Note: Descriptions are shown in the official language in which they were submitted.
WO 92/1156 PCT/US91/09328
°-1-
IMPROVED SPECIMEN TEST SLIDE AND METEOD OF
TESTING FORFECAL OCC17LT BLOOD
By: ~osefina T. Baker
Field Of The Invention
The present invention relates to devices and
methods for testing specimens and more particularly to
an improved specimen test slide for fecal occult blood.
Background Of The Invention
Fecal occult blood testing has become a papu-
lar, widely used procedure useful in the detection of
relatively small amounts of blood in fecal specimens,
This wide use and popularity arises primarily because
fecal occult blood testing is non-invasive, simple and
inexpensive to perform. Because the presence of fecal
occult blood in a specimen is a symptom that may be
associated with colon cancer or a precursor to colon
cancer, fecal. occult blood testing is often routinely
used as a screening tool. The routine screening of
patients by means of fecal occult blood testing has
helped to detect colon cancer at a stage where the
disease is readily treatable.
A popular form of fecal occult blood testing
utili2es a guaiac treated test sheet where a fecal
20 material specimen is smeared on a front, or, "test
material placement" side of the test sheet. The fecal
material has a tendency to diffuse through the test
sheet, defining a region on a side of the sheet opposite
to the test material placement side. The portion of the
sheet opposite to where the test material is smeared is
the back, or, °°developing" side of the sheet, and the
WO 92/11536 PCT/US91/093.'
_2_
area on the developing side of the test sheet where the
fecal material diffuses through from the test material
placement side of the test sheet is referred to herein '
as the "diffused region" or the "diffused area". A .
developing solution is applied to the developing side of '
the sheet directly onto the diffused region, and if a
color change is indicated. blood may be present in the
fecal specimen.
There has been an on-going need to abtain,
transport and process the specimens of the fecal occult
blood test in a manner that is as convenient and as
aesthetically acceptable as possible. One form.of
specimen collection device that has gained wide
popularity is a slide formed from folded paper or
cardboard. The slide includes guaiac treated paper to
which the fecal specimen is applied through a "test
window" or "aperture" located on the test material
placement side of the test sheet, and a cover which is
closed once the specimen application is completed. A
flap in the back of the slide may be opened to reveal
the developing side of the guaiac treated paper for
subsequent application of developer onto an area of the
devoloping side of the test sheet directly opposite to
the aperture or apertures, i.e.r directly onto the
diffused region. A positive result. that is, one
indicating the presence of blood in the fecal sample, is
determined by the presence of (usuallyy a blue color,
and the intensity thereof provides further information
as to the amount of blood present in the fecal sample. ,
Specimen slides for fecal occult blood tests
generally have test windows of varying sizes. In an
attempt to standardize the sample amount applied through
the test windows and in an effort to mitigate against
over- or under- application of sample, instructions for
iV0 92111536 ~ ~ ~'~ ~ ~~ ' P~'iL~S91/09328
-3-
sample application are ordinarily provided to the
patient. These instructions vary, but are generally
intended to provide direction to the patient in an .
effort to limit the amount of sample smeared into the
test window, i.e., "apply a thin smear"; "a pea size";~
or "the size of a match head". Thequantity of the
sample on the slides returned to the laboratory varies
from trace amounts, which axe insufficient for proper
testing, to very excessive amounts, which also create
technical, as well as aesthetic, problems. It is often
the case that when there. is too much sample on the test
material placement side, the developing side of the
slide is fully covered with the colored stain of the
diffused sample. This makes reading of test results
based on color intensity difficult and usually impos-
sible.
Errors on the part of the technician developing
the test slide may also occur. For example, if the
technician inadvertently adds developer to the test
material placement side of the test slide, as opposed to
the developing side, the sample can be flooded with the
developing solution, leading to incomprehensible,
incorrect or misleading results, due to reconstitution
of the sample. Furthermore, the instructions for
application of the developing solutions require
application thereof directly onto the diffused region.
It is often the,case, particularly when an insufficient
amount of sample is present, that far too much
developing solution will be added directly onto this
area in an attempt to compensate for the lack of
sufficient sample. This also has the effect of flooding.
the diffused region, which can result in
incomprehensible, incorrect or misleading results, i.e.
the intensity of the color can be artificially altered.
WO 92/i 1536 ~ ~ ~ ~ ~ ~ J P~1'/1iS91/093
Most of the problems associated with differing
amounts of sample added to the test slide by the patient
are predicted upon the inexperience of the patient with '
such test slides. Given the very nature of, for
example, fecal sample materials, different individuals '
will react differently to applying such a sample .to a
test slide. Therefore, while instructions can be
provided to the patient as to how much of a sample
should be placed in the test window area, consistent
amounts of sample across of wide-ranging group of
patients are not obtainable, as experience has demon-
strated. Additionally, the technician who attempts to
develop a test on a test slide that includes either
inadequate or excessive amounts of sample could possibly
provide incorrect results to the patient or the
patient's physician; thus the medical technician; who
attempts, albeit incorrectly, to compensate for
incorrect sample amounts could possibly provide the
patient or the patient's physician with results that do
not lead to additional (and necessary) tests, or with
results that lead to additional (but unnecessary)
tests. Thus, the technician who provides such erroneous
results could be exposed to legal liability.
Because the performance of the test is depen-
dent on the reproducibility, ease of use by patients, as
well as efficient, yet simple, samplzng/developing
procedures, an improved specimen test slide taking the
above factors into account is not only desirable, but
necessary.
Summary Of The Invention
The present invention overcomes the limitations
and drawbacks noted above.
d'CT/tJS91 /09328
WO 92/11536
-5-
A most prefered embodiment of a specimen slide
in accordance with the present invention comprises a
front panel including a test material placement side and
a portion of a test sheet, the test material placement,
side including at least one aperture which is configured
to accept a test sample through the aperture onto the
test sheet, and a back panel including a developing side
through which the opposite side of the test sheet is
exposed. An indicating means is located on the
developing side portion of the test sheet within
proximity to, but not directly on, the portion of the
test sheet directly opposite to the aperture. The
location of the indicating means relative to the
aperture is critical and essential to the performance of
the specimen slide. The indicating means is most
preferably a target printed directly onto the test
sheet.
The size and shape of the aperture is
2U configured such that the aperture is completely filled
with the test sample. The width of the aperture is
preferably less than the height of the aperture. Two
appertures are most preferably included in the front
panel, and the juxtaposition of the apertures is such
that the inner edges thereof are substantially non-
parallel with each other. Most preferably, the
appertures have,a "letter C" configuration such that the
interior portion of each "letter C" aperture faces the
indicating means.
The location of the indicating means relative
to the aperture is such that when a developing solution
is added onto the indicating means, the solution
migrates completely through the portion of the test
sheet directly opposite to the aperture.
PCT/L'S99/093.'
I~YO 92/1153b
-6-
To use the specimen slide, the patient
completely fills the aperture with, e.g., a fecal
specimen. A developing solution is then added onto the '
indicating means. The solution, having a tendency to
S migrate across the test sheet, migrates through an area ~ '
of the test sheet directly opposite to the aperture,
including the diffused region. By specifically locating
the indicating means at a defined location relative to
the aperture, the developed solution migrates away from
this stained region, which surprisingly and unexpectedly
enhances the color intensity of the developed solution,
and hence the readability of the color intensity, as
compared to previous specimen test slides.
Hrief Description Of The Drawings
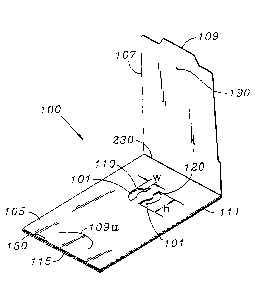
FIG. 1 is a perspective view of the front side -
of a preferred embodiment specimen test slide in
accordance with the present invention where the test
windows have a letter C configuration and each test
window faces the other;
FIG. 2 is a perspective view of the back panel
of the device shown in FIG. 1 and
Z5
FIG. 3 is a plan view of the back panel of the
device shown in FIG. 1 where the test windows are
designated as phantom lines.
35
WO 92/11536 9'CT/LS91/0932~
_7_
Detailed Description Of Preferred Embodiment
With reference to FIG. 1 and 2, a most
preferred embodiment of a device in accordance with the
present invention is in the form of a specimen slide 100
and includes ~a front panel 105, with a corresponding
front panel cover flap 107 (shown in an open position in
FIG. 1) and a back panel 115, with a corresponding back
panel cover flap 117. Panel 105 includes in the central
region thereof a test material (sample) placement area
which preferably includes two apertures 110, 120 and ,
test sheet 101 of absorbent material fixed between the
front panel 105 and back panel 115.
Cover flap 117 (shown in its open position in
FIG. 2) is defined in the back panel 115 by an outline
of perforations 111 and a crease 130 which serves as a
hinge. The perforations 111 are spaced to define a
plurality of bridges, each bridge comprising bridge
portions 117a and 117b. The bridges hold flap 117 in
place until the bridges axe broken as flag 117 is opened
along the perforations 111 to reveal the backside of
test sheet 101. In the embodiment disclosed herein,
sheet 101 is filter paper which carries a reagent which
will react with hemoglobin components from blood and a
peroxide solution to form a visible colored compound.
In the embodiment disclosed herein, test sheet 101 is
Whatman Grade #l. filter paper (Whatman Paper Ltd.,
Springfield Mill, Kent, United Kingdom). The reagent
may be, for example, guaiac, tetramethyl benzidine,
ortho tolidine, and other similar chromogens. In the
embodiment disclosed herein, the reagent carried by the
sheet 7.01 is guaiac. An area defining monitors suitable
for indicating the performance of the guaiae carrying
test sheet 101 and reagents which may be applied thereto
W~ 92/ii536 ~ ~ ~ ~ -~ ~ ~ Pt.'f/US91/093'
_8_
is indicated at 200 and may be of the form described,
far example, in U.S. Patent No. 4,365,970.
The specimen slide 100 is preferably formed
from a single sheet or panel of paper or cardboard. The '
cardboard is die-cut to form apertures 1.10 and 120 (best
viewed in FIG. 1 and shown in phantom in FIG. 3), as
well as the perforations to define flaps 107 and 117. A
tab 109 is also formed at the other edge of flap 107.
Tab 109 is adapted to engage a semi-circular slit 109a
formed near an outer edge 150 of the Front panel 105.
The slit 109a is also formed by, for example, die-
cutting during the manufacturing process of the slide
100.
Specimen sheet 101 is positioned and fixed by a
suitable adhesive or glue. The front and back panels
105 and 115 are folded along the edge 150 and are
pressed and held together by means of a suitable glue or
adhesive. A drop of glue 190 holds the.front panel 105
and front panel cover flap 107 together until slide 100
is ready for use.
In order to effectuate the placement of
developing solution onto test sheet 101, means are
provided for indicating the placement of the developing
solution onto test sheet 101 onto an area of the test
sheet opposite t.o appertures 110 and 120. For example,
a target area, preferably in the form of a target 830
(FIG. 2 and FIG. 3), can be printed directly onto test
sheet 101. Target 830 is the most preferred means for
directing the medical technician to apply the developing
solution onto the test sheet 101. Alternative means for
indicating can include, for example, any printed. locator
for directing the placement of the developing solution
onto test sheet 101.
~~ $ ~ P~ PC'T/U~91/09328
WO 92/11536
_g_
the location of the indicating means is
critical. By ensuring that the indicating means is
properly located relative to the aperture(s), then
irrespective of the size, shape or specific
juxtaposition of the aperture(s), or the specific type
of filter paper utilized, the advantages derived herein
can be realized. In effect, the location of the
aperture relative to the indicating means is determined
by the objective of ensuring that the developing
solution can contact the test sample..react therewith to
form a reaction product (if any), and carry any reaction
product away from the stained region onto a relatively
clean area of test sheet 101 adjacent to such a~stained
region. ~'or these reasons, the location of the
indicating means is advantageously located relative to
the location of the aperture, as will be described in
detail below.
With reference to FIG. 3, and with respect to
the embodiment disclosed herein, target 830 is
advantageously located between about 1.5 and about 2.5
times, and preferably about 2.0 times the width of an
aperture from outer edges 110a and 120a of apertures 110
and 120, respectively. As used herein, the term "outer
edge" is defined as the edge of an aperture located
furthest from the target area as defined, including
target 830, "inn.er edge" is defined as the edge of the
aperture opposite to the outer edge. and "times" is an
alternative term to the mathematical expression referred
to as °'multiplication" or "multiplied°'.
As an alternative method for locating the
indicating means relative to the aperture, the distance
between the outer edge of one aperture and the outer
edge of at least one other aperture is advantageously
WO 92/11536 PCTtUS91l093? ""'
-10-
between about 3.0 and about 5.0 times, and preferably
about 4.0 times the width of an aperture such, that the
indicating means is located at the midpoint between the
apertures. As a further alternative, the indicating
means is advantageously located between about 2.0 and
about 4.0 times and preferably about 3.0 times one half
of the width of the aperture from the approximate center
of the aperture. As used herein, the "approximate
center" of each aperture is defined as a point
approximating call-out designations 110d and 120d (FIG.
3). i:e, a point located interior to an aperture at a
point approximating about one-half of the height and
about one-half of the width.
Preferably, the width of the aperture is less
than the height of the aperture. "Height" is defined as
the measured distance "h" in FIG. 1 and width is~defined
as the measured distance '°w" in FIG. 1. The height of
the aperture is advantageously about 5 times to about
1.5 times than the width of the aperture. Most
preferably, the height is about 4 times the width of the
aperture. The width is advantageously about .25cm to
about l.OOcm and most preferably about .50cm in length.
When two apertures are utilized, it is
essential that the inner edges of the apertures be
substantially non-parallel with each other. As used
herein, the term "parallel" is accorded its usual
definition, e.g. everywhere equidistant. "Substantially
non-parallel" as used herein is indicative of the
relative realtionship between the inner edges of the
aperture. Thus, the inner edges of the two apertures
are substantially non-parallel if the distances between
the inner edges are substantially non-equidistant. When
more than two apertures are utilized, it is essential
that the inner edges of the apertures be substantially
r
'. 'WO 92/11536 '~ '~ ~ ~ ~ PCT/L'S91/0932~
-11-
non-partallel with each other as previously disclosed.
When a single aperture having a straight line inner edge
is utilized, it is essential that the distance between
the top inner edge corner of the apertuxe and the
indicating means be substantially greater or
substantially less than the distance bei:ween the inner
edge corner opposite to the top inner edge corner and
the indicating means. Under this scheme, the indicating
means can be described as the apex of a triangle,
whereby the triangle is formed by: a line from the
indicating means to the top inner edge corner; the inner
edge; and a line from the inner edge corner opposite to
the top inner edge corner and the indicating means. In
this scenario, the triangle thus formed is not an
equilateral triange, given the length differentials
described above.
Most preferably, the inner edge of each apertures)
is curvilinear in nature such that a portion of the
inner edge, or the entire inner edge, is bounded by a
curved line. If only a portion of the inner edge of an
aperture~ia curvilinear, the remaining portion thereof
can be parallel with the inner edge of another
aperture. In such a situation, at least one-half of the
inner edge is preferably curvilinear. The curved line
is advantageously positioned interiorly to the
apsrture(s). By way of example and not limitation and
referencing FIG.3, inner edge portion 110c and inner
edge portion 120c are parallel to each other; however,
inner edge portion LIOb and inner edge portion 120b are
curvilinear such that the inner edges of aperture 110
and 120 are substantially non-parallel to each other.
The outer edge of each aperture can also be
curvilinear in nature. Most preferably, the entire
outer edge is curvilinear. Accordingly, in the most
preferred embodiment, the apertures each have a "letter
W~ 92/11536 ~ ~~ ~ ~ ~ ~ ~ PC'T/tJS91/093?''"~
-12-
C" configuration. As used herein, the term "letter C".
configuration is meant. to describe the configurational
shape of an aperture as depicted in FIG.1 and FIG.3.
Most preferably, the apertures each face one another.
For the embodiment disclosed herein, the most
preferred measurements far the apertures would be such
that the height of each aperture is about 2.Ocm, the
width of each aperture is about 0.5cm, and the distance
between the outer edges of the apertures is about 2.Ocm,
such that the distance between the outer edge of each
aperture and target 830 is about l.Ocm and the distance
between the approximate midpoint of each aperture and
target 830 is about .75cm.
In using the specimen slide 100, fecal speci-
mens are planed onto test sheet 101 through apertures
110 and 120 such that the specimens completely fill
apertures 110 and 120. The patient closes the specimen
slide 100 by folding front panel cover flap 107 along
crease 230 and inserting tab 109 beneath slit 109a. The
specimen slide 100 is transported to the physician's
office or laboratory for analysis. With reference to
FIG. 2, the analysis of the fecal specimens carried by
specimen slide 100 may be accomplished in an
advantageous manner, i.e. without reopening the specimen
slide 100 at front panel cover flap 107 to gain access
to apertures 110 and 120. Back panel cover flap 117 is
opened by separating bridge portions 117a from 117b. A
developing solution is applied to the back of the test
sheet 101 onto target 830 to form a screening test for
occult blood in the specimen.
Developing solution is most usually applied via
a drop-wise application to the developing side of filter
paper 101. As such, as these drops come in contact with
s
'WO 92J11536 ~ ~ ~ ~ f ~ Pf."T/1JS91/09328
-13-
test sheet 101 and are absorbed therein, there is a
tendency for the solution to migrate outwardly from the
point of ccntact, usually in a radial direction. Two to
three drops of developing solution are added to the test ,.
sheet 101 by addition thereto onto target 830. After
testing, the entire slide 100 may be properly disposed
of .
Sheet 101 may be sensitized for other analytes
and the device may be adapted for collecting other types
of specimens, such as mucosic, viscous materials.
Several slides may be attached side-by-side, each having
a different reagent on sheet 101, such that testing of
several analytes can be effectuated.
Different filter papers can be utilized for
test sheet 101 such that adjustment of the location of
the outer edges of the apertures) relative to the
location of the target area or to one another is
possible. Preferably, the composition of the filter
paper is cotton fiber, although wood/cotton fiber or
glass/cotton fiber combinations can be utilized. Three
factors are of importance in determining the location of
the outer edges of the apertures relative to the target
area, these being the thickness, particle size relation
and flow rate of the filter paper.
The thickness of the filter paper is preferably
between about .lOmm and about .26mm. Most preferably
the thickness of tht filter paper is about 0.175mm.
When a filter paper is used that has a thickness in
excess of about 0.175mm, it is possible that the area in
which developing solution travels may correspondingly
decrease; the opposite is possible for a filter paper
that has a thickness less than about 0.175mm. In order
to compensate for these variables, at least two
WO 92!11536 ro ~ ~ ~ ~ g '~~y PCTlL~S9110937"'
-14-
approaches are possible: (1) altering the amount of
developing solution added to the filter paper; or (2)
adjusting the location of the outer edges of the
apertures relative to the target area. Thus, for a
filter paper having a thickness greater than about
0.175mm, more than two to'.three drops of developing
solution can be utilized (about three to four drops), or
the aperature, and hence, the outer edges of the
aperture, can be moved closer to the target area. The
opposite is suggested.for filter paper having a
thickness less than about 0.1?5mm. I.e., less than
about two drops of developing solution can be utilized
(about one drop) or the aperature, and hence the outer
edge of the aperture, can be moved further from the
target area.
The particle size retention of the filter paper
is defined herein as the average size of a spherical
particle retained by a given filter paper with a 98%
efficiency as determined using an electronic particle
counter. Preferably, this value is relatively small, on
the order of from less than about 1.0 micron to about
7.0 microns. Most preferably, the particle size
retention is about 4.0 microns. The value for the
particle size retention cannot be such a value that too
much of the sample is able to "leak'° through the~filter
paper, while at,the same time if the value is too small,
the sample will not properly diffuse through the filter
paper. Adjustment of the distance of the outer edge of
the aperture relative to the particle size retention is
made in the same manner as that of the thickness of the
filter paper. Accordingly, as the particle size
retention value increases, the outer edge distance from
the target area increases. and as the particle size
retention value decreases, the outer edge distance from
the target area decreases.
W~ 92/11536 ~ ~~ ~' ~ ~.~~ PCT/l,'S91/09328
-15-
Related to particle size retention is the flow
rate or linear wicking of the filter paper. This value
can be defined in a variety of ways depending on the
manufacturer of the filter paper. Preferably, the flow
rate (which can be defined in terms of the time
necessary for distilled water to rise a specified
distance an the filter paper) is between about
.3cm/minute and about .9cm/minute. Most preferably, the
flow rate is about 0.6cm/minute. The flow rate value is
of import in that diffusion of the sample is related to
the flow rate of the filter paper. Thus, as flow rate
increases, the outer edge distance from the target area
increases, and as the flow rate decreases, the outer
edge distance from the target area decreases.
Advantages of the Invention
The advantages derived from the present inven-
tion include the placement of a consistent quantity of
sample applied in a consistent manner to a consistent
location on test sheet 101 such that addition of a
developing solution close to but not directly into the
diffusion area allows the developer to migrate through
the diffusion area, carryincJ the resultant color (e.g
blue when guaiac treated paper is utilized) away from
the dark background of the diffused region, thus
increasing and enhancing the readability of the
reaction. These advantanges are derived form locating
the aperture relative to the indicating means.
k'urthermore, because the patient is instructed to
completely fill the aperture, variations in the amount
of sample added to a specimen slide are avoided as well
as the aforementioned concerns associated with such
variations. Additionally, because specimen slide 100
does not yield any additional sub-parts or components
WO 92/11531 f~ ~ ~ ~ ~. ~ ~ PCTiUS91/093,'""
_15_
which may require separate disposal, the entire slide
can be disposed upon completion of development, thereby
reducing the potential for improper disposal of a
clinical material.
While the present invention has been set forth
in considerable detail, the invention disclosed herein
is not to be limited to the detailed description, but is
to be afforded the full scope of the appended claims and
all equivalents thereto.
20
5
35