to refer to such products. Typically, roasted
zinc sulphide concentrate~ contain zinc oxide and other
components, including zinc sulphate, zinc sulphide,
mixtures of metal oxides such as copper, lead, calcium,
cadmium, and msgnesium, zinc ferrite, magnetite,
possibly some haemetite~, and usually ~ome metal
silicates, ~ulphate and sulphides.
Zinc sulphide bearing concentrates are commonly
roasted in a fluid bed roaster. In the fluid bed
roast~ng of zinc concentrates, the concentrates are
continuously fed into the roaster chamber, wherein air
blown up through the chamber converts sulphides to
oxides rQleasing sulphur into the gas stream chi~fly as
2 2~7~l1
sulphur di~xide, with the development of heat. Smaller
and lighter particles in the feed concentrate tend to
be carried out of the chamber with the ga~ stream and
are collected by downstream equipment. Larger and
heavier particles tend to remain in the roaster
fluidized bed through which the moving air i8 blown.
Since the bed i8 full during continuous operations, a
bed overflow stream outlet is provided by which further
accumulation of coarse ~olids is prevented by allowing
bed material to overflow out of the chamber. This
material is referred to as ~bed overflow calcine~. In
some zinc refineries, calcine is compo~ed of an
approximately 50:50 by weight mixture of bed overflow
calcine and fine material that is recovered from the
gas stream, as described above. This mixture is then
used as a feed material in the production of zinc metal
and iB referred to as normal calcine at least in some
cases, such as at the zinc refining from which samples
were used in the U. S. Patent No. 5,028,410 hereafter
referred to below.
In accordance with a previous invention, U.S.
Patent No. S~028,410 aqueous ammoniacal ammonium
carbonate solutions are used to selectively leach zinc
from roasted zinc sulphide concentrates, leaving a
substantial portion of the iron components in the leach
residue.
Ammoniacal ammoniumS'carbonate solutions have been
suggested for treating zinc scrap and mini-steel plant
baghouse dusts to recover zinc oxide. However, it was
not previously recognized that such solutions could be
used to leach roasted zinc sulphide concentrates,
which are dissimilar to zinc scrap and baghou6e dust,
to produce such high quality, high surface area zinc
oxide as are obtained by U. S. Patent 5,028,41d. Zinc
2~7~7~
oxid~ with the high specific ~urface area produced by
U. S. Patent No. 5,028,410 can be classed as premium
zinc oxide.
A new di~covery now reveals that by the use of
S bed overflow calcine alone, rather than normal calcine
as discussed above, a number of improvements result.
Thi~ is the essence of the present invention.
I~ is to be noted here that the ratio of bed
overflow calcine to dust carryover calcine can vary
widely at different zinc refineries. The ratio will
not always be approximately 50:50. This would, in no
way, detract from the practice of the pre~ent
invention.
BRIFF SUMMARY OF THE INVENTION
It has now been discovered that by using bed
overflow calcine as oppo~ed to normal calcine as feed
material to the ammoniacal ammonium carbonate leaching
pxoce~s for normal calcine, a number of very important
improvements result, which should result in greater
commercial utilization of the process in its improved
form, as opposed to the process described in U.S.
Patent No. 5,028,410.
These will be described in the forthcoming
section entitled, "Description of the Preferred
Embodiment".
Reference is made t'o U. S. Patent No. 5,028,410
which is incorporated herein by reference.
DESCRIPTION OF THE PREFE M ED EMBODIMENTS
In one embodiment, the bed overflow calcine i3
fed directly to ammoniacal a~monium carbonate leaching
ves6els operating continuously under similar conditions
as in U. S. Patent No. 5,028,410.
4 2~727:~
The slurry leaving the above-described leaching
ves~els is then fed to a liquid-601id separation vessel
as described in U. S. Patent No. 5,028,410 to separate
a pregnant solution containing most of the zinc that
Swa~ in the calcine from a solid residue containing
nearly all of the iron thnt wss in the calcine. The
solid residue i8 returned to the zinc refinery for
further treatment or can be treated otherwise according
to conventional means.
10The pregnant solution is then fed to a
cementation step as described in U. S. Patent No.
5,028,410 for removal of impurities such as cadmium and
copper where zinc dust is used for cementation
purposes.
15A liquid-solid separation step as described in
U. S. Patent No. 5,028,410 is then employed to separate
the purified solutions from the solid residue.
The purified solution is then fed to thermal
decomposition vessels as described in U. S. Patent No.
205,028,410 wherein basic zinc carbonate i8 precipitated
and much of the ammonia is stripped off and sent to
ammonia recovery equipment, also described in U. S.
Patent No. 5,028,410.
The solid phase containing the basic zinc
25carbonate is separated from,,the remaining liquid phase
by liquid-solid separation techniques with the liquid
phase being recycled to an' earlier step in the process.
The basic zinc carbonate solid is then washed to
remove entrained impurities and can be marketed as
3~such or fed to a calciner where its carbon dioxide
content and water content can be driven off in order to
produce zinc oxide.
The calcining step is operated at a temperature
between 275C and 600C to produce an essentially pure,
20727~
high surface area zinc oxide having a range of 10 to 75
m2/g~
In a second embodiment, the procedure of the
first embodiment is followed except that the calcining
temperature is operated at a temperature of from 600 to
1000C to produce a zinc oxide product that can be made
to be essentially equivalent to French Process zinc
oxide.
In the third embodiment, both the first and
second embodiments would both be used so that both high
surface area zinc oxide could be produced to serve
certain markets and low surface area zinc oxide could
b~ produced for other markets.
Some of the benefits of the improved invention
over the previous invention are described below.
Details of the variou~ improvements are shown in
the examples to follow. The improvements apply to both
embodiments.
In the first instance, the bed overflow is
greatly superior composition to the normal calcine,
such as used in U. S. Patent No. 5,028,410. In
addition, if the normal calcine consisted only of
roaster dust carryover material, the comparison
between the two materials would be substantially
greater insofar as to the improvement characteristics
of the bed overflow~ material are concerned.
Unfortunately, we do not have any examples yet to
compare such differences.
Secondly by using bed overflow calcine as a feed
material, downstream processing during continuous
operations becomes greatly simplified, particularly in
relation to the costs and complexities incurred in the
treatment of the recycle liquor returned as a feed
I
6 21~72~
material to the ammoniacal ammonium carbonate leaching
system.
Thirdly, the leach liquor purification step i8
simplified by the reduction in cadmium be removed.
Fourthly, the chemical analyses of the
intermedlate product, namely basic zinc carbonate, and
the final zinc oxide product are improved.
Fifthly, there is no desire or need for a pre-
- washing ~tep and subsequent liquid-solid separation
step prior to feeding to the ammoniacal ammonium
carbonate to leaching ~essels.
In the sixth place, the liquid-Rolid separation
step following the ammoniacnl ammonium leaching step i~
improved because, of the larger particles for one
thing.
There are other interesting advantages, such as
improved water washing steps and other items not
specifically mentioned here but will become obvious
from a study of the examples.
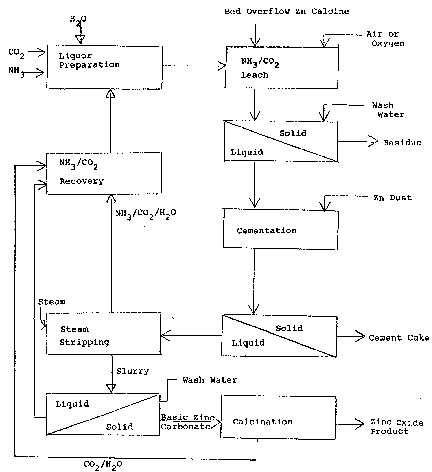
Brief Description of Drawinq
Fig. 1 provides a description of the process in
the form of a block diagram.
Example 1
In this example a specimen of normal zinc calcine
and a specimen of bed overflow zinc calcine were
obtained and analyzed. The results of the analysis are
reported in Table 1. The bed overflow calcine
contained lesser amounts of arsenic and sulphur
compared to the normal calcine. The bed overflow
- 30 calc.~ne contained 61.2% extractable zinc, comprising
61.0% in an ammonia-soluble form and 0.2% in a water-
soluble form. The normal calcine contained 57.7%
2~7~
extractable zinc ~ comprising 54.9~ in an ammonia-
~oluble form and 2.8% in a watsr-soluble form.
A ~pecimen of normal and bed overflow calcine
were separately processed in accordance with the
process described below.
The calcines were leached in a 28% aqueous
ammoniacal ammonium carbonate solution which was
prepared by feeding gaseous carbon dioxide into a
concentrated ammonium hydroxide solution with vigorous
stirring. The final pH of this solution was 11Ø An
amount of 450 g of calcine was leached in 1.5 litres of
this aqueous ammoniacal ammonium carbonate solution
with agitation at a temperature of 50C. Leaching was
conducted for 40 minutes with the occasional addition
of gaseous carbon dioxide gas to maintain the pH at
approximately 11Ø Th~ resulting slurry was filtered
through a filter and the resultinq filtrate and residue
were analyzed. The assay of the resultant filtrate and
residue is reported in Table 2.
A mixture comprising 0.5 l/minute of air and O.S
l/minute of oxygen was sparged into the leaching
vessel. The leach filtrate from the bed overflow
calcine contained 0.013 g/l arsenic and 0.22 g/l
sulphur, whereas the filtrate from the normal calcine
contained 0.052 g/l arsenic and 3.99 g/l sulphur.
The leach filtrate from the bed overflow calcine
was then purified by ~inc dust cementation for 30
minutes at a solution pH of approximately 11.0 and a
solution tempexature of approximately 40C. 8 g/l of
fine zinc dust was used to cement out almost all the
copper, cadmium, lead and cobalt in the filtrate;
arsenic and sulphur were not affected. Analysis of the
feed solution, and the cementation residue and filtrate
are provided in Table 3.
2~72~
The purified zinc solution obtained from the zinc
dust cementation step af ter liquid xolid separation was
steam-stripped by in~ecting live ~team into the
purified zinc solution until most of the ammonia was
5 expelled and basic zinc carbonate precipitated,
approximately. AnalyE~es of the purified zinc
solution, the resulting precipitate, and the depleted
solution are reported in Table 4. The p~ of the f inal
solution was reduced to between approximately 8 to 8.5.
In this particular example, it is obvious that
the bed overflow calcine was of superior quality to
normal calcine in that the extractable zinc is
substantially higher, 61.296 versus 57.796. Also the
total zinc is higher 67.0% versus 63.796.
The cadmium content of the bed overflow calcine is
very much lower than the normal calcine, thus
resulting in simpler leach lîquor purification.
The particle size of the bed overflow is much
larger, thus resulting in easier liquid-solid
separation.
The zinc sulphate content of the bed overflow is
essentially negligible as compared to that of normal
calcine thus indicating a much simpler and less costly
recycle system for the improved process.
It i8 also obvious in this example that there is
no need for a pre-water-washing step and consequent
liquid-solvent separation~'since this step is sometimes
desirable to remove sulphates and it can be seen that
the sulphate content of the bed overflow material is so
low that ~uch a washing step would not be either
desirable or necessary.
9 2~727.~
Example 2
In this example a ~pecimen of normal zinc calcine
and a specimen of bed overflow calcine were treated in
general accordance with the process disclosed in
Example 1 above.
The basic zinc carbonate was then calcined at
l,000C. Analysis of the resulting calcine i~
presented in Table 5.
It is appsrent from Example 2 that bed overflow
calcine produces a higher purity zinc oxide as compared
to normal calcine with respect to the following
elements:
Bed OverflowNormal Calcine
Calcine (wt %) lwt %
Arsenic - 0.006 0.024
Sulphur - 0.004 0.02
Cadmium - ~0.0005 ~0.00004
Iron - 0.003 0-0059
Silicon - <0.001 0.016
2~727
~r~
CALCINE ANALYSIS
Element Normal Calcine Bed Overflow
N~ight ~ Weight % Calcine ~eight %
__ . ____ __
Zn 63.7 67.0
Cu 0.85 0.92
Cd 0.33 0.14
Co 0.02 0.017
Fe 10.1 9.31
Pb 0.05 0.022
Ca 0.22 0.12
Mg 0.063 0.068
Si 0.55 0.01
As 0.04 0.015
Cl 0.002 0.005
S 1.8 0.14
Mn 0.02 0.01
Zinc ComPonents
Normal Bed Overflow
Calcine Calcine
wt % Zn wt % Zn
Zn So4 (water soluble) 2.8 0.2
Zn O (ammonia soluble) 54.9 61.0
Zn SiO2 (acetic acid soluble) 1.8 2.1
Zn Fe2O4 (H3 PO4/HCL soluble) 4,4 4.2
Total 63.9 67.5
Size Analvsis
Normal Bed Overflow
Calcine Calcine
wt % Pas~ing wt % Pas~ing
100 Mesh 94.4 1 52.4
200 Mesh 87.3 28.7
400 Mesh 68.0 2.6
7 2 7 ~
TA~I~ 2
NO~mA 1 Calcine sed Overfluw Calcine
~lement Filtrate Residue Filtrate Residue
g~l wt % g/l wt %
zn 150 29.5 185 30.5
Cu 1.58 1.01 1.58 1.53
Cd 0.48 80.2 0.27 0.17
Co 0.017 0.027 0.022 0.023
Fe 0.008 35.2 0.035 35.5
Pb 0.009 0.14 0.011 0.064
Ca 0.026 0.45 0.048 0.37
Mg 0.033 0.15 0.055 0.18
Si 0.01 1.03 0.012 1.06
A~ O . 052 0.085 0.013 0.043
Cl 0.03 _ ~.035 0.005
S 3.99 0.25 0.22 0.05
Mn <O.0002 0.03 0.0003 0.038
12 2~7
TABLB 3
CEMENTATION OF BED OVERFLOW CALCINE LEACH LIQUOR
Leach ~iquor
Element Feed Solution Filtrate Residue
g/l g/l wt %
. . . _ . . _ _ .
Zn 185 190 65.6
Cu 1.58 0.0002 26.7
Cd 0.27 0.001 5.29
Co 0.022 0.0004 0.42
Fe 0.035 0.014 0.37
Pb 0.011 0.0008 0.25
Ca 0.048 0.051 0.005
Mg 0.055 0.061 0.009
Si 0.012 0.017 0.06
As 0.013 0.009 0.017
Cl 0.035 0.044 0.005
S 0.22 0.29 0.014
Mn O.0003 0.0003 0.O
13 ~727~'~
TAJ3IJ3 4
STEAII STRIPPING
Final
l~ t Feed SolutionSolution Precipitate
g/l g/l w~: %
.
Zn 190 ~.005 60 r 0
Cu 0.0002 0.0002 0.005
Cd 0.001 0.0002 0.004
Co 0.~004 0.0003 0.003
Fe 0.014 0.0003 0.013
Pb o.ooO~ 0.001 0.011
Ca 0.051 0.002 Q.023
Mg 0.061 0.0002 0.025
'S~ 0.017 0.003 O.OS
As O.Oo9 0.001 0.014
Cl 0.044 0.036 0.005
S 0.29 0.081 0.09
Mn 0.0003 0.0001 0.0009
NH3 190 0.46 0.24
Amount 0.875L 1.139L 251.57g
The amount of feed solution used was 0.875 1,
the final solution amounted to 1.139 1, and the dried
precipitate weighted 251.57 g.
i~
14 2 ~3 7 2 1 L `~
TAsL~ 5
~ed Overf low
Element Normal Calcine Calcine
Rt % Wt %
. .
Zn 81.5 81.2
Cu O . 0019 0 . 001
Cd <0.0005 <0.00004
Co ~0.0005 0.001
Fe 0.0059 0.003
Pb <0.0005 0.0006
Ca 0.021 0.031
Mg 0.022 0.026
Si 0.016 <0.001
A8 0.024 0.006
Cl <0.004 <0.004
S 0.02 0.004
Mn <O .0002 0.0001
NH3 <0.0006 0.046
Li 0.00003 0.00004
Al 0.002 0.002