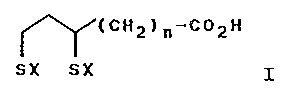Note: Descriptions are shown in the official language in which they were submitted.
2C°'~~~DB
- 1 -
The invention relates to the use of compounds of formula
~(CH2)p-C02H
SIX SIX I
where X is a hydrogen atom or both X represent a single bond
between the two sulphur atoms and n is a number between 1 and
l0, and their therapeutically .applicable salts to combat
physiologically-induced excitatory disorders and diseases
related thereto as well as allergic diseases and also the
preparation of corresponding medicaments.
The amounts by weight cited in this specification relate in
each case to the pure compounds of Formula I and to their
enantiomers, that is not to their salts. If salts are used,
the appropriate dosages must be used in each case and be
appropriately increased to suit the altered molecular weight.
The compounds of Formula I are for example alpha-lipoic acid
(racemate and the two optical isomers R(+)- and S(-)-form) as
well as dihydrolipoic acid (racemate and the two optical
isomers R(+)- and S(-)-form), that is compounds of Formula I
where n is the number 4 and X has the meanings given.
Alpha-lipoic acid and the S(-)-form of alpha-lipoic acid are
particularly effective in respect of the effects described in
the present patent application. This applies in particular to
the effect in withdrawal symptoms and drug abuse.
Alpha-lipoic acid is widespread in plants and animals in the
form of the racemate (ThioctacidR); it acts as a coenzyme in
many enzymatic reactions, constitutes a growth factor for
various bacteria and protozoa and is used in death-head
poisoning. The alpha-lipoic acid racemate also has
anti-inflammatory, antinociceptive (analgesic) and
cytoprotective properties.
Thioctacid is marketed for the following indications:
fatty liver and fatty cirrhosis,
chronic liver disease due in particular to
alcohol,
liver damage due to mushroom poisoning,
diabetic neuropathy,
alcoholic neuropathy.
Dihydrolipoic acid is 6,8-dimercapto-octanic acid. It is~known
from animal experiments that dihydrolipoic acid inactivates
''snake venom. These investigations were for example conducted in
.rats and mice, using solutions in water or physiological salt
solution which contained the snake venom and dihydrolipoic acid.
The compounds of Formula I may also be used in the form of their
therapeutically applicable salts. Salts of this kind are
prepared in the conventional manner.
Salt-formers that may for example be used are conventional bases
or.cations which are physiologically acceptable in the salt
form. Examples hereof are:
2~'"~~~~3'~
- 3 -
alkaline or alkaline earth metals, ammonium hydroxide, basic
amino acids such as arginine and lysine, amines of formula
NR~R2R3 where the radicals R~, R2 and R~ are the
same or different and represent hydrogen, C~-C4-alkyl or
C~-C4-oxyalkyl such as monoethanolamine and diethanolamine,
1-amino-2-propanol, 3-amino-1-propanol; alkylene diamine with
one alkylene chain of 2 to 6 carbon atoms such as ethylene
diamine or hexamethylenetetramin, saturated cyclic amino
compounds with 4 to 6 ring carbon atoms such as piperidine,
piperazine, pyrrolidine, morpholine; N-methylglucamine,
creatine, tromethamine.
The compounds of the invention of Formula~I have a favourable
effect in pathophysiologieally caused excitatory disorders,
particularly as a consequence of degenerative (preferably
neurodegenerative) diseases of the central nervous system and
the heart and the changes in excitability associated therewith.
The compounds of the invention are in particular also suitable
to combat withdrawal symptoms such as occur after the abuse of
alcohol, sedatives or drugs. Appropriate medicaments containing
the compounds of formula I as active substances have for example
and antidegenerative and antiatrophising as well as
excitability-stabilizing effect on the central nervous system
and the heart as well as an anti-arrythmic,
rmetabolism-regulating, memory performance increasing, general
geriatric and also anti-allergic effect.
In addition, the compounds of Formula I are able, because of
their excitability-stabilizing effect to exert a favourable
influence on functional disturbances of the central nervous
system. Examples that may be cited are withdrawal symptoms after
drug abuse and the symptoms of "panic disorder".
The compounds of Formula I also display an effect in damage to
the brain and heart, for example in Morbus Alzheimer, Korsakow
syndrome, particularly in view of the memory impairment
associated with these illnesses, such as also occur in the
context of cerebro-organic psychosyndrome.
- rG~~a~~~~
The compounds of the invention of Formula I display for example
in t:he model to determine the prevention of changes in the EEG
of t:he rat caused by hypoglycaemic or ischaemic insult (as
compared to the pre-value) in parenteral application (i.p. -
intraperitoneal) at 30 mg/kg (i.p.) of the racemate of
alpha-lipoic acid 118 %, in 30 mg/kg i.p. of the (+)-enantiomer
157 % and at 30 mg/kg i.p. of the (-)-enantiomer 85 % change in
the performance density of the beta-2-band in the frontal
cortex.
The dose range for an effect in the above mentioned'experimental
model is for example 10 - 100 mg/kg, in particular 15 - 45 mg/kg
far the parenteral (intraperitoneal) application.
This investigation was conducted by analogy with the method by
Dimpfel et al., Neuropsychobiology 16: 163 - 168, (1986) except
that the lead was taken from the hippocampus instead of the
thalamus.
(Radioelectroencephalography (Tele-Stereo-EEG) in the rat as a
pharmacological model to differentiate the central action of
flupirtine from that of opiates, diazepam and phenobarbital).
This test demonstrates effect in the following clinical
pictures:
allergic and metabolic-disturbance-related central and
peripheral neuropathies with disturbed memory performance as
'.well as nerve conductivity speed for example age-related
deterioration in brain performance (minor brain dysfunction).
A brief description of the above mentioned test method: 4
bipolar concentric electrodes together with a microplug on a
single baseplate were implanted in 4 - 5-month-old male
Fischer-344 rats. The plug was used to take up a 4-channel
transmitter for telemetric transmission of the field potentials
emitted by the frontal cortex, hippocampus, striatum and
Formatio Reticularis. The signals were subjected to a Fast
Fourier transformation in a Pro Science Computer System in real
time and the performance density spectra meaned in each case
r~i~ '~.~~~~~
- 5 -
over 15 minutes. The subdivision of the spectra in 6 different
frequency ranges permits the recordal of pharmaceutical-specific
changes in relation to the pre-values within these frequency
bands measured in each case before application.
Information on the application protocol on the substances:
The substances were injected intraperitoneally 45 minutes after
cammencement of measurements (pre-value). Measurements resumed
five minutes later, were analysed continuously for at least the
next 2 hours and summarized in 15-minute periods. The test
substances (for example Thioctacid) were applied in a dosage of
30 mg/kg.
The experimental series began with the injection of placebo.
There followed the first trial of deprenyl. After 4 weeks the
chronic application of, for example, R(+)-alpha-lipoic acid
began and continued for 7 days with 2 injections/day. Two days
after the last injection the effect of deprenyl was measured
again. After a 1-week pause for the animals, the effect of
R(+)-alpha-lipoic acid was checked again and then once more, 1
week later. After a subsequent Wash-out period of a further week
and another placebo series, the chronic application of
S(-)-alpha-lipoic acid began (total of 4 weeks after.completion
'=~of the first chronic application). In conclusion 2 days after
.the last S(-)-alpha-lipoic acid application the effect of
deprenyl was recorded once again. During the chronic application
of alpha-lipoic acid (racemate) the effect was measured on days
1, 3, 5 and 7.
Statistical comparison of the trials was effected using a
multivariant analysis after Ahrens and Lauter (1974) on the
basis of changes within the individual frequency bands in all
regions of the brain as variables.
- 6 -
The anti-allergic effect is inter olio proven by investigations
in PAF (platelet activating factor) acether-oedema in the rat.
The principle of the method is to induce a localized oedema or
inflammation in the rat paw using subplantar injection of
PAF-acether 2 ug/animal. The investigations are conducted
according to the method after SWINGLE and REITER (Agents and
actions, 18, 358 - 365, (1986). The animals were given the test
substances 0.5 hours before triggering of the oedema. 1 hour
thereafter the oedema was determined plethysmographically.
The following results were obtained:
Oedema inhibition in % Dose mg/kg
intraperitoneallv
alpha-lipoic acid 16 50
14 25
dihydrolipoic acid 63 50
73 200
Platelet activating factor is one of the partial mediators for
the immediate allergic reaction. In the previously mentioned
experimental model, oedema inhibition is therefore also proof of
an anti-allergic effect.
~tndications that may for example be considered for the compounds
of Formula I are:
tissue-damaging processes of the central nervous system and the
heart as well as the tissue necroses resulting therefrom; .
ischaemic or hypoxically-induced late organ lesions, in
particular of the central nervous system and the heart, such as
micro- and macroangiopathies and the hyperexcitability phenomena
associated therewith; rhythm disturbances of the heart; damage
to the brain or heart, for example Morbus Alzheimer, Korsakow
syndrome, in particular in respect of the memory impairment
associated with these illnesses also occurring in the context of
the eerebro-organic psychosyndrome; allergy-related and
2C~'~~4~~~
- 7 -
metabolic disorder-related (for example due to diabetes)
central and peripheral neuropathies and polyneuropathies
(wit:h impaired memory performance and nerve conductivity
speed) age-related deterioration of brain performance (minor
brain dysfunction). Neuropathophysiological dysfunction in
central excitability such as in the clinical picture of panic
disorder (Margraf J. and Schneider S.: Panik, published by
Springer Verlag 1990) as well as withdrawal symptoms after
drug abuse.
The latter indication is for example confirmed by trials in
rats treated daily for 2 weeks with higher doses of alcohol
or morphine. After withdrawal for 16 hours, the brains of
these animals showed hyperexcitability clearly recordable in
vitro under controlled conditions, which is totally
normalized by the presence of 100 uM of a compound of Formula
I. A comprehensive description of these trials is given in
Annex A. This Annex A is a constituent part of the
specification of this patent application.
The daily doses of the dosage forms of the invention.for the
above mentioned effects consist for example of 0.1 to 800 mg,
preferably 15 to 400 mg and in particular 50 to 300 mg of
Compound I (for example alpha-lipoic acid or dihydrolipoic
acid or their enantiomers).
In accordance with the invention a daily dose of Compound I
(for example alpha-lipoic acid or dihydrolipoic acid or their
enantiomers) of 0.1 preferably 10 to 800 mg, for example 25
to 400 mg, in particular 10 to 300 mg is given.
The maximum daily dose should not exceed 800 mg. The daily
doses may be used in the form of a single administration of
the entire amount or in the form of 1 to 6, in particular 1
- 7a -
to 4 partial doses per day. In general an administration of
1 t.o 4 times, in particular 1 to 3 times daily is preferred.
The preferred daily dose is for example 80 to 350 mg for the
parenteral form of
l0
_ 2(''7~~8F'~
application and 800 mg for the oral form. The daily dose for the
parenteral form of application is in particular 50 mg and 400 mg
for the oral form.
The medicaments are preferably given orally.
The compounds of Formula I {for example alpha-lipoic acid and
dihydrolipoic acid or their enantiomers) may also be applied in
the form of a solution, for example by the peroral, topical,
parenteral, intravenous, intramuscular, subcutaneous, nasal,
inhalative, rectal, transdermal route.
Medicaments containing as active substance one or several
compounds of Formula I (for example alpha-lipoic acid or
dihydrolipoic acid or their enantiomers) may for example be
formulated in the form of tablets, capsules, pills or coated
tablets, granulates, suppositories, pellets, solutions or
emulsions, the active substance being combined with appropriate
auxiliary substances and carriers. Solutions contain for example
0.5 to 20 % by weight, preferably 1 to 10 % by weight of the
compound of Formula I.
The dosage unit of the medicaments containing one or several
compounds of Formula I or a therapeutically applicable salt
thereof as active substance may for example contain:
a) in the case of peroral medicinal forms:
in particular 50 to 400 mg compound/active substance I (for
example alpha-lipoic acid or dihydrolipoic acid). The doses
may for example be given 1 to 6, preferably 1 to 4, in
particular 1 to 3 times daily. A total dose of $00 mg per
daily should, however, not be exceeded. The same also
applies to the following medicinal forms listed under b) to
e).
- ~;~'7~~8'~
b) in the case of parenteral medicinal forms (for example
intravenous, intramuscular):
to 350 mg, preferably 15 to 350 mg, in particular.20 to
150 mg compound/active substance I. The doses may for
example be given 1 to 6, preferably 1 to 4, in particular 1
to 3 times daily.
c) in the case of medicinal forms for rectal application:
10 to 500 mg, preferably 40 to 400, in particular 50 to 200
mg compound/active substance I. These doses may for example-
be given 1 to 6, preferably 1 to 4, in particular 1 to 3
times daily.
d) in the case of medicinal forms for application to the skin
and mucous membranes (for example as solutions, lotions,
emulsions, ointments and the like):
10 to 500 mg compound/active substance I, preferably 40 to
250 mg, in particular 5 to 200 mg. The doses may for example
be given 1 to 6, preferably 1 to 4, in particular 1 to 3
times daily.
e) In the case of medicinal forms for the inhalation of
solutions or aerosols:
0.1 to 300 mg, preferably 0.25 to 150 mg, in particular 0.5
to 80 mg compound/active substance I. These doses may for
example be given 1 to 6, preferably 1 to 4, in particular 1
to 3 times daily.
If solutions are used, the compound/active substance I is
preferably used in the form of a salt.
It is of course also possible to prepare pharmaceutical
formulations which contain the above mentioned dosage units 2 to
for example 6 times.
o~~.~~ e'1YC1' t
In particular tablets or capsules contain 20 to 600 mg; pellets,
powders or granulates 20 to 500 mg; suppositories 20 to 400 mg
compound/active substance I.
The medicaments with one or several active substances of Formula
I appropriately also contain 0.001 to 1 part by weight of
antioxidant (related to 1 part by weight of compound I).
Antioxidants that may for example be considered are: sodium
sulphite, sodium hydrogen sulphite, sodium metabisulphite,
ascorbic acid, ascorbyl palmitate, ascorbyl myristate, aseorbyl.
stearate, gallic acid, gallic acid alkyl ester,
butylhydroxyanisol, nordihydroguaiacic acid, tocopherols as well
as synergists (substances which bind heavy metals through
complex formation, for example lecithin, ascorbic acid,
phosphoric acid, ethylenediaminetetra-acetic acid, citrates,
tartrates). The addition of synergists considerably enhances the
antioxygenic effect of antioxidants.
Preservatives that may for example be considered for the
medicaments of the invention are sorbic acid, p-hydroxybenzoic
acid esters (for example lower alkyl esters), benzoic acid,
sodium benzoate, trichloroisobutyl alcohol, phenol, cresol,
benzethonium chloride, chlorhexidine and formalin derivatives.
-Complex formers that may for example be considered are: chelate
.~formers such as ethylenediaminetetra-acetic acid,
nitrilotriacetic acid, diethylenetriaminepentaacetic acid and
their salts.
The complex formers may also be those which enclose
dihydrolipoic acid in a cavity. Examples of these are urea,
thiourea, cyclodextrins, amylose.
Adjustment to a pH range of approx 6 to 9 with physiologically
acceptable bases or buffers may optionally be necessary to
stabilize the active substance molecule. As neutral or weakly
basic a pH value (up to pH 8) is generally preferred.
~~'~;~~D8!~
_ 11 _
Examples for pharmaceutical formulations
Examinle 1:
Tablets containing 50 mg S- or R-alpha-lipoic acid
250 g S-alpha-lipoic acid are evenly ground with 750 g
microcrystalline cellulose. After seiving the mixture, 250 g
starch (starch 1500/Colorcon), 732.5 g lactose, 15 g magnesium-
stearate and 2.5 g highly disperse silicon dioxide are mixed in
and the mixture pressed into tablets weighing 400.0 mg.
One tablet contains 50 mg S-alpha-lipoic acid.
In the same way it is possible to prepare tablets containing 50
mg R-alpha-lipoic acid by using the same amount of
R-alpha-lipoic acid instead of 250 g S-alpha-lipoic acid.
The tablets may optionally be provided with a gastric
juice-soluble or gastric juice-permeable film coating using
conventional methods.
Example 2:
Ampoules containing 50 mg S- or R-alpha-lipoic acid as
tromethamine salt in 2 ml
250 g S-alpha-lipoic acid are dissolved together with 352.3 g
tromethamine (2-amino-2-(hydroxymethyl)-1,3-propandiol) in a
mixture of 9 litres of water for injection purposes and 200 g
1,2-propylene glycol with stirring. The solution is made up to
litres with water for injection purposes and then filtered
through a membrane filter of pore size 0.2 um with a glass fibre
pre-filter. The filtrate is filled under aseptic conditions in 2
ml portions into sterilized 2 ml ampoules.
L
- 12 -
One ampoule contains 50 mg S-alpha-lipoic acid as tromethamine
salt in 2 ml injection solution.
In ;similax manner it is possible to prepare ampoules with
R-alpha-lipoic acid by using the same amount of R-alpha-lipoic
acid instead of 250 g S-algha-lipoic acid.
Examgle 3:
Ampoules with 250 mg dihydrolipoic acid in 10 ml infection
solution
60 g tromethamine and 1 g ethylenediaminetetraacetic acid,
disodium salt are dissolved in 1.8 litres water for injection
purposes. The solution is gassed with nitrogen for 30 minutes. 2
g sodium disulphite and then 50 g dihydrolipoic acid are
dissolved in the mixture with continued nitrogen gassing. The
solution is made up to a volume of 2 litres with water for
injection purposes gassed with nitrogen. After careful mixing
the solution is filtered over a membrane filter of pore size 0.2
um and the filtrate is filled under aseptic conditions and with
pre- and post-gassing with nitrogen into ampoules~having a
filling volume of 10 ml.
One ampoule contains 250 mg dihydrolipoic acid as tromethamine
salt in 10 ml solution.
2C''7~~43~
- 13 -
The effect of compounds of Formula I (for example the L-
enantiomer of alpha-lipoic acid) on the hippocampus section
preparation of ethanol-dependent rats.
Results:
Preparations removed from the hippocampus of rats 1.6 hours
after discontinuation of ethanol (5g/kg/day ethanol'over a
period of 2 weeks) were subjected to decreasing magnesium
concentrations in the superfusion medium i,h order to detect
changes in plasticity changes in the excitability of the
pyramidal cells. The electrically triggered and spontaneous
discharges were monitored in the absence and presence of the
S(-)-form of alpha-lipoic acid.
Triggered discharges were found to be totally dampened by the
(-)-enantiomer of alpha-lipoic acid.
Material and methods:
Two groups of fully grown, male CD rats (Charles River Wiga)
received conventional rat feed ad libitum. The drinking
water was sweetened by addition of 1 g glucose/litre. One
group received water containi.zg 5 % ethanol. The amounts of
water taken up by the animals was determined daily and was an
average of 4O m1 in both groups. It follows that, during the
two-week trial - the ethanol group took up about 5 g/kg
ethanol within 24 hours starting from their average weight.
The animals received the modified drinking water over a
period of 2 weeks. Their body weight was measured daily, no
difference being observed between the two groups. 16 hours
before measurement of excitability commenced ex vivo, the
- 14 -
animals treated with ethanol also received glucose-containing
drinking water to initiate the withdrawal.
The animals were exsanguinated under slight anaesthetic, the
brain was removed in toto and the isolation of the Formatio
hippocampi monitored under a stereomicroscope. The middle
portion of the hippocampus was fixed using a cyanacrylate
adhesive in cooled phosphate-buffered salt solution'(NaCl 124
mM; KG1 5 mM; CaCl2 mM; MgS04 1.25 mM; NaHC03 26 mM; glucose
lOmM) on the table of a Vibratom and sliced into 400 um thick
sections. All sections were pre-incubated in a pre-chamber
for at least one hour before use in carbon-saturated salt
solution. During the experiment the sections were fixed
according to the method of Haas et al. (1979) and treated in
a special superfusion chamber. The preparation was flushed
with 180-240 ml/hour artificial liquor (see above).
Electrical stimulation (200 uA, 100 us) of the Schaffner-
collaterals within the CA2 range and recording of the
pyramidal cell section from CA1 was conducted by means of
conventional electrophysiological methods using the Pro
Science Laboratory Computer System "LabTEAM" and the software
package "NeuroT00L". For each reaction measurement the mean
value of 4 stimulations was determined at 20-second
intervals. Measurements were conducted regularly every 20
minutes in order to exclude potentiation characteristics.
After three stable values had been recorded before the
administration of compound T (active substance T) the
perfusion was switched over to the artificial liquor with
active substance T and maintained for at least 30 minutes.
The mean amplitude was determined from the three signals
before administration of active substance I and regarded as
100 ~. All mean changes after addition of active substance I
2C'~ :~D8~
- 15 -
relate to this percentage value. At least 6 independent
measurements were made under each experimental condition.
Ths~ amount of Mg++ was reduced stepwise in order to activate
the transfer controlled by the NDMA receptor. In addition to
the earlier measurements, the spontaneous activity of the
section preparation was monitored during this experiment.
The spontaneous activity in the form of spontaneous'
population peaks or even discharges was determined by sending
the signal through a shielding discriminator adjusted in such
a way that the appropriate signals of the individual sections
could be recognized.
The ~(-)-form of alpha-lipoic acid was tested at a dose of
246 ug/ml.
The effect of compounds of Formula I (for example the L-
enantiomer of alpha-lipoic acid) on the hippocampus section
preparation of morphine-dependent rats.
Results:
Preparations removed from the hippocampus of rats 16 hours
after withdrawal of morphine (2 mg/kg morphine subcutaneously
2 x daily for 2 weeks) were exposed to decreasing magnesium
concentrations in the superfusion medium in order to
demonstrate plasticity changes in the excitability of the
pyramidal cells. The electrically triggered and spontaneous
discharges were observed in the absence and presence of the
S(-)-form of alpha-lipoic acid. The (-)-enantiomer of alpha-
lipoic acid was able to diminish the spontaneous discharges
and reduce the peak amplitudes of the population to normal
values which were intensified with lower magnesium
'~~"~~.~8~ -
- 16 -
concentrations. This suggests and application/use of alpha-
lipoic acid in withdrawal symptoms in drug dependence.
Material and methods
Two groups of fully grown, male CD rats (Charles River Wiga)
received commercial rat feed ad libitum. During the twa '
weeks of the trial the animals of the first group received 2
mg/kg morphine twice daily. The control group received
injections of salt solution. Body weight~was recorded daily.
16 hours before measurement of excitability commenced ex
vivo, injections were discontinued in the animals treated
with morphine in order to initiate withdrawal.
The animals were exsanguinated under slight anaesthetic, the
brain was removed in toto and the isolation of the Formatio
hippocampi monitored under a stereomicroscope. The middle
portion of the hippocampus was fixed using a cyanacrylate
adhesive in cooled phosphate-buffered salt solution (NaCl 124
mM: KC1 5 mM~ CaCl2 mM1 MgS04 1.25 mM; NaHC03 26 mM: glucose
lOmM) on the table of a Vibratom and sliced into 400. um thick
sections. All sections were pre-incubated in a pre-chamber
for at least one hour before use in carbon-saturated salt
solution. During the experiment the sections were fixed
according to the method of Haas et al. (1979) and treated in
a special superfusion chamber. The preparation was flushed
with 180-240 m1/hour artificial liguor (see above).
Electrical stimulation (200 uA, 200 us) of the Schaffner-
collaterals within the CA2 range and recording of the
pyramidal cell section of CA1 was conducted by means of
conventional electrophysiological methods using the Pro
Science Laboratory Computer System "LabTEAM" and the software
package "NeuroTOOL". For each reaction measurement the mean
- 17 -
value of 4 stimulations was determined at 20-second
intervals. Measurements were conducted regularly every 10
minutes in order to exclude potentiation characteristics.
After three stable values had been recorded before the
administration of compound I (active substance I) the
perfusion was switched over to the artificial liquor with
active substance I (see above) and maintained for at least 30
minutes.
The mean amplitude was determined from the three signals
before administration of active substance I and regarded as
100 %. All mean changes after addition of active substance I
relate to this percentage value. At least 6 independent
measurements were made under each experimental condition.
The amount of Mg++ was reduced stepwise in order to activate
the transfer controlled by the NDMA receptor. Apart from the
earlier measurements, the spontaneous activity of the section
preparation was monitored during this experiment. The
spontaneous activity in the form of spontaneous population
peaks or even discharges was determined by sending the signal
through a shielding discriminator adjusted in such a way that
the appropriate signals of the individual sections could be
recognized.
The S(-j-form of alpha-lipoic acid was tested at a dose of
100 uM.