Note: Descriptions are shown in the official language in which they were submitted.
GLVCrROL FUNC~IONAL POLYSI~OXANES
This invention relates to personal care and more particu-
larly to certain glycerol functional polysiloxanes useful as
humectants in skin care applications.
The water content of the outer layers of the stratum
corneum of the human epidermis is a controlling factor in the
appearance of dry skin symptoms. When the stratum corneum
contains an adequate amount of waier within the range of about
10 to 20 percent, the skin remains flexible. However, when the
water content falls below about ten percent, the s.ratum orneum
often becomes brittle and rough and can e~hibit scaling and
cracking.
The stratum corneum receives its water from the deep
layers of the epidermis by diffusion or when it is brought int~
direct contact with water. The dif~usion process is controlled
by the water content of the skin as well as by the concentration
gradient. In a very dry environment for example, the water loss
from the external skin layers can be significant and often
Iexceeds the rate of replenishment by the diffusion process.
IIt is not uncommon therefore to include in skin
condltioning compositions a humactant which is capable of intro-
ducins moisture to the skin from t~e atmosphere in conditions of
modexate or high humidity. In conditions of low humidity
humectants attract mois~ure from the lower layers of the skin.
Humectants are materials which are hygroscopic and are therefore
capable of retaining moisture. Among the most well known water
retentive humectants capable of preventing drying out of the
skin is glycerol. Glycerol is known to be an ef,ective
humectant and is generally considered harmless in cosmetic
applications. It is a clear water white viscous li~uid having
the chemical formula HOCH2CHOHCH20H. However, glycerol exhibits
no bonding to the skin and hence is not dura~le or substartive
, ~
2~7~
- 2 -
wi~h the result that it can be washed from the skin surface. It
is used in many creams and lotions ior the purpose of keeping
the skin soft and for replacing skin moisture. -
The prior art is replete with various formulations which
contain glycerol as an ingredient. One such prior art compo-
sition is described in United States pat~nt application Serial
No. 07/489117 .iled 5th ~arch 1990 of Andrew H. Ward entitled
"Glyceroxyfunctional org2nosilicon Compounds", which application
is assigned to Dow Corning Corporation. The Ward application
discloses a silicone compound having the group -OCH~CH(OH)C~2OH
directly bonded to a silicon atom in the polymer main chain.
Upon contact with water, the silicone compound undergoes
hydrolysis with the result that the si-o bond is cleaved and
free glycerol is released onto the skin accom-panied by the
simultaneous formation of silanol groups. However, as already
noted, glycerol is not durable and can be easily removed from
the skin surface by washing.
In accordance with the present invention, there are
provided silicone compounds in which there is directly bonded to
a silicon atom in the main chain of the polymer the group
-(CH2)zOCH2CH(OH)C~2QH. These compounds differ from the
compounds disclosed by the Ward application in the presence of
the intervening spacer group -(CH2)z-. Because the Si-C bond is
not broken by water there is no hydrolysis to free glycerol as
in the Ward application. Rather, the compounds of the present
invention are durable and skin substantive which is an advantage
over the prior art as represented by the Ward application.
Because of this ad~antage of d~ability and substantivity, the
compounds of the present invention provide the benefit of longar
lasting moisturisation and exhibit humectant characteristics of
attracting water because of the presence in the molecule of the
glycerol functionality. The silicone portion of the molecule
contributes its benefits of skin softening, ~ilm forming and the
. .
~ ' ' ' ' ' ' ~
.:
~, , .
.~ . , .
- 3 -
facilitation of spreading of the composition over the skin
surfaces.
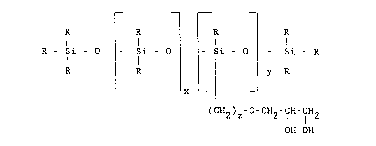
The present invention relates to new and novel compounds
which are glycerol functional polysiloxanes having the Lormula
R - Si - O ~- Si - O~ Si - o~ - Si - R
R ~ ¦ y R
L x
(cx2)z-o-cH2-c~-CH~
OH OH
wherein R is an alkyl group having from one to 5iX carbon atoms
or a phenyl group; x has a value of from zero to six hundred; y
has a value of from one to 5iX hundred and z has a ~alue o~ from
two to eight. While x can ~e 0 to 600 x preferably has a value
of from one to three hundred. More preferably x has a value of
from one to one hundred, and more particularly x has a value of
from forty-five to one hundred. While y can be 1 to 600,
preferably y has a value of from one to 100 and more prefera~ly
1 to ~0. While z can be 2 to 8 preferably z has a value o~ from
three to six. In the most preferred embodiment of the present
invention R is methyl; x has a value of 45 to 98; y has a value
of 1 to 10 and z is three.
While ~he compounds of the present invention can be used
for direct application to the skin, it is preferred to include
them as an ingredient in topical skin conditioning formulations.
The glycerol functional polysiloxanes may be present in the skin
conditioning formulation in an amount of about one to five per-
cent by weight, and the compounds are particularly suitable for
use in skin conditioning com~ositions which are in the form of
creams although the compounds are equally effective in other
delivered forms such as ointments, gels, lotions and emulsions.
.
.: . , : .: :
-- : -
. ~ :
~ ~3 PI~ 3
The process for preparlng the glycerol functional poly-
siloxanes of the present inventiOn involves contacting
alkenyloxy-1,2-propanediol with a polysiloxane having a reactive
site. Preferably the alkenyloxy group is 3-allyloxy but other
groups such 25 vinyloxy or ~-butenyloxy are also acceptable.
The diol and the siloxane are preferably heated under nitrogen
to about 75C in the presence of a solvent such as isopropanol.
The reaction mixture may be catalysed with a noble metal
catalyst such as chloroplat,nic acid and allowed to reflux for
thirty minutes. Any active metal catalyst is suitable ~or the
reaction, and particularly preferred are platinum catalysts such
as platinum acetylacetonate or chloroplatinic acid. The mixture
may then be cooled and the solvent e.g. isopropanol removed
under vacuum for example at about 110C. The reaction mechanism
as shown below has as reactive site R' hydrogen.
R [ R ~ L R ~ r R
H2P~Cl6.6H20
CH2 = CH - CH2 - O - CH - CH2 -~ -->
¦ l Isopropanol
OH OH
R ~ R ~ R R
R - Si - O _ ~x
CH2 ~ z-0-CH2-CE~-CH2
OH OH
Following are examples illustrating the preparation of
glycerol functional polysiloxanes according to the present
invention.
,
,
,:, . :- ', .
; ' , :'
~ E I
A mixture containing 16.43 weight percent of 3-allyloxy-
1,2-propanediol, 58.47 weight percent of the siloxane
Me3SiO(Me2SiO)45(Me~SiO)5SiMe3, O.2 percent by weight of sodium
acetate and 24.75 weight percent of isopropanol, was heated to
75~C under nitrogen. The mixture was catalysed with 0.15 weight
percent of the catalyst HZPtCl6 in the form of a 0.7688 percent
solu,ion in isopropanol. The mixture was allowed to reflux for
thirty mlnutes. The reaction mixture was allowed to cool and
the isopropanol solvent was removed by the application of a
vacuum OI 50 mm/Hg and heating the mixture to 110C. A product
was isolated and identified 2S the glycerol functional poly-
siloxane Me3SiO(Me2SiO)45(MeRSiO)5SiMe3 in which Me is methyl
and R is the group -cH2cH2cH2-o-cH2-c~3H-cH2oH.
E~ LE II
A mixture containing 9.57 weight percent of 3-allyloxy-
1,2-propanediol, 65.17 weight percent of the siloxane
Me3SiO(Me2SiO)47 5(MeHSiO)2 5SiMe3, 0.2 percent by weight of
sodium acetate and 24.91 weight percent of isopropanol, was
heated to 75OC under nitrogen. The mixture was catalysed with
O.15 weight percent of the catalyst H2PtC16 in the form of a
0.7688 percent solution in isoproanol. The mixture was allowed
to reflux for thirty minutes. The reaction mixture was allowed
to cool and the isopropanol solvent was removed by the appli-
cation of a vacuum of 50 mm/Hg and heating ~he mixture to 110C.
A product was isolated and identified as the glycerol functional
3 ( 2SiO)47.5(MeRSiO)2 5SiMe3 in which Me is
methyl and R is the group -CH2CH2CH2-O-CH2-CHOH-CH2OH.
EXAMPLE III
A mixturP containing 4.39 weight percent of 3-allyloxy-
1,2-propanediol, 70.35 weight percent of the siloxane
Me3SiO(Me~SiO)49(MeHSiO)lSiMe3, O.2 percent by weight of sodium
acetate and 24.91 welght percent of isopropanol, was heated to
: : : , . . .
~. :
- ~ - ,; .
:: . - .: . , ,
- 6 -
75OC under nitrogen. The mixture W2S catalysed with 0.15 weight
percent of the catalyst ~2PtC16 in the form of a 0.7688 percent
solution in isoproanol. The mixture was allowed to reflux for
thirty minutes. The reaction mixture was allowed to cool and
the isopropanol solvent was removed by the application of a
vacuum of ~0 mm/Hg and heating the mixture to 110C. A product
was isolated and identified as the glycerol functional poly-
siloxane Me3SiO(Me2SiO)49(MeRSiO)1SiMe3 in which Me is methyl
and R is the group -CH2CH2CH2-O-C~2-C~O~-C~2OH.
E~MP~E IV
A mixture containing 16.57 weight percent of 3-allyloxy-
1,2-propanediol, 58.30 weight percent of the siloxane
Me3SiO(Me2SiO)90(Me~Sio)10SiMe3, O.2 percent by weight of sodium
acetate and 24.78 weight percent of isopropanol, was heated to
75C under nitrogen. The mixture was catalysed with 0.15 weight
percent of the catalyst H2PtC16 in the form of a 0.7688 percent
solution in isoproanol. The mixture was allowed to reflux for
thirty minutes. The reaction mixture was allowed to cool and
the isopropanol solvent was removed by the application of a
vacuum of 50 mm/Hg and heating the mixture to 110C. A product
was isolated and identified as the glycerol functional poly-
siloxane Me3SiO(Me2SiO)90(MeRSiO)10SiMe3 in which Me is methyl
and R is the group -C~2CH2CH2-O-CX2-C~OH-C~2OH.
EXAMPLE V
A mixture containing 9.57 w~ight percent of 3-allyloxy-
1,2-propanediol, 65.17 weight percent of the siloxane
Me3SiO(Me2SiO)95(MeHSiO)5SiMe3, 0.2 percent by weight of sodium
acetate and 24.91 weight percent of isopropanol, was heated to
75C under nitrogen. The mixture was catalysed with 0.15 weight
percent of the catalyst H2PtC16 in the form of a 0.7688 percent
solution in isoproanol. The mixture was allowed to reflux for
thirty minutes. The reaction mixture was allowed to cool and
the isopropanol solvent was removed by the application of a
''
. ~ , , . - . .
- . . . .
.. , . . . :
!
vacuum OL 50 mm/Hg and hea~ing the mixture to 110C. A product
was isolated and identified as the glycerol functional poly-
siloxane Me3SlO(Me2SiO)95(MeRSiO)5SiMe3 in which Me is methyl
and R is the 5roup -C~2c~2c~2--cx2-cHH-c~2H
The polymerisation catalyst for the reaction can include a
variety of hydrosilation catalysts known to promote the reaction
of vinyl-functional radicals with silicon-bonded hydrogen akoms.
Ac~ive me.al catalysts such as platinum or rhodium-containing
metal compound are included in this class of catalysts.
Platinum catalysts such as platinum acetylacetonate or chloro-
platinic acid are representative of these compounds and suitable
for US2 . A preferred catalyst mixture is a chloroplatinic acid
complex of divinyltetramethyldisiloxane diluted in dimethyl-
vinylsiloxy endblocked polydimethylsiloxane which may be
prepared according to methods described by Willing in U.S.
Patent No. 3,419,593. Most preferably this mixLure contains
about 0.6 weight percent platinum.
Hyd.osilation catalysts are well known in the art and t~e
interes~ed reader is referred to the following patents for
detailed descriptions regarding their preparation and use:
Speier, U.S. Patent No. 2,823,218; Willing, U.S. Patent No.
3,419,359; Kookootsedes, U.S. Patent No. 3,445,420; Polmanteer
et al, U.S. Patent No. 3,697,473; Nitzsche, UOS. Patent No.
3,814,731~ Chandra, U.S. Patent No. 3,890,3S9 and Sandford, U.5.
Patent No. 4,123,604. Many of the catalysts known in the art
require the reactants to be heated in or~er for reaction to
occur. When such catalysts are employed this requirement must
be taken into consideration.
When platinum catalysts are used an inhibitor may be
required in ordex to improve the shelf life of the starting
materials and to control the viscosity-time profile of the
compositions. These inhibitors are also known in the art and
lnclude ethylenically unsa,ura~ed isocyanurates, such as
.
,
.
; , . - , .~ ~ .
- :
trialkylisocyanurate, dialkylacetylenedicarboxylates, alkyl
maleates, phosphines, phosphites, aminoalkyl silanes,
sulphoxides, acrylonotrile derivatives and others. Particular
inhibitors prererably used are diethyl ~umarate, bis(2-methoxy-
1-methylene)maleate, bis(2-methoxy~ methylethyl)maleate and
similar compounds.
The concentrations of catalyst and inhibitor to be used in
the present invention may be determined by routine experimen-
tation. Typically, the effective amount of catalyst should be
in a range so as to provide from about 0.1 to 1000 parts per
million (ppm) of platinum by weight in the compositions of the
present invention. As an example, when the preferred catalyst
mixture (i.e. the chloroplatinic acid complex of divinyltetra-
methyldisiloxane containing about 0.6~ by weight of platinum)
and inhibitor (i.e. bis(2-methoxy-1-methylethyl)maleate) are
employed, a ratio by weight of inhibitor to catalyst mixture
ranging from zero to about 0.6 provides a suitably wide range of
inhibition which is adequate under mos~ practical conditions of
manufacture.
The substantivity of the waxy glycerol functional poly
siloxanes of the present invention wa~ verified ~y the use of in
vivo Fourier transform infrared spectroscopy with attenuated
total reflectance (ATR/~TIR) in accordance with the procedure
described in the Journal of the SocietY of Cosmetic Chemists,
Volume 37, pages 73 to 89, March/April 1986. Test data
indicated that the waxy glycerol functional polysiloxanes were
capable of providing an occlusive ~ilm on the skin similar to
petrolatum which enhances moisturisation of the skin.
It will be apparent from the foregoing tha~ many other
variations and modifications may be made in the compounds and
compositions described herein without departing from the
essential essence of the invention. It should be understood
:
,
~'
that the forms of the invention described herein are exemplary
and not intended as limitations on the scope o~ the present
invention as defined in the appended claims.
, ~ ;
~ - :