Note: Descriptions are shown in the official language in which they were submitted.
207~33~
WO91/12228 PCT/US91/00371
.. . .
ARTHROPODICIDAL TRICHLOROMETHYL~ENZYLAMINES
FIELD OF TH~ I~VENTION
Trichloromethylbenzylamines useful as
arthropodicides, compositions containing them and
methods for using them.
S~ATE O~ THE ~T
DD 123049 discloses N-(benzyl)amines as plant
growth regulators. Div. Agric. Chem.; Ind. Agric.
Res. Inst. Ind. J. Chem., Sect. B 16B~3), 250 to 252,
discloses the preparation of 2-(trichloromethyl-
benzyl)anilin~s. J. Agric. Food Chem.; 24(4) 724 to
727, 1976, discloses N-(~-CC13benzyl)aniline).
JP 49/101526 and DE 2,301,397 disclose
~-(CC13benzyl)aniline insecticides. J. Agr. Food
Chem., 20~4) 818 to 824, 1972, discloses
~-(CC13benzyl)anilines and phenylethers with D~T-like
activity.
SV~MARX ~F T~ YENTIO~
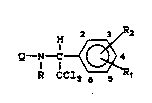
The invention pertains to compounds of Formula
I, includin~ all geometric and stereoisomers,
agriculturally suitable ~alts thersof, agricultural
compositions containing th~m and their use as
arthropsdic~des in both ~gronomic and nonagronomic :~environments. The compounds are:
Q-N-
R CC13
'
''``` " ' ' ' ' ' ', .' ,; ~ '' ',, ' ' ' " .
.. , . , . ',
, ,' ' ' ` ',
,,: ' ' `~' ' ' ~ ' ',, ' ' '
,
- `
WO91/12228 2 ~ 7 S 3 8 2 PCT/US91/00371 -
wherein
5Q i5 ~elected from the group
c ~ R,
Q-1 Q-2
R is selected from the group H, Cl-C3 alkyl,
CHO, C2-C5 alkylcarbonyl, C2-C~
haloalkylcarbonyl, benzyl optionally
substituted by W, C2-C5 alkoxycarbonyl,
Cl-C4 alkylsulfonyl, Cl-C4 haloalkylsulfonyl
and SR5;
Rl i6 selected rom the group halogen, Cl-C4
alkyl~ ClC4 haloalkyl, Cl-C4 alko~y, C3-C4
alkenyloxy, C3-C4 Hlkynylo~y, Cl-C4
halo~lkosy, ~l-C4 alkylthio, Cl-C4
haloalkylthio, Cl-C~ alkylsulfinyl, Cl-C~
haloalkylsulfinyl, Cl-C4 alkylsulfonyl,
Cl-C4 haloalkylsulfonyl, NO2 and CN;
R2 is ~elected from the group H, halogen, Cl-C4 :~
alkyl, Cl-C4 haloalkyl, Cl-C4 alkoxy, Cl-C~
haloalko~y, C2-C4 alkenylo~y, C2-C4
~5
.' ~
~ .
WO91/12228 2 0 7 5 3 8 2 PCT/US91/00371
alkynylozy, C2-C4 alkenyl, C2-C4
haloalkenyl, C2-C4 alkynyl, C2-C4
alko~ycarbonyl, Cl-C~ alkylamino, di-Cl-C4
alkylamino, NO2 and CN,
R3 and X are independently selected from the
group H, halogen, Cl-C4 alkyl, Cl-C4
haloalkyl, Cl-C4 alkoxy, Cl-C4 haloalkoxy,
C2-C4 alko~ycarbonyl, Cl-C4 alkylthio, Cl-C4
haloalkylthio, Cl-C4 alkylsulfinyl, Cl-C4
haloalkylsulfinyl, Cl-C4 alkylsulfonyl,
Cl-C4 haloalkylsulfonyl, NO2 and CN;
R4 is selected from the group H, halogen, Cl-C4
alkyl, Cl-C4 haloalkyl, Cl-C4 alko~y, Cl-C~
haloalko3y and CN;
R5 is selected from the group CC13, N(R6)COR7,
phenyl optionally substituted with W, and
benzyl optionally substituted with W;
W is selected ~rom the group halogen, CF3,
OCF3, Cl-C4 alkyl, nitro, and Cl-C9
~lkoxycarbonyl;
is selected ~rom the ~roup H and halogen;
R6 is selected from the group Cl-C4 alkyl, and
~7 is selected ~rom the group Cl-C4 alkyl.
Preferred Compounds A are those of Formula I
wherein Rl is in the para po~ition and R ~s H.
P~reerred Compounds B are those of Preferred A
30 wherein Q is Q-1. .
Pre~erred Compounds C are those of Preferred A
wherein Q is Q-2.
Preferred Compounds D are those of Preferred C
wherein Rl is selected from the group F, Cl, Br, CF3,
OCH2C-CH, OCHF2 and OCF3.
.. , ,........... . ~ . . , ........................... .. . - . , .
,' ~ ' ,
WO~1/12228 2 0 ~ ~ ~ 8 2 PCT/US91/00371
In the abo~e recitations, the term ~alkyl~,
used either alone or in compound words sueh as
~alkythio" or haloalkyl~, denotes straight chain or
branched alkyl such as methyl, ethyl, ~-propyl,
isopropyl or the different butyl isomers. Alko~y
denotes methoxy, etho~y, n-ProPylo~y, isopropylo~y
and the different butoxy isomers. Alkenyl denotes
straight chain or branched alkenes such as vinyl,
l-propenyl, 2-propenyl, 2-propenyl and the different
butenyl isomers. Alkynyl denotes straight chain or
branched alkynes such as ethynyl, l-propynyl,
3-propynyl and the different butynyl isomers.
Alkylthio denotes methylthio, ethylthio and the
different propylthio and butylthio isomers.
Alkylsulfinyl, alkylsulfonyl, alkylamino, etc., are
~efined analogously to the above examples.
The term "halogen", either alone or in compound
words such as ~haloalkyl~, denotes fluorine,
chlorine, bromine or iodine. Further, when used in
compound words such as ~haloalkyl" said alkyl can be
partially or fully substituted with halogen atoms,
which can be the same or different. E~amples of
haloalkyl include CH2CH2F, C~2CF3 and CH2CHFCl. The
terms "haloalkylthio~ haloalkenyl~ and ~haloalko~y~ -
are defined analogously to th~ term ~haloalkyln.
The total number of carbon atoms in a
substituent group is indicated by the ~Ci-Cj n prefi~
where i and j are numbers from l to 5. For e~ample,
Cl-C3 alkysulfonyl designates methylsulfonyl through
propylsulfonyl; C2 alkylcarbonyl designates C(O)CH3
and C4 alkylcarbonyl includes C(O)CH2CH2CH3 and
C(O)CH(CH3)2; and as a final e~ample, C3
alkoxycarbonyl designates CO2CH2CH3 and C4
alko~ycarbonyl includes COzCHzCH2CH3 and CO2CH(CH3)2.
.
.
WO91/12228 2 0 7 ~ 3 8 2 PCT/US91/00371
Compounds of Formula I can be prepared by
5 various methods. One reaction ~equence is the
formation of an imine from a desired aryl or
heteroaryl amine by reaction with an appropriate
aldehyde by conventional methods as illustrated in
Scheme l.
SCH~E l
Q- NH2 ~ HC~ ~ Q- N= CH~R,
R2 R2
Imines of Scheme l can further react with
trichloroacetic acid according to procedures
described by Hirwe et al., J. Ag Food Chem. ~Q, 818 to
24, 1972 and Yost e~aZ., J.A~. ~oo~ Chem., ~, 724 to
25 727, 1976 to give compounds of Formula I ~s shown in
.Scheme 2.
&~
Q-N=C~ ~ ~ HOCCCl -- ~Q MH-C~ ~ R7
3S R2 CCl3
~ .
,
~c ~
WO91/12228 PCT/US91/00371~-
The addition of the trichloromethyl group to imines
can be accomplished by other methods to form
compounds of Formula I. One such method is the
addi-tion of chloroform to imines under basic
conditions în an aprotic solvent such as
dimethylformamide in a manner similar to the
synthesis of a-trichloromethylbenzyl carbinols from
aldehydes (Wyvratt et a~., J. Org. Chem. 52, 944, 1987) .
This method is illustrated in Scheme 3.
~CHEME 3
Q-N=C~ ~ HCC13 + XOH~ Form~la I
Alternati~ely, the ~ddition of the trichloromethyl
group to imines can be accomplished through the
utilization of trichloromethyl lithium ~s shown in
Scheme 4.
Q-N=CH ~ ~ LiCCl3 --~ For~ula I
_ 90
R2
. .
..
.. , . . . . . . :
. : . - . . . : ., :- . . . ~ . :
. ., .. , , . : .. , :,: . . :
- . -. . . . .: .: .. . . ~ . . . .
WO91/lZ~23 2 0 7 ~ 3 8 2 PCT/US9l/U0371
The ~ri~hloromethyl lithium reagen~ is prepared from
chloroform and butyl lithium as described by
Kobrich, G. e~al., Angew. Chem., 76,536 (1964~.
Compounds o Formula I where R is other than
hydrogen can be prepared by reaction of the
corresponding imine of Scheme 1 with R-X (wherein X
is a leaving group) followed by the reaction of the
resulting iminium salt with trichloromethyl lithium
in a manner similar to a procedure described by ~rook
etal., Syn. Commun. L~, 893 to 898 (19~8~ for the
reaction of imines with trimethylsilyl triflate
followed by the addition of nucleophiles and as
illustrated in Scheme 5.
~C~E~ 5
~-N-C~ ~ ~ R C13CLi I ~ R~
R2 ~C13
Compounds of Formula I ~where R is SR5) can be
prepared from compounds of Formula I by reaction with
the appropriate CISR5 in the presence of a base such
as a tertiary amine as shown in Scheme ~.
. . . . .
WO 91/t2228 2 0 7 5 3 ~ 2 P~uS91~003~
E;CHE;~iE b
,h~R1 Et~N SRg ~R
Q-N~CH~ ) I ClSR5 - - ~ Q~N-CH--
9cc13 \$/ CHaClz CCl~ ~/
R2 ~2
Sulfenyl halide~ of the type ClSR5 wherein R5 is as
specified in this disclosure are known in the
literature. A review arti~le by Englebert Ruhle
(Synthesis, 561 (1970)) describes the chemistry and
the preparation of the majority of sulfenyl halides
utilized in this invention. The synthesis of
N-chlorosulfenylcarbamates is déscribed in U.S.
3,843,689.
The following Esamples illustrate the invention.
N-(4-chlQro-a-t~hlQL~methyl~nzylL-3
is(t~ifluo~Qm~thyl~aniline ~
To a solution of 4-chlorobenzaldehyde (2.B g,
0.02 mol) in 20 ml of toluene was added
3,~-bis(trifluoromethyl)aniline (4.6 g, OrO2 mol)
Molecular sieves 5A ~20 g) were added to the mixture
and stirred at room temperature overnight. The
sieves were filtered and washed with C~2C12 ~2 2 50
ml) and the solvents were evaporate.d under vacuum, to
gi~e N-(4-chlorobenzylidene1-3,5-bis(trifluoro-
methyl)aniline (lA). The product was a ~olid; mp
S5-57C.
.: .-.: , :. :. ;. . . . . : .. . .... , . .-.. . :, .. .. .. . . . .
wosl/l2228 2 0 7 ~ 3 8 2 PCT~US91/00371
Then, 3O3 9 (0.01 mol) of lA was mi~ed with
trichloroacetic acid (2.0 9, 0.012 mol) and heated
slowly to 120C under a nitrogen ~tmo~phere. The
temperature was maintained between 120 to 125C until
gas evolution ceased. The reaction was cooled to
room temperature and 100 ml of CH2C12 was added. The
solution was washed with 10% sodium carbonate (2 ~ 20
ml), then with water (2 ~ 20 ml) and dried over
anhydrous MgSO4. Evaporation of the solvent followed
by chromatography on silica gel (hexane) gave l; mp
81~ to 83C. lH-NMR spectrum of 1 in CDC13 showed
the following chemical shifts (in ppm): ~ 7~54 (d,
2H), 7.38 (d, 2H), 7.24 (m, lH), 7.0 (m, 2H), 5.12
and ~.13 ~s, 2H).
EXAMPLE 2
2~ Cll,lnro~ hlorome~.hylbenzyl ~inQ,)-
3,5-di~hlorop~ridine (2~
To a solution of Z-amino-3,5-dichloropyridine
(9.9 ~, 0.06 mol) in 60 ml of CH2C12 was added
4-chlorobenzaldehyde (8.4 g, 0.06 mol) followed by
60 9 of mol~cular sieves SA. The mi~ture was stirred
at room temperature for three days. The reaction
2~ mi~ture was filt~red and the sieves were washed with
CH2C12 ~5 ~ 50 ml). The sol~ent was evaporated un~r
~scuum to give 2-~N-4-chlorobenzylidene)amino-
3,5-dichloropyridine (2A) a~ a solid; mp 101-103C.
Then,` 15.0 g 2A (0.053 mol) was mixed with
trichloroacetic acid (25 ~, 0.153 mol) under a
nitrogen atmosphere. The mi~ture was heated slowly
up to 132C and maintained at this temperature for
two hours until gas evolution ceased. The misture
was cooled to room temperature and CH2C12 (3D0 ml)
3~ was added. The solution was washed with 10% sodium
bicarbonate solution (2 x 100 ml), then with water (2
x 20 ml), and the organic phase was dried over
,
- - .- . ~ .: . ; . . :,
, . .. .- . . . .
. .. .. .,: . , . , . -, .
, - .,, . . ~ ", ., ~ . .
.,, . ~, "
. -, .. ;.. ...
- :.
wosl/l2228 ,~ Q,~ PcT/us91/oo37l -~
MgSO4. The solvent wa~ evaporated under vacuum and
the residue was chromatographed on ~ilica ~el
(hexane) to give 2 as a solid; mp 105 to lOBC.
lH-NNR spectrum of 2 in CDC13 showed the followi~g
chemical shits (in ppm): ~ 7O95 ~m, lH), 7.53 (m,
3H~, 7.35 (d, 2H), 6.24 (d, lH), 6.05 ~d, lH~.
~XAMPI,~ ~,
~L~-ch~Qro-a-t~hlorQm~hyL~enzyl~-N-
rlN'_4~Qxy~arbo~y~ -meth~3mino sulçnYll-
3,~-bis~ fluorom~~hyl~iline (3)
To a cold solution (0C) of N-(4-chloro-a-
trichloromethylbenzyl)-3,5-bis(trifluoromethyl)-
aniline (1.0 g, 2.6 mmol, from E~ample 1) in 10 ml
C~2C12 was added triz~hylamine ~0.6 g, 6 mmol).
Butyl (chlorosulfenyl)(methyl~carbamate (1.2 g, ,
6 mmol) was add~d dropwise with stirring at 0C`.
After complete addition of thè carbamate, the
temperature wa~ allowed to rise to room temperatureand stirring was continued overnight. The solvent
was evaporated under vacuum and the residue was
chromatographed on silica gel column (he2ane) to give
(3) as a viscous oil. lH-NMR o~ 3 in CDC13 showed
the characteristic chemical shift (in ppm) of ~ 3.58
(S, 3H) for ~SNCH3.
~LE~
3.5-Di~loro-N-(2l2.2-tri~hlo
2-yl~phenYl)~hy~ 2-~Yr~di.namine (4)
Ste~ ~: 3,5-Dichloro-N-(2,2,2-trichloroethylidene)-
2-pyridinamine
To a solution of 2-amino-3,5-dichloropyridine
~S 116.3 9, 0.10 mole) in 125 ml,of chlorobutane was
- added 5A molecular sieves (20g). At 0C, anhydrous
. , ~ . , . . . , ., ,: . .
'. . ,
' ,', . ~ .
WO91/122~8 2 ~ ~ ~ 3 8 2 PCT/US91/00371
11
chloral (15 g, O.lO mol) was added dropwise, and the
mi~ture was allowed to warm to room ~emperature and
to stir overni~ht.
The reaction mi~ture was filtered through
Celite and the sieves were washed with chlorobutane.
The filtered solution was cooled in an ice bath, and
thionyl chloride (35.5 9, 0.30 mol) was added
dropwise. The resulting white suspension was then
heated to reflu~ for six hours, producing a clear
solution. The excess thionyl chloride W3S evaporated
under vacuum, and the residue was dissolved in 150 ml
of ethyl ether. To this ~olution was added
triethylamine tl2.1 9, 0.12 mol) at 0C, producing a
white precipitate. After 15 minutes, this mi2ture
was filtered and the filter cake was washed w;th
hexanes. The filtrate was concentrated ~nder vacuum
to give the imine 4~ as a brown solid, 23.67 g. The
lH N~R spectrum of this compound in CDCl3 showed the
following chemical shifts (in ppm): ~ 8.59 (s, lH),
8.35 (fine d, lH), 7.85 ~fine d, lH).
Sten ~: To a solution of 4-bromobenzaldehyde (5.55
g, 30 mmol) in 25 ml of toluene was add~d ethylene
glycol (2.23 g, 36 mmol) and p-toluenesulfonic acid
monohydrate (0.27 g, 1.4 mmol). This mi~ture was
stirr~d under nitrogen and heated overnight with
azeotropic removal of water. The reaction mixture
~0 was washed with saturated aqueous sodium bi~arbonate
sol~tion, then with water and with brine ~olution.
The organic phase was dried over MgSO~ and filtered.
The solvent was evaporated under vacuum.
The residue (2.29 g, lO mmol) was then
dissolved in 30 ml of tetrahydrofuran and cooled to
-70C. A solution of n-BuLi (~.40 ml of a 2.5 M
.
' ,,, '- ' ' : : . ',
WO91/12Z28 2.0 7 S 3 8 2 PCT/U$91/00371
~olution in hezanes, ll mmol) was then added
dropwise. Solid magnesium bromide etherate (3.l0 g,
12 mmol~ was then added in one portion, and the
mi~ture was stirred for 30 minutes at -70C. A
solution of the compound from Step A (2.63 9, 9.0
mmol) in 5 ml of tetrahydrofuran was then added
dropwise. The reaction mixture was stirred at low
tempera~ure for 30 minutes, and 0.6 ml of glacial
acetic acid was added. At room temperature, the
reaction mixture was diluted with an equal amount of . -
ether and washed with saturated aqueous sodium
bicarbonate, water, and brine. The organic phase was
dried over M~SO4 and filtered, and the solvent was
removed under va~uum.
The residue was chromatographed on silica gel
with 10% ethyl acetate/hexane to give 4 as a viscous
oil~ 2.14 g.
lH NMR spectrum of ~ in CDC13 showed the
following chemic~l shifts (in ppm~ 6 7.96 (fine d,
lH), 7.63 (d, 2H~, 7.47-7.52 (m, 3H), 7.~8 (m, lH),
6.29 (d, lH), 6.07 (d, lH), 5.81 (~, lH), 4.00-4.l2
(m, 9H).
~ D~E_~
3.5-Di~blQ~Q-N-(2~2.2~ hLoro-l-
~ . .
To a solution of the compound from E~ample 4
~l.OO g, 2.26 mmol) in 3~ ml of acetone was added
p-toluenesulfonic acid (0.~3 g, O.l~ mmol). This
solution was stirred at room temperature overnight.
The solvent was removed under vacuum. The residue
was dissolved in etherJethyl acetate and was washed
with aqueous sodium bicarbonate, water, and b~ine.
The or~anic phase was dried over MgSO4 and the
solvent was removed under vacuum. The resultant oil
:. :
: : , . . :
~ . , .
~. . ,, ~ ' ' , ' ' .' :' ' '.
WO91/12228 2 0 7 ~ ~ 8 2 PCT/US91/00371
crystallized on standing. The solid was triturated
with he~ane, filtered, and dried to obtain 0.55 9 of
a white ~olid (compound 30, mp 152-155C).
A mixture of the above intermediate (0.29 g,
0.73 mmol) and hydroxylamine hydrochloride (0.12 9,
l.7 mmol) in 5 ml of 96% ~ormic acid was heated at
reflu~ for 3.5 to ~ hours, cooled to room
temperature, and poured over ice. Ether was added,
and the mi~ture was made basic with lN NaOH. The
layers were separated, and the organic phase was
washed with water and with brine, and dried with
MgSO4. The solvent was removed under vacuum, and the
residue was chromatographed in silica gel (5~ ethyl
acetate/he2ane) to afford compound 5; mp 175-176C.
EXAMPLE 6
~ 5-Dichlor~-N-(2.2 2-~richlQr~
(2.4-dichlorophenyl)ethylL-~-p~ridin2mine (6)
To a solution of 2,4-di~hlorobenzaldehyde (8.68
g, 50 mmol) in 6S ml of toluene was added
2-amino-3,5-dichloropyridine (8.08 9, 50 mm0l)
followed by 50 g of molecular sieves 5A. This
mi~ture was stirred overnight and then filtered
2~ through Celite. The filter cake was washed with a
50:50 mixture of CH2C12/he~ane. The solvents were
evaporated under Yacuum to afford 5.64 g of
2 (N-2,4-dichlorobenzylidene)amino-3,5-dichloro-
pyridine (6A). An additional 8.83 y of this
intermediate was obtained by washing the filter cake
: with CH2Cl2 and concentrating the filtrate under
vacuum.
To a cold (-70C) solution of 6A (0.g6 9, 3.0
mmol~ in 30 ml of tetrahydrofuran was added a
solution of lithium diisopropylamide in
heptaneJtetrahydrofuran (4.30 ml of 2 1 M solution,
.. . . . . . .. . . .. . . . . . . . .
: : . . ., .. , ,: '...... .. , . : .' - .. .
. ~ . . ,
. ~- , . . , ., , : . .
- ' ~ ' ` ' . ...... . . . . . . .
:, , ~. ':
- . . . . .
wo 91/12228 2 0 7 ~ 3 8 2 pcT/ussl/oo371
14
9.0 mmol). A soluti~n of CHC13 11.4~ g, 12,5 mmol)
in 5 ml of tetrahydrofuran was then added dropwise
over a 15 minute period. A solution of acetic acid
(0.6 ml, 10 mmol) in 3 ml of ether was added. At
room temperature, the reaction mi~ture was diluted
with ether and washed with saturated aqueous
bicarbonate, water, and ~rine. The organic phase was
dried over MgS09, and the solvents were evaporated
under vacuum. The residue was chromatographed on
silica gel with hexane to af~ord 0.48 g of 6 as a
viscous oil. The lH NMR spectrum of this compound in
CDC13 showed the following chemical shifts (in ppm):
~ 8.01 (d, lH), 7.65 (d, 1~), 7.52 (d, lH), 70-46 (d,
lH), 7.29 (dd, lH), 6.S8 (d, lH), 6.04 (d, lH).
~ y the general procedures described herein, or
obvious modifications thereof, the compounds of Table
20 1 can be prepared. . .
3~
.
.
. . .
,
:, ~ . . . :
.. . ~
, . , . ~ ,
"WO91/12228 2 D 7 ~ 3 ~ 2 PCT/~S~1/00371
's
0 ~N
CF3
~LQ~ B3 :
a 2-F
b 2-Br
c 2-CN
d 3-F
e 3~Cl
~ 3-Br
9 3-CN
h 3-NO2
i 3-OCH3
j 3-OC~F2
k 3-SCH3
l 4-Cl
m 4-C~
Compounds of Table 1 wherein R, Rl and R2 are
as set out therein can be prepared havi~g the recited
values of Groups a through m. That is for each value
of R, Rl and R~ in Table l, R3 can be F, ~r, CN, Cl,
NO2, OCH3, OCHF2 or SCH3. All of said compounds are
35 specifically included within the scope of this
invention .
....... . . . . , , ~ , . .............. .. . .. .. .
, . ~ .. - . . . , . . ~ .. . .. .: .
WO91/12228 2: d 7 ~ 3 ~ 2 PCT/US9l/00371
!
16
~ .
This Table contains a larye number of ~ompounds
of Formula I in a format adopted to a~oid mechanical
reproduction of substituent values that do not vary.
For example, the first table entry (where R is H, R
is 4-F and R2 is H) actually specifies 13 separate
and distinct compounds because the Key for Table 1
identifies additional substituent values of R3,
namely Group a through m for each Table 1 entry.
:
.
. . , ,, , ,, , . , .: : . -
: . . .. , ~ ,i .; ;; ~ ... . . .
WO 91/12228 2 ~ 7 ~ 3 8 2 pcr/us91/oo37l
!
17
R~H R~CH3
~l ~2 ~1 ~2
4-F H 4-F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 4-CH3 H
4-CF3 H g_CF3 H
4-OCH2CH3 H 4-OCH2CH3 H
3-OCH2C-CH H 3-OCH2C_CH H
4-OCHF2 H 4-OCHF2 H
4-OCH2CF3 ~ 4-OCH2CF3 H
9-SCH3 H 4 -SCH3 H
4-SO2CH3 H - 4-SO2CH3 H
4-SO2CF3 H 4-SO2CF3 H
4-CN H 4-CN H
4-NO2 H 4-NV2 H
3-F H 3-F H
3-Cl H . 3-Cl H
3-F 4-Cl 3-F 4-Cl
3-Cl 4-Cl 3-Cl 4-Cl
3-CH3 4-Cl 3-~H3 4-Cl
3-F 4-CH3 3-F 4 CH3
3-Cl 4--CH3 3-Cl 4 CH3
2-F 4-F 2 F 4-F
2-F 4-C1 2-F 4-Cl
2-F , 4-Br 2-F 4-~r
2-F 4-CH3 2-F 9-CH3
2-F 3-Cl 2-F 3-Cl
WO91/12228 2 ~ 7 5 3 g 2 PCT/US91/0~371
lB
R~CHO R.C(O)CH3
E~l B2 ~1 B2 : '
4-F H 4-F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 4-CH3 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 9-OCH2CH3 H
3-OCH2C-CH H 3-OCH2C_CH H
4-OCHF2 H 4-OCHF2 H
4-OCH2CF3 H 4-OCH2CF3 H
~-SCH~ H 4-SCH3 H
4-SO2C~3 H 4-SO2CH3 H
4-SO2CF3 H 4-SO~CF3
4-CN H 4-CN H
4-NO2 H 4-NO2 H
3-F H 3-F H
3-Cl H 3-Cl H ~.
3-F 4-Cl 3-F 4-Cl
3-Cl 4-~1 3-~l 4-Cl
3-CH3 4-Cl 3-CH3 4-Cl
3-F 4-CH3 3-F 4-CH3
3-Cl ~-C~3 3-Cl 4-~H3
2-F 4-F Z-F 4-F
2-F 4-Cl 2-F 4-Cl
2-F 4-Br 2-F 4-Br
2-F 4-CH3 2-F 4-CH3
2-F 3-Cl 2-F 3-Cl
. :.
- . , ~ . . . ; ~ . ; : .
' ' ' ~ ~', ', . , : : , '
:- , - . , - .
,:
,, , . : . . ..
WO 9]/12228 2 0 7 5 3 8 ~ pcr/us91/oo37l
19
R~ SN ( CH3 ) C ( O ) OC,4 Hg R~ S02 CF3
Bl ~2 Bl B2
4-F H 4-F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 'I-CH3 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 4-OCH2CH3 H
3-OCH2C_CH H 3~0CH2C-CH H
4-OCHF2 H 4-OCHF2 H
4-OCH2CF3 H 9-OCH2CF3
4-SCH3 H 4-SCH3 H
~ S02CH3 H 4-S02CH3 H
4 S2 CF3 H 4 ~ S2 CF3 H
9-CN H 4-CN
4-N02 H 4-N2 H
3-F H 3-F H
3-Cl H 3-Cl H
3-F 4-Cl 3-E` 4-Cl
3-Cl 4-C1 3-~l 4-Cl
3~~3 9-Cl 3-CH3 4-Cl
3-F 4-CH3 3-F 4-CH3
3-C1 4 -CN3 3-Cl ~ H3
2 F 4-F 2~ ~ 4 F
2-F 4-Cl 2-F 4-Cl
2-F 4~Br 2-F 4~Br
2-F 4-CH3 2-~ 4-CH3
2-F 3 Cl 2-F 3-Cl
:: : , . : . -
WO91/122?8 2~5~ Pcr/usgl/oo37l .
g~y~FQR 3~LE
10 ~_~; ,
Group ~3 ~4
a 3~C1 5-Cl H
b 3-~r 5-~r H
~ 3-C1 5-CF3 H
d 3-CF3 5-CF~ H
e 4-CF3 6-CF3 H
f 3-C1 6-CF3 H
g ~Cl 6-CF3. H
:h 5-C1 6-CF3 H
i 3-CN 5-Cl H
j 3-CN 6-Cl H
k 3-C1 5-CN H
1 3-C~ 5-CF3 N
m 3-CN ~-CF3 H
n 5-CN 6-CF3
o 5-CN C~3 CH3
p 3-C1 5-CF3 H
~ .
Compounds of Table 2 wherein R, Rl and ~2 are
as set out therein can be prepared having the recited
values of Groups a through p. That is, for each
value of R, Rl and R2, R3, R4 a~d Rx can be H, Cl,
: .
,, .. ; . . ..
,,, . . . ; ,
20~382
WO91~12228 .~. ; PCT/U~91/00371
Zl
Er, CH3, CF3 or CN. All of ~aid compounds are
specifically i~cluded within the scope of this
invention.
This Table contains a large number of compounds
of Formula I in a format adopted to avoid mechanical
reproduction of substituent values that do not vary.
For example, the first table entry (where R is H, R
is 4-F and R2 is H) actually specifies 16 separate
and distinct compounds because the Key for Table 2
l~ identifies additional substituent values of R3, R4
and R~, namely Groups a through p for each Table 2
entry.
.... . ....
Wo 91/12228 2 ~ 7 5 3 ~ 2 Pcr/US91/00371 - --
R~H R-CH3
~l ~2 ~1 B2
4-F H 4-F H
4-Cl H 9-Cl
4-Br H 4-~r H
4-CH3 H 4-CH3 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 4-OCH2CH3
3-OCH2C_CH H 3-OCH2C-CH H
4-OCHF2 H . 4-OCHF2 H
4-OCH2CF3 H ~ 4-OCH2CF3 H
4 SCH3 H 4-SCH3 H
4-S02CH3 H 4-S02CH3 H
4 -S2~ F3 H 4-S02CF3 H
4-CN H 4-CN H
4-N02 H 4-N02
3-F H 3-F H .
3-Cl H 3-Cl
3-F 4-C:1 3-F 4-Cl
3-C1 4-Cl 3-Cl ~-Cl
3-CH3 4-Cl 3-CH3 4-Cl
3--~ 9--C~3 3--F r 4--CH3
3-Cl 4-CH3 3-Cl 4-CH3
2-F 4~F 2-F 4-F
2~F 4-C1 2-F - 4-Cl
~-F 4-~r 2-F 4-Br
2 F 4-CH3 2-F ~ 4-CH3
2-F 3-CI . 2-F 3-Cl
.
:
WO 91/1~228 2 0 ~ 5 3 8 2 P~/US91/00371
R~C~IO R8C ( O ) CH3
~l ~2
4-F H 4-F H
9-Cl H 4-Cl H
4-~r H 9-Br H
4-CH3 H 4-C~33 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 4-OCH2CH3 H
3-OCH2C_CH H 3-OCH2C_C~I H
4-OCHF2 H 4-OCHF2 H
4-OCH2CF3 H 4-OCH2CF3 H
4-SCH3 H 4-SC:H3 H
4-SO2CH3 H 4-SO2CH3 H
4 -SO2CF3 H 4 -So2cF3
4-CN H 4-CN H
4-NO2 H 4-NO2 H
3-F H 3-F
3-Cl H 3-Cl H
3-F 4-C1 3-F 4-Cl
3-Cl 4-Cl 3-C1 4-Cl
3-CH3 4-Cl - 3-CH3 4-Cl
3-F ~I-CH3 3-F 4-CH3
3-C1 4--CH3 3-Cl 4 CH3
2--F 4 F 2--F 4-F
2-F 4-Cl 2-F 4-(:l
2-F 4-~r 2-F 9-~r
2-F 4-CH3 2-F 4-CH3
2-F 3-Cl 2-F 3~ C:l
, - , - ,, , : ,:, . . ;,,: . .. .
-, :
~O91/lZ22~ 2 ~ Z ~ PCT/US91/00371 -
24
R~SN(cH3)C(O)Oc4H9 R~SO2)CF3
~l B2 ~1 ~2
~-F H 4-F H
4-Cl H 9-Cl H
4-Br H 4~Br H
4-CH3 H 4-CH3 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 4~OCH2CH3 H
3-OCH2C_CH H 3-OCH2C-CH H
9-OCHF2 H 4-OCHF2 H
4-OCH2CF3 4 OCH2CF3
4-SCH3 H 4-SCH3 H
4-SO2CH3 H 4-SO2CH3 H
4-SO2CF3 H 4-SO2CF3 H
4-CN H 4-C~ H
4-NO2 H 9-NO2
3-F H 3-F H
3-Cl H 3-Cl H
3-F 4 Cl 3-F 4-Cl
3-Cl 4-C1 3-~1 4-Cl
3-CH3 4-Cl 3-CH3 4-Cl
3-F 4 CH3 3-~ 4 CH3
3-Cl 4-C~3 3-Cl ~ C~3
2-F ~ 4 F 2-F 9-F
2-F 4-Cl 2-F 4-Cl
2-F 4-Br 2-F 4-B~
2-F 4-CH3 2-F 4-CH3
2-F 3-Cl 2-F 3-Cl
.
Wo91/12228 2 ~ 7 5 i3 8 2 PCT/~Sgl/00371
R,~
~Ql~ ~3 Bg
a 2-CF3 6 CF3
b 2-C1 6-Cl
c 3-C1 5 Cl
d 2-F 6-F
2 0 e 2 -CH3 6 CH3
Compounds of Table 3 wherein R,.Rl and R2 are
as set out therein can be prepared having the recited
values of Groups a through e. That is, or each
2~ value of R, Rl and~R2, R3 and ~4 can be CF3, Cl, ~ or
C~3. All of ~aid compounds are specifically included , ~;
within the ~cope of this invention~
~ :'
3~ : .
This:T~ble contains a large number o~ compounds
of Formula I~in a format adoptéd to avoid mechanical
reproduction of substituent values that do not vary.
For e~ample, the first table entry (where R is H, Rl -
is 4-F and R2 is H) actually specif,ies 5 separate and
di~tinct compounds because the Key for Table 3
identifi~es addi~ ~nal substituent ~alues of R3 and
R4, namely Groups a through e for each Table 3 en~ry.
,
.:
: .
WO 91/12228 2 0 7 ~ 3 ~ 2 pcrtus9l/oo371 --
. .
R~H R~CH3
Bl ~2 ~l B2
4-F H 4-F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 4-CH3 H
4-CF3 H 4 CF3 H
4-OCH2CH3 H 4-OCH2CH3 H
3-OCH2C_CH H 3-OCH2C--CH H
9-OCHF2 H 4-OCHF2 H
4-OCH2CF3 H 4-OCH2CF3 H
4-SC~3 H 4-SCH3 H
4-SO2C~3 H 4-SO2CH3 H
g S2~F3 H 4-SO2CF3 H
4-CN H 4-CN H
4-NO2 H 9-NO2 H
3-F H 3-F H
3-Cl H 3-Cl H
3-F 4--Cl 3--F 4-Cl
3-Cl 4-Cl 3-Cl 4-Cl
3-CH3 4-Cl 3-CH3 4-Cl
3-F 4--CH3 3-F 4-CH3
3-Cl 4-CH3 3-Cl 4-CH
2-F 4-F 2-F 4-F
2-F 4-Cl 2-F 4-Cl
2-F 4-Br 2-F 4-Br
2-F 4-CH3 2-F 4-C:H3
2-F 3-Cl 2-F 3-Cl
.
.
:- . ~: . ' .... . .. .
.. .
,
.
' , " , ,. ~ ,
', ' .
WO 91/12228 2 0 7 5 3~8 2 Pcr/usg1/oo37l
,
27
R~CHO R-zC ( O ) CH3
2 ~l ~2
4--F H 4--F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 4-CH3 H
4-CF3 H 4-CF3 H
4-OCHzCH3H 9-OCH2CH3 H
3-OCH2C_CH H 3-OCH2C_CH H
4-OCHF2 H 4-OCHF2 H
4~0CH2CF3 H 4-OC112CF3 H
4-SCH3 H 4-SCH~ H
4-S02CH3 H 4-S02CH3
4-S02CF3 ~ 4-S02CF3 H
4-CN H 4-CN H `
4-No2 H 4-N02 H
3-~ H 3-F . H
3-C:l H 3-Cl H
3-F 4-C1 3-F 4-Cl
3-C1 4-Cl 3-Cl 4-C
3--CH3 4--Cl 3--CH3 4--Cl
3--F 4-CH3 . 3~~ 4-CH3
3-Cl 4--CH3 3--Cl 4-CH3
2--F 4-F 2-~ 4--F
2-F 4-Cl 2-F 4-Cl
2-F 4-E3r 2-F 4-Br
2-F 4-CH3 2-F 4-CH3
2-F 3-Cl 2-F 3-Cl
.
wo 91/12228 2 ~ 7 S 3 8 2 PCT/US91/00371 ~
28
R~SN(CH3)C(O)OC4Hg R-SO2CF3
~1 R2 ~1 B2
4-F H 4-F H
4-Cl H 4-Cl H
4-Br H 4-Br H
4-CH3 H 9-CH3 H
4-CF3 H 4-CF3 H
4-OCH2CH3 H 4-OCH2CH3 H
3-OCH2C_CH H 3-OCH2C-CH H
4-OCHF2 H 4-OCHF2 H
4-OCH2CF3 H 4-OCH2CF3
4-SCH3 H 4-SCH3 H
4-SO2CH3 H 9-SO2CH3 H
q SO2CF3 H 4-SO2CF3 H
4-CN H 4 CN H
4-NO2 H 4-NO2 H
3-F H 3-~ H
3-Cl H 3-Cl H
3-F 4-Cl 3-F 4-Cl
3-Cl 4-Cl 3-Cl 4-Cl
3-CH3 4-Cl 3-CH3 4-Cl
3-F 4-CH3 - 3-F 4-CH3
3-Cl ~-CH3 3-Cl 4-CH3
2 F 4-F 2-F . 4-F
2-F 4-Cl 2 F 4-Cl
2-F 4-Br 2-F 4-Br
2-F 4-CH3 2 F 4-CH3
2-F 3-Cl 2-F 3-Cl
WO91tl2228 2 0 7 5 ~ 8 2 PCT/US91/00371
29
F~rmuLa~ion an~ Use
The compounds of this invention will generally
be used ;n formulation with an agriculturally
suitable carrier comprising a liquid or solid diluent
or an organic solvent. Useful formulations of the
compounds of Formula I can be prepared in
conventional ways. They include dusts, granules,
lQ baits, pellets, solutions, suspensions, emulsions,
wettable powders, emulsifiable concentrates, dry
flowables and the like. Many of these can be applied
directly. Sprayable formulations can be extended in
suitable media and used at spray volumes of frsm
about one to several hundred liters per hectare.
High strength compositions are pr;marily used as
intermediates for further formulation. The
formulations, broadly, contain from less than about
1% to 99% by weight o~ active ingredient(s) and at
least one of a) about 0.1% to 20% surfactant~s) and
b) about 5~ to 99% solid or liquid diluent(s). More
specifically, they will contain effective amounts of
these ingredients in the following appro~imate
proportions:
_ Pe~n~ by WeiQh~
Active
II~LJ~1~&1~ Diluent(s~ E~L5~ S1
Wettable Powders 25-90 0-74 1-10
Oil ~uspensions, 5-50 40-95 0-15
30 Emulsions, Solutions,
(including Emulsifiable
Concentrates)
Dusts 1-25 70-99 0-5
Gran~les, Baits 0.01-95 5-99 0-15
and Pellets
3~
High Strength 90-99 0-10 0-2
Compositions
.. . . - . .. . .. . . . . . .
. ~ . . .. . ~ . . : . ,
- . . .: . . :
.:
. . .
, , : : . .
W091/12228 PcT/US91/00371 -
20~382
~o
Lower or higher levels of active ingredient can,
of course, be present depending on the intended use
and the physical properties of the ~ompound. ~igher
ratios of surfactant to active ingredient are some-
times desirable, and are achieved by incorporation
into the formulation or by tank mi~ing.
Typical solid diluents are described in Watkins,
et al., "Handbook of Insecticide Dust Diluents and
Carriers", ~nd Ed., Dorland Books, Caldwell, New
Jersey. The more absorptive diluents are preferred
for wettable powders and the denser ones for du~ts.
Typical liquid diluents and solvents are described in
1~ Marsden, "Solvents Guide,~ 2nd Ed., Interscience, New
York, 1950. Solubility under 0.1% is preferred for
suspension concentrates; solution concentrates are
~referably stable against phase separation at O~C.
"McCutcheon's Detergents and Emulsifiers Annualn,
Allured Publ. Corp., Ridgewood, New Jersey, as well as
Sisely and Wood, "Encyclopedia of Surface Active
Agents~, Chemical Publ. Co., Inc., New York, 1~64,
list sur~actants and recommended uses. All formula-
tions can contain minor amounts of additives to reduce
foam, caking, corrosion, microbiological growth, etc.
Preferably, ingr~dient~ should be approved by the U.S.
Environmental Protection Agency for the use intended.
The methods of making such compositions are well
known. Solutions are prepared by simply mi~ing the
ingredients. Fine solid compositions are made by
blending and, usually, grinding as in a hammer or
fluid energy mill. Suspensions are prepared by wet
milling (see, for example, U.S. 3,06D,084). Granules
and pellets can be made by spraying the active
material upon preformed granular carriers or hy
ag~lomeration techniques. See J. E. Browning,
~Agglomeration", ~hemi~a~_En~in~Li~, December q,
, . I , . - . ~ . ~: , -
.- . - ... . . .: . . . . . . . .. . .. . .
- ... - - - ., .. ,. . . - , . .. . .. . .
- . . ,, , , :, .. .. : . . . . ,, . ~ ~
- ..... , ., - , .. .. :. .. , . .. : . . . . . .
- - : .,. , , .:, . .
.,. , , : . . . .
- WO9]/122~8 2 0 7 ~ 3 8 2 Pcr/Us9l/oo371
1967, pages 147 and following, and ~Perry~s Chemical
Engineer's Handbook~, 4th Ed., McGraw-Hill, New York,
1963, pages 8 to 59 and following.
~ample~a
Emulsi~i~ble Conce~S~
N-t4-chloro-a-~richloromethylbenzyl)-3,5-bis
(trifluoromethyl)aniline 20%
blend of oil soluble sulfonates
and polyo~yethylene ethers 10%
isophorone 70~ ~-
The ingredients are combined and stirred with
gentle warming to speed solutio~. A fine screen . --
filter is included in packaging operation to insure
the absence of any e2traneous undissolved material in
the product.
E~am~le 8
Wetta~le powdeI-
N-(4-chloro-a-trichloromethylbenzyl)-3,5-bis
(trifluoromethyl)aniline 30%
sodium alkylnaphthalenesulfonate 2%
sodium ligninsulfonate 2%
synthetic amorphous silica 3%.
kaolinite 63%
The acti~e i~gr~dient is mised with the inert
materials i~ a blender-. After grindi~g in 2 hammer-
mill, the material is re-blended and sifted through a
50 mesh screen.
Exam~le_~
1~U ~i tL
Wettable powder of E~ampl~ ~ 10% .
3S pyrophyllite (powder) 90%
. ~
~, . ~ , . ,. .. . .. ~.
WO9]/12~28 2 0 7 ~ 3 8 2 PCT/US9l/00371--
32
The wettable powder and the pyrophyllite diluent
are thoroughly blended and then packaged. The product
is suitable for use as a dust.
Exampl~_~
Gr~nul~
N-~9-chloro-~-trichloromethylbenzyl)-3,5-bis
(trifluoromethyl)aniline 10%
attapulgite granules ~low volative
matter, 0.71/0.30 mm; U.S.S. No.
25-50 sieves) 90%
The active ingredient i~ dissolved in a volatile
solvent such as acetone and sprayed upon dedusted and
pre-warmed attapulgite granules in a double cone
blender. The acetone is then driven off by heating.
The granules are then allowed to cool and are packaged.
Ex~mple
G~anulQ
Wettable powder of Example B 15%
gypsum 69%
potassium sulfate 16%
The ingredients are ~lended in a rotating mi~er
and water sprayed on to acromplish granulation. Wh~n
most of the material has reach~d ~he de~ired range of
0.1 to 0.42 mm (U.SOS. No. lB to 40 siev~), the gran-
ules are removed, dried, and s~reened. Oversize
material is crushed to produce additional material in
the desired range. These granules contain 4.5~ active
ingredient.
ExamDl~ F
35 SolutiQn
N-(4-chloro-a-trichloromethylbenzyl)-3,5~bis
(trifluoromethyl)aniline 25%
, . .. . . . . . . . . . . .
- ... ~ . . , ............ ... : , . ..
.. . . , . . , . - . ,. . , . . . .: : . :
: . . : : , : , . . . . .
,'-WO91/læ28 20~382 PC'r/USg1/00371
33
N-methyl-pyrrolidone 75%
The ~ngredients are combined and stirred to
produce a solution suitable for dir~ct, low volume
application.
Example G .
A~ueo~ Su~Pension
N-(4-chloro--trichloromethylbenzyl)-3,5-bis
(trifluoromethyl)aniline 40%
polyacrylic acid thickener 0.3%
dodecyclophenol polyethylene glycol
ether 0.5%
disodium phosphate 1.0
monosodium phosphate 0.5%
polyvinyl alcohol 1.0%
water 56.7~
The ingredients are blended and ground together
in a sand mill to produce particles suhstantially all
under 5 microns in size.
~ ;amP
Oil._~usp~nsion .
N-(4-chloro-a-trichloromethylbenzyl~-3,5-bis
~trifluoromethyl)aniline 35.0%
blend of polyalcohol carbo~ylic 6~0~ -
esters and oil soluble petroleum
sulfonates
xylene range solvent s9~o%
The ingredients are combined and ground together
in a sand mill to produce particles substantially all
below 5 microns. The product can be used directly,
extended with oils, or emulsified in water.
,
.. . .
, , , . ~ . ~ . . .. .
. .
. . . .
- . :, . . . .
,,, , ~
; ~222~ 2 ~ 7 5 ~ ~ 2 PCT/US9~/00371 ~'1
. .
34
~m~
Bait ~ranules
N-(4-chloro-a-triChlrmethylbenzyl)-3,5-bis
(trifluoromethYl)aniline 3 0%
blend of polyetho~lated nonyl- 9.0%
phenols and sodium dodecyl-
benzene sulfonates
ground up corn cobs 88.0~
The active ingredient and surfactant blend are
dissolved in a suitable solvent such as acetone and
sprayed onto the ground corn cobs. The granules are
then dried and pa~kaged.
l~ Compounds of Formula I can also be mi~ed with
one or more other insecticides, fungicides,
nematocides, bacteric;des, acaricides, or other
biologically active compounds to form a
multi-component pesticide giving an even;broader
spectrum of effective agricultural protection~
Examples of other agricultural protectants
with which compounds of this invention can be
formulatPd ,are:
25 I~C~:
3-hydrosy-N-methylcrotonamide(dimethylphosphate)ester
(monocrotophos)
m~thylcarbami~ acid, ester with 2,3-dihydro-2,2-
dimethyl-7-benzofuranol ~carbofuran)
0-~2,4,5-trichloro-a-(~hloromsthyl)benzyl]phosphoric
acid, O~,O'-dimethyl ester (tetrachlorvinphos)
2-mercaptosuccinic acid, diethyl est~r, S-ester with
thionophosphoric acid, dimethyl ester (malathion~
phosphorothioic acid, O,O-dimethyl, O-D-nitrophenyl
3~ ester (methyl parathion)
.
- . . . . . ..
- : .
' I
' ~ ~ ' . , ' . '
.' .'' ~ ' '~ . ' ', ,' ' :,
- Wogl/12228 . . PCT/US91/0037]
2~382-
methylcarbamic acid, ester with a-naphthol (carbaryl)
methyl 0-(methylcarbamoyl)thiolacetohydro~amate
(methomyl)
N'-(4-c:hloro-Q-tolyl)-N,N-dimethylformamidine
(chlordimeform)
0,0-diethyl-0-(2-isopropyl-4-methyl-6-pyrimidylphos-
phorothioate (diazinon)
octachlorocamphene (toxaphene~
0-ethyl 0-p-nitrophenyl phenylphosphonothioate (EPN)
(S)-a-cyano-~-phenoxybenzyl(lR,3R)-3-~2,2-dibromovinyl)
-2,2-dimethylcyclopropanecarboxylate (deltamethrin)
Methyl-N',N'-dimethyl-N-t(methylcarbamoyl)o~y]~
thioos amimidate (osamyl)
cyano(3~pheno~yphenyl~-methyl-4-chloro-a-(1-methyl-
ethyl)benzeneacetate (fen~alerate) :
~3-phenoxyphenyl)methyl( )-~,~n-3-(2,2-dichloro
ethenyl)-2,2-dimethylcyclopropanecarbo~ylate (perme-
thrin)
a-cyano-3-pheno~ybenzyl 3 (2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carbo2ylate (cypermethrin)
0-ethyl-S~(~-chlorophenyl~ethylphosphonodithioate
(profenofos)
phosphorothiolothionic acid,
O-ethyl-O-t4-(methylthio)-phenyl]-S-n propyl este
(sulprofo6).
Additional insecticides are listed hereafter by
their common names: triflumuron, diflubenzuron,
methoprene, buprofezin, thiodicarb, acephate, azinphos-
methyl, chlorpyrifos, dimethoate, fonophos, isofenphos,
methidathion, methamidiphos, monocrotphos, phosmet,
phosphamidon, phosalone, pirimicarb, phorate,
profenofos, terbufos, trichlorfon, methoxychlor,
bifenthrin, biphenate, cyfluthrin, fenpropathrin,
:~ , . . . .
. . . . .
.
WO91/12228 2~0~7 5 3 8. 2 PCT/US91/00371
fluvalinate, flucythrinate, tralomethrin, metal-
dehyde and rotenone.
Funqi~
methyl 2-benzimidazolecarbamate (carbendazim)
tetramethylthiuram disulfide (thiuram)
n-dodecylguanidine acetate (dodine)
manganese ethylenebisdithiocarbamate ~maneb)
1,4-dichloro-2,~-dimethoxybenzene (chloroneb)
methyl l-(butylcarbamoly)-2-benzimidazolecarbamate
~benomyl)
1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-
ylmethyl]-lH-1,2,4-triazole (propiconazole)
2-cyano-N-ethylcarbamoy-2-metho~yiminoacetamide
(cymo~anil)
1-(4-chloropheno~y)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-
yl)-2-butanone (triadimefo~)
N-(trichloromethylthio)tetrahydrophthalimide (captan)
N-(trichloromethylthio)phthalimide (folpet)
l-[t~bis~4-fluorophenyl)~[methyl~silyl~methyl]-lH-
1,2,4-triazole.
- 25 Ne~ti~ides: .
S-methyl l-~dimethylcarbamoyl)-N-(methylcarbamoyloxy)-
thioformimidate
S-methyl
l-carbamoyl-N-~methyl~arbamoylo~y)thioformimidate
N-isopropylphosphoramidic acid, O-ethyl
O'-C4-(methylthio)-m-tolyl~diester (fenamiphos)
~actc~i~i~5:
tribasic copper sulfate
streptomycin sulfate
.. . ~ .......... . . , . ~: :,
- .. -: . . , ,: . .. .
wosl/l2z2B 2 0 7 5 3 8 2 PCTtUS91/00371
caricides:
senecioic acid, ester with 2~~-butyl-4,6-dinitro-
phenol (binapacryl)
6-methyl-1,3 cithiolot4,5-B]quinoxalin-2-one
(o~ythioquinox)
ethyl 4,4'-dichlorobenzilate (chlorobenzilate)
1,1-bis(P-chlorophenyl)-2,2,2-trichloroethanol
(dicofol)
bis(pentachloro-2,4-cyclopentadien-l-yl) (dienochlor)
tricyclohexyltin hyd~oxide (cyhexatin)
trans-5-(4-chlorophenyl)-N-cyclohe~yl-4-methyl-2-oxo-
thiazolidine-3-carboxamide (hexythiazo~)
amitraz
propargite
fenbutatin-oxide
~iolosi~l
Bacillus thuringiensis
Avermectin B.
~tili~y
The compounds of this invention eshibit activity
against a wide spectrum of foliar and soil inhabiting
arthropods which ar~ pests of growing and stored
agronomic crops, forestry, greenhouse ~rops,
ornamentals, nursery crops, stored food and ~iber
products, li~estock, household, and public and animal
health. Those skilled in the art will recognize that
not all compounds are~ equally effective against all
pests but the compounds of this invention display
activity against economically important agronomic,
forestry, greenhouse, ornamental food and fiber
product, stored product, domestic structure, and
nursery pests, such as:
- . - , . ~ . . . . . ................................. . .
,
- : . . : , ~ , . . . .~
WO91/12228 2 0 7 S 3 8 2 PCT/US91/00371 -
38
larvae of the order ~ a including
fall and beet armyworm and other ~DodoPte~a
S~P., tobacco budworm, corn earworm and o~her
Helio~hi~ ., European corn borer, navel
orangeworm, stalk/stem borers and other
pyralids, cabbage and soybean loopers and
other loopers, codling moth, grape berry moth
and other tortricids, black cutworm, spotted
cutworm, other cutworms and other noctuids,
diamondback moth, green cloverworm,
velvetbean caterpillar, green cloverworm,
pink bollworm, gypsy moth, and spruce budworm;
foliar feeding larvae and adults of the order
Col~optera including Colorado potato beetle,
Mexican bean beetle, flea beetle, Japanese
~eetles, and other leaf beetles, boll weevil,
rice water weevil, granary weevil, rice
weevil and other weevil pests, and soil
inhabiting insects such as Western corn
rootworm and other Piabrotica sp~., Japanese
beetle, European chafer and other cvleopteran
grubs, and wireworms;
adults ~nd 1 rvae of the orders Hemi~ Q a~d
~55~t~1~ includi~g tarnished plant bug and
- other plant bugs (miLig~ aster lea~hopper
- 30 and other leafhoppers (cicadelLi~e), rice
planthopper, brown planthopper, and other
planthoppers (fulQQ~oidea), psylids,
whi~eflies (9~ L~I), aphids (aphidae),
s~ales (cQccid~ and diasp.i~idaç), lace bugs
(~i~gi~gQ), stink bugs (Den.tatomidaç), cinch
bugs and other seed bugs (~Y~çi~a~), cicadas
(sic~Lda~), spittlebu~s (c~r~Qpi~), squash
:,
.
- : . . - . . .. :, . , -... .. , ,, , . , ~: . .
2~5382`
-- WO91/12228 PCrtUS91/0037
. 39
bugs (s~silgg), red bugs and cotton stainers
(DYrrhocorida~);
adults and larvae of the order a~ari (mites)
including European red mite, two spotted
spider mite, rust mites, McDaniel mite, and
foliar feeding mites,
adults and immatures of the order Orthopt~ra
including grasshoppers;
adults and immatures of the order Diptera
including leafminers, midges, fruit flies
(te~hritidae), and soil maggots,
~ .
adults and immatures of the order
Thys~nopter~ including onion thrips and other
foliar feeding thrips.
The compounds are also acti~e against
economically important livestock, household, public
and animal health pests such as:
insect pests of the order HYmenoPt~ra
in~luding carpenter ants, bees, hornets, and
wasps;
insect pests of the order Di~t~L~ including
house flies, stable flies, face flies, horn
flies, blow ~lies, and other muscoid fly
pests, horse flies, deer flies and other
8rachYce~, mosquitoes, black flies, biting
~35 midges, sand flies, sciarids, and other
~emato~era;
207~382
WO91/12228 PCr/US91/00371 -
i~sect pests of the order Q5~h~2~L~
including cockroaches and crickets;
insect p~sts of the order I~QE~ÇL~ including
the Eastern subterranean term.ite and other
termites;
insect pests of the order Malloph~ and
Anol~LLa including the head louse, body
louse, chicken head louse and other sucking
and chewing parasitic lice that attack man
and animals;
insect pests of the order Siphonoptera
including the cat flea, dog flea and other
flea~.
The specific species for which cont ol is
exemplified are: fall armyworm, Spodop~er~
r~i~i~L~a; tobacco budworm, ~eliot~ vir~sce~;
boll weevil, Anthonom~ sI~n~i~; aster leafhopper,
~ac3s~beic~ fa~s~llaIla; black bean aphid, (~hi~
E~k~): southern corn rootworm, ~igL~Q~
~ C~ . The pest control protection a~forded
by the compounds of the pr~sent in~ention is not
limited, however, to these sp~ies. The compounds o~
this invention ma~ also be utilized as rodenticides.
App~ica~iL~
Arthropo~ pests are controlled and protection of
a~ronomic crops, animal and human health is achieved
by applying~one or more of the Formula I compounds of
this invention, in an effective amount, to the
environment of the pests including the agronomic
: and/or nonagronomic locus o~ infestation, to the area
to be ~rotected, or directly on the pests to be
~ .
:: - : , . . . ........... . . . -. - . . ..
. . . ., , . , ,, , .. . ~ .. . . . .. .
- WO91/12228 2 ~ 7 5 ~ 8 2 PCT/~S91/00371
41
controlled. ~ecause of the diversity of habitat and
behavior of these arthropo~ pest species, many
different methods of application are employed. A
preferred method of application is by spraying with
equipment that distributes the compound in the
environment of the pests, on the folia~e, animal,
person, or premise, in the soil or animal, to the
l~ plant part that is infested or needs to be protected.
Alternatively, granular formulations of these toxicant
compounds can be applied to or incorporated into the
soil. Other methods of application can also be
employed including direct and residual sprays, aerial
sprays, baits, eartags, boluses, foggers, aerosols,
and many others. The compounds can be incorporated
into baits that are consumed by the arthropods or in
devices such as traps and the like which entice them
to ingest or otherwise contact the compounds.
The compounds of this invention can be applied
in their pure state, but most o~ten application will
be of a formulation comprising one or more compounds
with suitable carriers, diluents, and surfactants and
possibly in combination with a food depending on the
contemplated end use. A preferred method of
application i~olves spraying a water dispersion or
refined oil solution of the compounds~ Combinations
with spray oil~, spray oil concentrations, and
synergists such as piperonyl buto~ide often enhance
the efficacy of the compounds of Formula I.
The rate of application of the Formula I
~ompounds required for effective control will depend
on such factors as the species of arthropod to be
controlled, the pest's life cycle, life stage, its
size, location, time of year, host crop or animal,
feeding behavior, mating behavior, ambient moisture,
temperature, etc. In general, application rates of
... ... . . ..
- ' ' "- . ' ' . ' '~ ' . . .
.
: ; .
. ' ' :
.
WO91/12228 æ ~ ~ 53 ~ 2 PCT/US91/00~71
0cOl to 2 kg of actiYe ingredient per hectare are
surficient to provide large-scale effective control of
pests in agronomic ecosystems und~r normal
circumstances, but as little as O.OOl kg/hectare or as
much as 8 kg hectare may be required. For
nonagronomic applications, effective use rates will
range from abou~ l to 50 mg/square meter but as little
as about O.l mg/square meter or as much as l50
mg/s~uare meter may be required.
The following Examples demonstrate the control
efficacy of compounds of Formula I on specific pests;
see Inde~ Tables A and B for compound descriptions.
Compounds not included in the E~amples were either not
screened or produced mortalities of less than 80%.
-, .. . . . . .. . .
- ~ . ' ~ . '-; ,. . .. .. . . ..
- : . . . - . . : . :
- ,,, ::
WO91/12228 2 ~ ~ ~ 3 8 2, PCT/US91/00371
. . .
43
x~
CF3
~m~ 3 B2 ~l Physical Sta~e
7 3-CF3 H 4-F H oil
8 3-CF3 H 4-F 2-F oil
9 3-CF3 H 4-OEt H oil
3-CF3 H 4-OCHF2 . H oil
ll 3-CF3 H 4-CH3 H solid; mp: 84-86C
12 3-CF3 H 4-Br H very viscous oil
13 3-CF3 H 4-OCH3 H viscous oil
14 3-CF3 H 3,4~OCH20- viscous oil
3-C~3 H 4-OCH2CF3. H viscous oil
16 3-CF3 H ~-U 3-Cl solid; mp: 96-99C
17 3-CF3 H 4-~CH2C-C~ H solid; mp: 76-78C
.. . . .. . .. . . .
. . .
.
:
WO 91/12228 2 ~ 7 ; Pcr/US91/00371 ~.
44
~3~2
B3 ~2 ~1 Phy$is;al
18 2-Cl H 4-Cl H viscous oi l
l9 2-Cl H 4-F 2-F solid; mp: 79-81C
20 2-Cl H 4-OCH2C-CH H ViSCOIlS oil
21~ 2-Cl H 4-OEt H viscous oil
22 2-Cl H 4-OCHF2 H viscous oil
23 2-Cl ~ 4-OCH2CF3 H viscous oil
24 2-Cl H 4-CH3 H viscous oil
25 2-Cl H 4-SCH3 H viscous oil
.. . ,. ~ . .. . .. . . . . .... . . .
~ - ~ . .. j.. ... .
: ' , .. ' '. ' ' . :
~075~8~
-` WO 91/12228 , PC~/US91/00371
1~7i~LI~
Exam~le ~s ~ ~3~2 ~1
26 H 3-Br S-Br 4-F . H oil
27 H 3-Br 5-~r 4-Cl H solid; mp: 113-115C
2B H 3-C1 5-C1 4-tBu H oil
29 H 3-C1 5-C1 4-OCH3 ~ oil
H 3-C1 5-C1 4-C~0 H mp: 152-155C
31 H 3-C1 5-C1 3-F H o~l
32 H 3-C1 5-C1 3-Cl H ~olid; mp: 89-91C
33 H 3-C1 5-C1 3-CH3 4-C1 601id: mp: 96.5-99C
34 H 3-C1 5-Cl ~-(4-C~3C6H4)CH20 vi~oou6 ~omi-~olid
H 3-C1 5-C1 4-(C~OCH3)2C~3~ ~olla; mp: 133-135C
36 ~ 3-C1 5-Cl ~-C~O)C~3 ~olid~ ~p: 137-140C
37 ~ 3-C1 5-C1 4-~C~3 o~
H 3-C1 5-Cl 4-S~O)C~3 pa~ty 6emi-601id
3~ ~ 3-C1 5-C1 4-S(0)2C~3 oil
H 3-C1 5-CF3 4-OCH2C-CH oil
.- - - : , , ,: :: :: :
' ` : ~ ..: .
WO 91/~:~128 2 0 7 ~ 3 8 2 Pcr/us9l/0037l
46
R~s R~
Q B4 ~3 B2 ~1 Phy~ al ~.ate
41 5-C1 ~ ~ 0~3t viscous oil
42 5-C1 H H Cl m.p. 1~8-141C
43 5-Cl H H CF3 very viscous oil
44 5-C:1 3-Cl H F viscous oil
6-Me 4-Me H CF3 m.p. 97-100C
46 4-Me H H CF3 m.pO 118-120C
47 5-Br H H F m.p. 128-129~C
48 5-Me H H F m. p . 123-125C
49 5-OMe H H F viscous oil :
6-Me 4~Me H F viscous oil
51 6-Me 4-Me H OE'c waxy solid
52 5--Cl H H OC}~F2 viscous oil
53 S-~e H H OCHF2 viscous oil
54 5-C1 3-Cl H OCHF2 viscous oil
6-Me 4-Me H OCHzCF3 viscous oil
56 5-CF3 H H C)C}12CF3 viscous oil
57 5-CF3 H H Cl m.p. 133-135C .
58 S-Cl 3-Cl H OCHzCF3 very viscous oil
59 5-C1 3-Cl H Br m.p. 105-107C ,
5-C1 3-Cl H CF3 m. p . 87-89C
61 S-Cl 3-Cl H Me m. p . 75-77C
62 5-C1 3-C1 3-Cl Cl solid
63 5-C~3 3~Cl ~ Cl æolid
.
.. . . . . . . .: . . . . . . .~ . . . .. : . . .. : .
. . .. . - ., ., . .. . ,. ~, . .... ... . .
- -, .. . . , . :. .. , . . . , :
'- ' - . .' ~.. ~ .;. :~ ' .' '''"'. '. "' ; ' '
W09i/122282 0 7 5 a 8 2 PCT/US91/00371
47
~Eh~ J
Fa~ myw~rm
5Test u~its, each con~isting of an 8-ounce (230
mL) plastic cup containing a layer of wheat germ
diet, appro~imately 0.5 cm thick, were prepared.
F;ve third-instar larvae of fall armyworm (Spodop~tera
f~ugi~e~a~ were placed into ea~h cup. Solutions of
each of the test compounds ~acetone/distilled water
75/25 solvent) were sprayed into the cups. Spraying
was accomplished by passing the cups, on a conveyer
belt, directly beneath a flat fan hydrauli~ nozzle
which discharged the spray at a rate of 0.5 pounds of
15 active ingredient per acre (about 0.55 kg/ha) at 30
p.s.i. (207 kPa). The cups were then covered and
held at 27C and 50~ relative humidity for 72 hours,
after which time readings were taken. Of the
compounds tested, the following gave mortality levels
20 of 80% or higher: 1, 2~, 3*, 6, 7, 8, 14, lS, 16,
18, 20, 21, 23, 26, 27, 29, 31, 32, 33, 37, 40, 41,
43, 49, 45, 47, 48, 51, 52, 53, 54, 5S, 57 and 58.
*Tested at 0.137 kg/ha.
~ .
Tolaol~L~E~d~o~m
The test procedure of E~ample J ~as repeated for
efficacy against third-instar larvae of the tobacco
budworm (Helio~hi~ virescens) except that mortality
was assessed at 48 hours. Of the compounds tested,
the fo}lowing gave mortality levels of 80% or
higher: 1, 3*, 7, 8, 14, 15, 16, 23, 25, 27, 28, 33,
42, 44, 47, 50, 54 and 57.
*Tested at 0.137 kg~ha.
' . ' ' '`; : :'
- ~ .
- , ~ , ' . : - -
,' , . .
, ,~ .i , :, ' . ' , . . :
Wo91/12228 PCT/US91tO0371
2~ 7~382
~8
Sout~rn 5~n RQotwo~m
The units, each consisting of an 8-ounce (230 mL~
plastic cup containing 1 sprouted corn ~eed, were
prepared. The test units were sprayed as described
in Example J with individual solutions of the test
compounds. After the spray on the cups had dried,
five third-instar larvae of the southern corn
rootworm (Dia~s~ Q undç~imp~ns~ a howardi) were
placed into each cup. A moistened dental wick was
inserted into each cup to prevent drying and the cups
were then covered. The cups were then held at 270C
and 50% relative humidity for 48 hours, after which
time mortality readings were t~ken. Of the ~ompounds
tested, the following gave mortality levels of 80~ or
higher: 1, 2~, 7, 8, 9, IO, 11, 12, 13, 14, 15, 29,
37, 41, 45, 46, 97, 48, 50, 51, 53 and 56.
~Tested at 0.137 kg/ha.
'
~ste~ LeafhopPe~
Test units were prepared from a series sf
12-ounce (350 mL) cups, each containing oat (~Y~n~ -
~iY~) ~eçdlings in a l-inch (2.54 cm) lAyer of
~terilized soil. The test unit~ were ~prayed as
described in Example J with individual solutions of
the below-listed compounds. After the oats had dried
from the spraying, between 10 and 15 a~ult aster
leafhoppers (M~ crQ~tele.s faseirons) were aspirated
into each of the covered cups. The cups were held at
- 27C and 50% relative humidity for 48 hours, after
3~ which time mortality readings were taken. Of the
compounds tested, the following gave mortality levels
. . . . .. . . . ... , ,, .. , . , ,, . " ... .... . ....... . . ... .
: . . . . .. . . . .. . . .. .. . . . . . . . .
. .-: , ,: '. - , ,' ., ' ' ' :. . '' '' ' ' .
: . . . . . . . . . . .
' ,' . '', ' :',' ' ' ''''. ' ' .. ' '. . ' ' '
W091/12228 2 0 ~ ~ 3 8 2 PCT/US91/00371
49
of 80% or higher: l, 2~, 7, 8, 9, 10, ll, 12, 13,
14, 15, 16, 18, l9, 21, ~2, 23, 24, 29, 41, 43, ~4,
45, 48, 54 and 57.
*Tested at 0.137 kg/ha.
EXAMPLE N
Boll Weevil
Five adult boll weevils (Anthonom~s ~randis
arandis) were placed into each of a series of 9 ounce
(260 mL) cups. The test procedure employed was then
otherwise the same as in Example J. Mortality
readings were taken 48 hours after treatment. Of the
compounds tested, the following gave mortality levels
of 80% or higher: 12, l3n, 16, 18, 43, 47 and 57.
~Tested at 0.137 kg/ha.
Black Bean Aphid
Tndividual nasturtium leaves were infested with 5
to lO aphids (all stages of ~p~ fabae) and sprayed
~5 with their undersides facing up on a hydraulic
sprayer as described in Esample J. The lea~es were
then set in l-i~ch diameter vials co~tainin~ sugar
water solution and covered ~ith a clear plasti~ 1 oz
- por~i~n cup to prevent escape of aphids that drop
from the leaves. The test units were held at 27C
and 50% relative humidity for 98 hours, after whi~h
time mortality readi~gs were taken. Of the compounds
tested, the following gave mortality levels of 80% or
higher: l, 6, 7, 8, lO, 12, 13, 14, l~, 18, 20, 24,
35 40, 41, 44, 45 and 55.
- .
. : . . . . ; . . . . ..
. : . :: , , . ~. , . :, ,., :. . .
- : . i - .. - : . ... ,
. .. : ::,
:, ... . :
WO91/12228 2 0 i 5 3 8 ~ P~T/US91/00371 _
~e~ .
One inch ~quares of kidney bean leaves that have
been infested on the undersides with 25 to 30 adult
mites (Tetr~ny~h~ ~L~i~l) were sprayed with their
undersides facing up on a hydraulic sprayer as
described in Test Ao The leaf squares were placed
underside up on a square of wet cotton in a petri
dish and the perimeter of the leaf square was tamped
down onto the cotton with forceps so that the mites
cannot escape onto untreated leaf surfa~e. The test
units were held at 27C and 50% relative humidity for
48 hours, after which time mortality readings were
taken. Of the compounds tested, the following gave
mortality levels of 80% or higher: l, 7, 9, l0, 15,
18, 22, 30, ~3, ~4, 47, 50, 56 and 57.
. .
...
- , . . . ~
.: , - ':,. ', , ' ,~ .. . .
.. . ., . .. : .
-'. ' ' ,, ' .: ~ '' : .. . ' . ' ~. ,