Note: Descriptions are shown in the official language in which they were submitted.
1 CPW 36~90
2~7~5
Sul~hur Removal Process
This invention relates to a process for the removal of
elemental sulphur from organic compou~ds.
Elemental sulphur is soluble, and is often present as a
contaminant, in organic liquids such as carbon tetrachloride and
hydrocarbons such as benzene and petroleum. ~lso organic
polysulphides are often present in such liquids and these readily
decompose to give elemental sulphur dissolved in the liquid.
Elemental sulphur can also result from the reaction of sulphur
compounds such as hydrogen sulphide with oxidising agents.
Furthermore elemental sulphur has an appreciable volatility and
may be present in gaseous hydrocarbon streams. It has been found
that such elemental sulph~r is re ctive with metals and can cause
severe corrosion problems in pumps and other equipment which are
used to handle the organic compounds: o~ particular concern is the
corrosion of submerged automobile fuel injection pumps which may
occur if the automobile fuel contains elemental sulphur.
The removal of reactive sulphur compounds, such as
hydrogen sulphide and carbonyl sulphide, from gaseous or liquid
streams is well known. Elemental sulphur, however, is
significantly more difficult to remove than reactive sulphur
compounds and existing sulphur removal processes are largely
ineffective in the removal of elemental sulphur.
It has now been found that an active form of metallic
copper, as produced through the reduction of a reducible copper
compound, can be used to remove elemental sulphur from streams of
gaseous or liquid organic compounds, particularly elemental
sulphur dissolved in liquid hydrocarbon streams.
Accordingly the present invention provides a process for
decreasing the content of elemental sulphur of a stream of gaseous
or liquid organic material contaminated with elemental sulphur
comprising contacting the stream with a sorbent containing
metallic copper as an active constituent.
The process of the present invention is preferably
conducted under conditions of temperature and pressure such that
~ : ;, .. , ;~ '
~ ': " :"": `., .
. ~ . . .
, ' ' ~: '
.
Z CPW 36490
207~4~
the organic stream is in the liquid state. Preferably the process
is effected at a temperature below 300C, particularly below
150~C, and at pressures up to to 100 bar abs. Organic liquids
suitable for treatment by the present invention include
hydrocarbon streams such as petroleum, kerosene, liquefied
petroleum gas (LPG), natural gas liquid (NGL), aromatic liquid
hydrocarbons, and liquefied natural gas (LNG).
The initial concentration of the elemental sulphur is
usually from 1 to 200 ppm, and typically from 1 to 50 ppm, by
1 weight
Conventional sorbents, such as those based on ~inc
oxide, which are used for the removal of reactive sulphur
compounds, are ineffective at removing elemental sulphur. It has
now bcen found that metallic copper can be used as the active
constituent in a sorbent which is effective to sorb elemental
suIphur from an organic gaseous or liquid stream: during the
sorption process the metallic copper is converted into copper
sulphide. Generally, the higher the copper content of the sorbent
the more elemental sulphur can be removed before replenishment of
the sorbent is required. Consequently, it is preferred that the
sorbent has a copper content of at least 30~ by weight ~expressed
as the percentage of the copper tlI) oxlde present in the loss
free sorbent after ignition of the sorbent at 900C), and more
usually from 50~ to 90~ by weight. The ability of the sorbent to
sorb elemental sulphur is also effected by the accessibility of
the copper metal by the elemental sulphur. Generally, a high
copper metal surface area sorbent is more efficient at sorbing
sulphur tha~ a sorbent of comparable copper content but lower
copper metal surface area. Particularly efficient sorbents are
those having copper metal surface areas in excess of 20 m2.g~l,
and especially those having copper metal surface areas in the
range 20-40 m2.g~l. Sorbents having greater copper metal surface
areas, eg in an excess of 50 m2.g~l, may also be used. A high
copper metal surface area sorbent may be formed by the reduction
of a copper compound, e.g. the oxide, carbonate, or nitrate, with
, :, .
- ... ~, .,-
3 CPW 36490
2~7~
a suitable reducing agent. Suitable reducing agents include
hydrogen, a compound decomposable to hydrogen in the presence of
the sorbent, carbon monoxide, ~nd mixtures of carbon monoxide and
hydrogen. The conditions under which the reduction of the copper
compound is conducted may be slmilar to those be employed in the
preparation of a copper based methanol synthesis catalyst from the
oxidic precursor to such a catalyst. The temperature at which the
sorbent is reduced is will depend to some extent on the nature of
the reducing agent: typically the temperature will be in the range
90 to 250C, and is usually in the range 150 to ~00C.
Conveniently, the sorbent may prepared in ~he form of
particulates similar in size to those conventionally used for the
removal of reactive sulphur compounds, such as described in US
patents US-4871710, US-499Sl81 and US-4983367. The sorbent may be
disposed in ~ single bed or more usually in a plurality o~
serially and/or concurrently arranged beds. Typically, the flow
of organic liquid through each bed would be at a rate sufficient
to give a liquid hourly space velocity ~L~SV) from 1 to 20 hr~l,
and more usually from 1 to lO hr~l.
Usually, the organic streams to be treated will contain
other contaminants in addition to elemental sulphur. T~ese other
contaminants may include hydrogen sulphide, carbonyl sulphide,
organic sulphur compounds, arsenides, and heavy metals such as
mercury. The metallic copper sorbent may be used to remove these
contaminants in addition to the elemental sulphur. It i8
preferred, however, to remove these contaminants from the organic
stream by conventional means, e.g. a sorbent comprising zinc oxide
and/or copper oxide, prior to contacting the metallic copper
sorbent, thereby minimising the amount required of the metallic
copper sorbent. Some of the byproducts, e.g. water &nd carbon
dioxide, of the reactions between the conthmlnants and the
conventionai sorbents may subsequently react with the metallic
copper sorbent, thereby reducing the effectiveness of the metallic
copper sorbent to remove elemental sulphur from the organic
liquid. It may thus thus also be preferred to remove these
., -. . . . . .
,
.. ..
.
4 CP~ 36490
reaction byproducts prior to contacting the me~allic coppe~ ~ 7
sorbent.
The present invention is further illustrated by
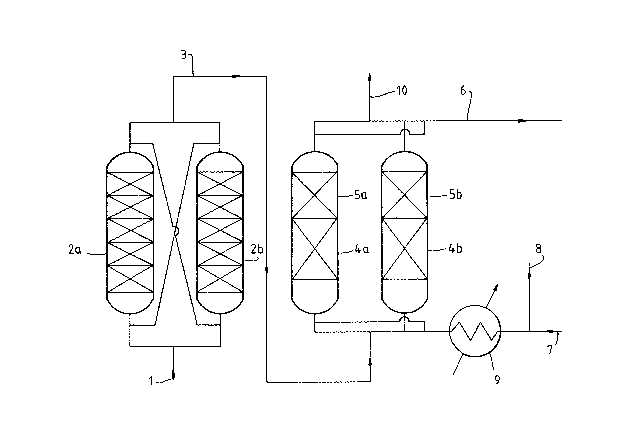
reference to the accompanying figure.
Figure 1 is a schematic diagram of an elemental sulphur
removal process of the present in~ention.
Figure l shows an organic liquid feed stream ~l)
con~acting one of two interchangeable contaminan~ removal reactors
~2a, Zb). The valving necessary to isolate each reactor and to
direct the li~uid stream between successive units has been omitted
for clarity. Reactors ~2R, 2b) are used to remove at least some
of the reactive contaminants such as hydrogen sulphide, carbonyl
sulphide as listed above and may contain a sorbent such as zlnc
andlor copper oxide. As a result of the reaction ocurring in beds
t~a. 2b), the stream ~3) is freed of the aforesaid reactive
contaminants but may contain by-products such as water and carbon
dioxide. Stream ~3) is then passed through one of two beds (4a,
4b~ effective to sorb at least some of the byproducts produced in
beds ~2~, 2b) from the stream (3). The byproduct-free stream then
flows directly into the respective metallic copper sorbent bed
tSa. 5b) wherein the dissolved elemental sulphur i3 removed to
produce a desulphurised product stream ~63. The metallic copper
sorbent is usually formed in situ by reduction of a precursor
comprising a reducible copper compound. Reduction of the
precursor may be accomplished by passing a hydrogen stream ~8)
which has been diluted with an inert stream ~7) and heated in a
heat exchanger ~9) to a suitable reduction temperature through the
bed of precursor and thereafter venting the effluent gas stream
(10).
3 In some cases it may be desirable to provide for
regeneration of the metallic copper sorbent. This may be achieved
by re-reducing the spent metallic copper sorbent with a stream of
hydrogen: typically the reduction may be effected using a hydrogen
stream at 200-300C. The copper sulphide formed by reaction of
the copper with tbe elemental sulphur is converted back to copper
.,..... . -
:. , ~ .. .. :
- . .. :, . ::
~ . : :, : . : ~ :
, . . . : : ,: ~ :
.:
5 CPW 36~90
~7~
metal with the concurren~ formation of hydrogen sulphide. The
hydrogen sulphide may be scrubbed from the hydrogen stream and
then fed to a sulphur recovery plant, eg a Claus plant.
The invention is further illustrated by the following
examples.
Exam~le 1
In this example two absorbent beds were employed in
series. The first bed was 300 g of granules of an absorbent
comprising a high surface area zinc oxide and a cement binder,
while the second bed was produced by reducing in situ 300g of
granules formed from mixture of high surface area copper and zinc
oxides and a cement binder. The mix~ure contained 55~ by weight
of copper oxide. The reduction was effected by means of a stream
of hydrogen st a temperature of 180C. It is estimated, from
measurements perfonmed on another sample of the mixture, that
after reduction, the copper surface area of the second bed was
20 m2.g-1
A liquid gasoline stream containing about 400-500 ppm by
weight of total sulphur of which about 20 ppm by velght was
elemental sulphur, was passed through st 20C at an average rate
of approximately 500 ml/h through the series of beds and the
elemental sulphur content of the effluent was monitored at
intervals. The experiment was terminated when the elemental
sulphur content of the effluent reached 5 ppm by weight which
ocurred after 87 days. The cumulative flow and effluent elemental
sulphur content at various times were BS set out in Table 1.
The spent copper/zinc oxide bed was then analysed and
found to have a sulphur content of about 7.2~ by weight. On
examination by XRD it was found that cupric æulphide was present
in the bed, but there was no cuprous sulphide, copper sulphate, or
zinc sulphide. The absence of zinc sulphide in the spent
copper/zinc oxide bsd indicates that re~ctive sulphur compounds, ~i
such as hydrogen sulphide, present in the gasoline were absorbed
by the first zinc oxide bed. The presence of cupric sulphide1
rather than cuprous sulphide, in the spent copper/zinc oxide bed
, . . ~ , . . . . .
., - .
.
6 CP~ 36490
2~7~55
indicates that the copper could be regenerated by raduction with
hydrogen since cupric sulphide is easier to reduce to metallic
copper than cuprous sulphide.
Table l
__________________________________ _________________________
I TLme (days) I Cumulative flow (1) 1 effluent sulphur (ppm) '
I__ ______ ___ _ ,
I 1 1 10.5 1 0
I11 ' 116.7 ' 0.2
121 122~3.2 1 0.2
'31 ~ 341.3 ' 0.7
1 41 , 486.0 ' 1.0
51 1 628.4 1 1.3
,61 1 765.4 1 2.5
173 1 905.0 1 1.6
179 1 974.0 I nm
5 ,81 I nm 1 2.~ 1
187 I nm 1 5.0
____________________________________________________________
nm ~ not measured
Example 2
One concern was that the metallic copper sorbent would
react with, or decompose, methyl cyclopentadiene manganese
tricarbonyl (MMT) which is often employed as an octane number
improver in gasoline. In order to examine this, a series of three
catalyst beds, viz a sample of the zinc oxide absorbent granules
of the first bed of Example 1, a sample of the spent, ie
sulphided, absorbent granules of the second bed of ~xflmple 1, and
a sample of the fresh copper oxidelzinc oxide absorbent granules
of the second bed of Example 1, were charged to a glass reactor.
The air in the reactor was displaced by nitrogen and the apparatus
wrapped in aluminium foil to shield it from light (which effects
decomposition o~ MMT). Hydrogen at 180C was then passed through
the series of beds to effect reduction of the copper compounds to
metallic copper. The reactor was then filled wi~h a mlxture of
. . .:
7 CPW 36490 2 o 7 ~
xylene (702 by weight) and heptane (30% by weight). A solution
containing 540 ppm by weight of MMT dissolved in the aforesaid
xylene/heptane mixture was then passed through the series of beds
at atmospher~c pressure and at 22-25C for 48 hours at a liquid
hourly space velocity of 2 h-l. Analysis of the solution before
and after passage through the beds f&iled to reveal any change in
the manganese content. The reactor was then flushed with fresh
solvent, purged with nitrogen. The absorbents were then dried at
110C and analysed for the presence of manganese. No manganese
was detected in the zine oxide sample while the reduced sulphided
copper containing adsorbents contained about 300-S00 ppm by weight
of manganese. Since manganese is a possible contaminant of the
cement employed as the granule binder, it is likely that this
found manganese resulted from that contamination in view of the
lack of detected change in the manganese content of the solution
during passage through the beds. If all the ~MT had been
decomposed and absorbed by the absorbents, the average manganese
content of the absorbent beds after the experiment would have been
about 3Z by weight.
~ .