Note: Descriptions are shown in the official language in which they were submitted.
2~7602~
ASSAY DEVICE AND METHOD OF D~TECTING CHITIN
Field of the Invention
Thi~ invention relates to the field of assays
to detect target substances, and particularly to assays-
to determine the presence of chitin and thereby determine
the presence of organisms which contain chitin such as
fungi, yeast, and insects in a sample.
Background
Infections caused by fungi and yeast affect
animals including humans and plants worldwide. There
exist a large number of such organisms which can
contaminate water and food supplies and cause infections
in various body tissues and fluids.
Conventional methods to detect the presence of
contaminating fungi, yeast and insects reguire obtaining
samples from an animal or plant suspected of containing
these organisms and culturing the samples to grow the
desired organisms present in the sample in sufficient
numbers to readily detect their presence visually.Typically, culturing the organisms requires specialized
media and lengthy culture times of up to several weeks.
Other methods involve the use of a hot basic
solution such as a 20% potassium hydroxide to clear
smears of specimens on a solid substrate. The cleared
specimen is then stained, for example using India ink,
then examined by microscope to detect the presence of
fungal structures remaining after this treatment.
2~76~2~
--2--
Fungi, yeasts and insects contain certain
substances, including proteins, that may be specific for
a particular species. Other substances are more widely
distri~uted. For example, chitin (N-acetylglucosamine
oligomer) is a polysaccharide component of cell walls
found in most fungi, yeasts and insects and is reactive
with reagents such as lectins (Galun et al., Arch.
Microbiol., 108(1):9-16 (197~)).
Assays for the presence of chitin-containing
organisms such as fungi are known but have been limited
to the detection of chitin using chemical analyses
including colorimetric determination (Sharma et al.,
Trans. Br. Mycol. Soc., 69(3):479-83 (1977)) and the use
of nitrous acid-3-methyl-2-benzothiazolinone hydrazone
hydrochloride-ferric chloride and light microscopy
[Kaminsky; et al., Can. ~. Bot., 60(12):2575-80 (19~2)).
Immunological assays to detect various microorganis~s
including fungi using antibodies are also known,
including those employing monoclonal antibodies reactive
with antigens associated with particular organisms.
(Goldstein, European Patent ~pplication EP 176,355
(1986)). Such assays are based on species-specific
proteins. In addition, to detect the organism the assays
typically require that the specific protein be isolated
or exposed for reaction with the antibody in the assay.
Labeled chitinase has been reported to be
useful for visualization of chiti~ with electron and
fluorescence microscopy. Chamberland et al.,
Histochemical Journal, 17:313_321 (1985), reported the
use of a chitinase-gold complex to localize chitin
ultrastructure in tomato root cells infected by Fusarium
sp. to study host-pathogen relationships. Molano et al.,
J. Cell Biol., 85:199-212 (1980), reported the use of
chitinase-gold complex to study the distribution of
2~7~
chitin in Saccharomyces cerevisiae. Both techniques
require the use of an electron microscope for
visualization. Molano et al. also reported the use of
fluorescein isothiocyanate (FITC)-chitinase to visualize
chitin with fluorescence microscopy.
Hadwiger et al., Plant Physiol., 67:170-175
(1981), reported the use of anti-chitosan and anti-fungal
cell wall antisera to study the localization of fungal
components in pea-Fusarium interaction. The antisera
were conjugated with FITC. Therefore, to detect chitin
in the tissue, the samples had to be examined with a W
microscope. Embedding and immunochemical staining of
sections were also prepared for transmission electron
microscopy. Procedures such as described by Chamberland
et al., Molano et al., and Hadwiger et al. necessitate
microscopy to visualize the chitin in the sample. These
procedures usually were accompanied by elaborate sample
preparation methods.
U.S. application serial no. 426,538, filed
20 October 24, 1989 (incorporated herein by reference),
disclosed a method of detection of fungi, yeasts and
insects without requiring in vivo or in vitro culturing
techniques or complex staining reagents, which method
relied on lectin or chitin antibodies to target the
chitin. The present invention offers substantial
improvements over this method.
Summary of the Invention
The basic inventive concept of the assay
methods and means for carrying out those methods is based
on the recognition that certain organisms including
fungi, yeasts and insects are rich in chitin.
Accordingly, the assay of the invention is for the direct
detection of chitin and by implication deducing the
2 ~ P~
--4-
presence of the chitin-containing organisms. An
essPntial feature of the present invention is that it
involves the use of enzymes which specifically bind to
chitin. Such enzymes are referred to as chitin-~inding
biological reagents and chitin-binding or chitin-specific
enzymes and include chitinase and lysozyme. Such chitin-
binding enzymes are directly or indirectly attached to a
label before or after they bind to chitin. Thereby the
assays of the invention can be used to selectively attach
detectable labels to chitin present within a sample.
The assay of the invention makes it possible to
detect chitin by the use of enzymes which specifically
bind chitin. Although the invention can be described in
terms of several different specific em~odiments, such
specific embodiments can be generally classified under
two subgeneric embodiments. Nore specifically, in
accordance with one subgeneric embodiment, an enzyme
which specifically binds chitin, such as chitinase, is
bound to the surface of a support such as the bottom of a
microtiter plate well, the surface of a glass slide or
the surface of a dipstick. Samples to be tested are then
brought in contact with the enzyme, bound to the surface
and various procedures are carried out in order to
determine and detect the presence of any chitin binding
to the enzyme on the surfaces. In accordance with a
second subgeneric embodiment, a fluid sample or a
fluidized sample is filtered through a membrane in order
to capture the solid components of the sample on the
surface of the membrane. Accordingly, any chitin-
containing organisms, chitin, or fragments thereof withinthe sample become bound to the membrane surface. The
bound sample is then contacted with an enzyme capable of
selectively binding to any chitin in the sample, for
~xample chitinase or lysozyme. The chitin-binding enzyme
- ~7~f~2
--5---
may be directly or indirectly labeled to permit detec-
tion. The presence of the chitin-containing organisms
such as fungi is determinPd by visually detecting the
label with the naked eye.
A primary object of the present invention is to
provide a rapid detection assay which uses a chitin-
binding enzyme for determining the presence of'chitin-
containing organisms without the need for in vitro or in
vivo culture techniques or time-consuming staining
procedures.
A feature of the present invention is that the
assay detects the cellular chitin component of all
organisms containing chitin such as fungi, yeasts and
insects, which chitin component is generally absent in
bacteria and mammalian and plant tissue, reducing
possible false positive readings.
An advantage of the present invention is that
it can ~e used to detect chitin and deduce the presence
of chitin-containing organisms in samples of all types of
biological (mammalian and plant) fluids and tissues as
well as food and water without the need for any culturing
techniques or staining procedures. The method is not
limited to particular species of fungi, yeasts or insects
since all such organisms contain chitin.
2S Another advantage of the present invention is
that it provides an efficient, economical, clinical
laboratory assay technique for the rapid diagnosis of
fungal infections in patients.
Another feature of the present invention is
that it pxovides an assay procedure which can be used by
agricultural and food laboratories to evaluate field
samples for the presence of fungi, insects, and related
pathogens and contaminants.
Another object of the invention is to provide a
portable (preferably disposa~le) diagnostic kit which can
be used to detect the presence of chitin-containing
organisms in an efficient ancl economic manner.
Another feature of the present invention is
that the fungal contaminants do not have to be directly
cultured nor do they require the use of classical
chemical staining procedures for ~he localization of the
contaminants.
Another feature of the present invention is
that neither lectins nor an~i-chitin antibodies are used
to detect chitin which is a desirable feature in that
lectins will often bind nonspecifically to nonfungal
carbohydrates other than chitin, whereas anti-chitin
antibodies are undesirable in that they are, at best,
difficult to generate due to chitin's poor
immunogenicity.
An advantage of the present invention is that
the detection means do not require the U52 of either
electron or fluorescent microscopy to effect detection.
These and other objects, advantages and
features of the present invention will become apparent to
those persons skilled in the art upon reading the details
of the structure and usage as more fully set forth below,
references being made to the accompanying figures forming
a part hereof.
Brief Description of the Drawinqs
The present invention will be described in
connection with the accompanying drawings in which: `
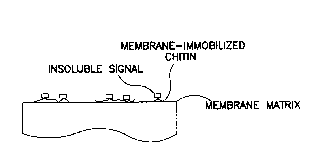
Figure 1 is a schematic view of an embodiment
of the invention wherein the chitin is bound on a
permeable memkrane matrix;
2~)76~3~ ~
Figure 2 is a schematic view wherein the
chitin-containing sample is p:resent in a coated
microtiter well;
Figure 3 is a schematic representation of the
components of an assay scheme which includes a membrane
support, chitinase, rabbit anti-chitinase antibody, and
alkaline phosphatase conjugated to goat anti-rabbit IgG
antibody, which enzyme can generate a colored product
from a chromogenic solution;
Figure 4 is a schematic representation of the
components of another assay embodiment of the invention
wherein the porous me~brane captures the chitin, a
chitin-binding enzyme attaches to the bound chitin,
thereafter a conjugate comprised of a signal generating
enzyme bound to an antibody reactive with the chitin-
binding reagent binds to the chitin-binding reagent and a
chromogenic solution is added, which solution contains
the substrate for the signal generating enzyme, which
substrate releases a color when contacted with the
enzyme;
Figure 5 is a schematic representation of the
components of yet another embodiment of an assay of the
invention which includes a support having bound thereto
chitin having bound thereto avidin-conjugated chitin-
binding biological reagent having bound theretobiotinylated enzyme and a chromogenic solution;
Figure 6 is a schematic view of the components
of yet another embodiment of the assay of the invention
wherein a solid support has thereon chitin which has
bound thereto an enzyme-con~ugated chitin-binding
biological reagent wherein the enzyme of the conjugate
will react with its substrate in a chromogenic solution
to form a colored product; and
2 ~ 7 ~
--8~-
Figure 7 is a schematic view of the components
of yet another embodiment of 1:he assay of the invention
which includes a chitin-binding biological reagent which
is chitinase coated on a soli~ support, chitin bound to
the coated cupport, chitin-binding biological reagent
bound to the chitin, rabbit anti-chitin-binding reagent
antibody bound thereto, an enzyme conjugated to goat
anti-rabbit IgG antibody and a chromogenic solution.
Detailed Description of the Preferred Embodiments
Before the present method of detecting chitin-
containing organisms such as fungi, yeasts and insects
and assay devices and kits for carrying out such are
described, it is to be understood that this invention is
not limited to ~he particular methods, assay devices or
kits described as such may, of course, vary. It is also
to be understood that the terminology used herein is for
the purpose of describing particular embodiments only,
and is not intended to be limiting since the scope of the
present invention will be limited only by the appended
- claims.
It must be noted that as used in this
sp~cification and the appended claims, the singular forms
"a," "an" and "the~ include plural referents unless the
context clearly dictates otherwise. Thus, for example,
reference to "an enzyme" includes mixtures of enæymes,
reference to "a sample for testing" includes reference to
mixtures of such samples and reference to "the method"
includes one or more of the methods described and so
forth.
The method of the present invention allows the
rapid detection of the presence of a variety of chitin-
containing organisms including fungi, yeast and insects,
without requiring costly and time-consuming culturing or
2 ~
staining of the organisms. The method provides an assay
for detecting chitin using chitin-specific enzyme
reagents and is not limited to any particular strain or
species of organism. In addition, the method does not
require that specific substances such as proteins or
car~ohydrates be isolated from a sample suspected of
containing organisms, prior to detection in the assay.
The substance that is detected in the method of
this invention is chitin, an N-acetyl-glucosamine
oli~omer present in the cell walls of nearly all fungi
and yeasts as well as the exoskeleton of many insects and
other arthropods.
To carry out the invention, a sample suspected
of containing target organisms is obtained. ~he sample
may include a fluid and non-fluid component and may
originate from humans, animals, plants or food items.
Typical samples include biological fluids such as urine,
spinal fluid, whole blood or serum. O~her samples
include biological specimens such as skin scrapings,
sputum, tissue homogenates, wound exudates, or hair. In
addition, samples may consist of materials from plants
including tissue, scrapings, fluids, exudates and
homogenates, or may be water or food suspected of
contamination.
Depending upon the type of sample obtained,
different embodiments of the invention are more readily
applicable for assaying for the presence of chitin in the
sample. Although the invention includes a wide variety
of different possible embodiments and different chemical
and biological reagents which can be used in connection
with each of these embodiments, the invention is
generaliy encompassed by two subgeneric embodiments. In
accordance with the first subgeneric embodiment, the
- sample includes fluid and nonfluid components or is
~7~02~
--10--
fluidized so as to contain both comp~nents and is
thereafter filtered through a porous membrane in order to
capture the nonfluid-containing components on the surface
of the membrane. The presence of chitin in these
nonfluid-containing components is then assayed by the use
of an enzyme which specifically binds to chitin. This
subgeneric embodiment is generally shown within Figure 1
and specific embodiments thereof are schematically
represented within Figures 3-6.
The second subgeneric embodiment of the
invention involves coating the surface of a material such
as a microtiter well with an enzyme which specifically
binds to chitin. Once the enzyme such as chitinase has
been bound to the surface, the sample to be assayed is
brought into contact with the bound chitinase for a
sufficient period of time to allow binding to occur
between any chitin in the sample and the chitinase bound
to the surface. This subgeneric embodiment of the
invention is shown within Figure 2. A specific
embodiment schematically demonstrating how this
subgeneric embodiment can be carried out is shown within
Figure 7. However, it should be noted that by various
manipulations which will be apparent to those skilled in
the art, the subgeneric embodiment shown within Figure 2
can also be carried out using the specific embodiments
shown within Figures 3-6, i.e., it need not be carried
out only using the specific embodiment of Figure 7.
When the chitin-specific enzyme is to be
adhered to a substrate surface, it may be adhered to the
surface of a glass slide, microtiter well, dipstick or
other suitable material. When the sample is to be
filtered through a membrane, any suitahle membrane filter
can be used, such as those composed of various synthetic
or natural polymeric materials.
2 ~
The two subgeneric embodiments of the invention
are shown in figures 1 and 2. In figure 1, the solid
components of a sample comprised of liquid and solid
components have been attached to a membrane matrix.
Various specific embodiments are shown in figures 3-6.
The surface of the matrix is preferably designed such
that it will hold the solid components and particularly
the chitin within the sample securely in place on the
surface of the matrix. It is often desirable to include
a blocking agent (described further below~ to protect
active binding sites that have not been occupied by
chitin. After the sample has been secured ko the matrix,
different procedures are carried out whereby a signal
(preferably a visually-observable color signal) is
generated and the presence or absence of chitin is
determined and thereby the presence or absence of chitin
containing microorganisms are determined.
The sites on the surface of the membrane which
do not have chitin bound thereto are preferably blocked,
for example by incubation of the fixed sample on the
membrane with an irrelevant protein solution such as
bovine serum albumin (BSA), casein or egg albumin.
Blocking reduces any nonspecific interactions (i.e.,
electrostatic interactions) of the binding sites which
may interfere with the assay to detect chitin.
In accordance with the embodiment shown in
figure 2, the surface of the well is coated with a
chitin-specific enzyme. A blocking agent is then applied
to occupy all remaining binding sites of the well. A
sample which may contain chitin is placed in the trea~ed
well surface. ~ specific embodiment is shown in figure
7. Ch.itinase is added and allowed to incubate with the
sample for a time period in the range of about 3 minutes
to about 1 hour to allow the chitinase to bind to any
~7~2~.
-12-
chitin present within the sample. The wells are then
washed with a ~uffer to remove any chitinase ~hich is not
bound to chitin bound to chitinase on the surface of the
microliter well. After the washing, any immobilized
chitin on the well plate would be detected by adding a
reagent which causes a color reaction after contacting a
label connected directly or indirectly to the chitinase.
This methodology can be used in connection with both
insoluble and soluble signal detection strategies. That
is, if an insoluble signal reagent is used, the signal
will appear coated on the bottom and sides of the well.
If a soluble signal reagent is used, the supernatant can
be read visually with the naked eye in situ or by highly
relia~le and commonly used automatic plate readers, or
drawn off for processing in a separate detector.
The sample can also be manually smeared onto a
glass slide with a cotton swab, scalpel blade, or other
similar device, allowed to dry completely and then fixed
with chemicals. Heat may also be used for fixation, for
example, the solid phase may be placed over a boiling
(100C) water bath until the sample adheres onto the
solid phase.
If the sample has no fluid component, it may be
manually placed on the solid phase and adhered using
~5 chemical fixatives, or it may be suspended in a fluid and
applied to a solid phase as described above.
The assay methodology of the present invention
is based on the use of the chitin-specific enæyme which
is directly or indirectly attached to a label. Accor-
dingly, the assay of the invention can be used to detectchitin-containing organisms present in situ on a surface
without having to remove or destroy the surface on which
the organisms might be present. For example, if one SU5-
pects a plant to have a fungal infection on its surface,
a biological reagent of the present invention containing
a chitin-spe ific enzyme can be sprayed onto the surface
of the plant. Thereafter, washing should be carried out
in oxder to wash away any unbound chitin-specific enzyme.
After washing, various procedures such as those shown
within the specific embodiments of Figures 3-7 can be
carried out in order to detect any chitin-specific enzyme
bound to the chitin which might be pxesent on the plant.
Such a method might be carried out by using chitinase
bound to another enzyme which generates a color when
placed in contact with a chromogenic solution. Such a
two-enzyme conjugate could b~ contacted with the plant
surface, followed by washing, ~ollowed by the application
of a chromogenic solution. If the surface changes color,
such would indicate the presence of a chitin-containing
organism on the plant surface.
The assay of the invention relies upon binding
of a chitin-binding enzyme reagent specifically binding
to chitin and the visualization of this binding by a
labeling system. The binding reagent is preferably
chitinase.
Polyclonal antibodies reactive with chitinase
may be obtained by recovery of serum-containing
antibodies following immunization of a mammalian hsst
using chitinase as the immunogen. Procedures for
producing polyclonal antibodies are well known and will
not be repeated here. The serum-containing polyclonal
antibodies are used for binding to chitinase which is
bound to chitin in an assay described below to detect
chitin-containing organisms.
Monoclonal antibodies reactive with chitinase
may also be used in an assay to bind to chitinase~ These
monoclonal antibodies may be derived using known tech-
niques following the procedures of Kohler and Milstein,
2 ~ 7 6 ~ 2 1
-14-
Nature, 256:~95 (1975), incorporated herein by reference,
to disclose methods of obtaining monoclonal antibodies.
In this procedure, hybridomas are prepared by fusing
antibody-producing cells (typically spleen cells of mice
previously immunized with an antigen) to cells from an
immortal cell line such as myeloma cells, using somatic
cell hybridization.
Although it has been found that chitin is
substantially nonimmunogenic and that, therefore, it is
difficult to generate chitin antibodies, it has been
found that anti-chitinase antibodies can be generated and
that such anti-chitinase antibodies are useful in
accordance with different embodiments of the present
invention. Polyclonal and/or monoclonal antibodies which
are anti-chitinase antibodies are useful in carrying out
differar.t specific em~odiments of the invention. We have
generated pol~clonal anti-chitinase antibodies in
rabbits, which antibodies can be used in the detection of
chitinase. Accordingly, if chitin is bound to a support
surface and chitinase is added and then attaches to the
chitin, these anti-chitinase antibodies will bind to the
chitinase. Such anti-chitinase antibodies are generally
labeled, such as with an enzyme, which enzyme generates a
color when brought into contact with a chromogenic
solution.
Polyclonal anti-chitinase antibodies were
generated using two New Zealand White rabbits. The
rabbits were immunized with 1 mg of chitinase from
Streptomyces griseus (obtainable from the Sigma catalog
C1525) present in Ribi adjuvant. The rabbits received a
second immunization three weeks later and were then
immunized twice more at two-week intervals. Serum was
collected from the rabbits and assayed for an~i-chitinase
antibodies by ELISA. The results obtained showed that
2~7~2 ~
-15-
both of the rabbits possessed high serum titers of anti-
chitinase antibodies. Resulting serum obtained from the
animals was stored at -30C and used in connection with
assays such as those described below.
Anti chitinase antibodies are believed to be
novel and therefore constitute another aspect of
applicants' invention. Further, conjugates formed
between anti-chitinase antibodies and enzymes capable of
generating a colorant upon contact with a chromogenic
solution include another aspect of applicants' invention.
It should be pointed out that the anti-chitinase anti-
bodies can be conjugated to other enzymes either directly
or indirectly. As an example of such indirect conjuga-
tion, other antibodies with respect to the anti-chitinase
antibodies can be generated, which antibodies are
connected to a label or enzyme capable of generating a
color upon contact with a chromogenic solution.
The assay to detect the presence of chitin-
containing fungi, may be a direct binding assay. In such
an assay the chitin-binding enzyme reagent, chitinase or
lysozyme, is reacted with chitin in fungi present in the
sample, by contacting the sample attached to the solid
phase with a labeled binding reagent. The label is then
visually detected to determine whether fungi are present.
The presence of fungi may be quantified, by
using predetermined amounts of the labeled assay reagent
and relating the intensity of the signal produced by the
label (which is a function of the amount of assay reagent
reacted)~ to the concentration of chitin using standard
binding curves. These curves are generated by measuring
the intensity of the signal produced using known amounts
of chitin.
The amount of chitin may also be quantified
using an indirect, competitive inhibition assay in which
2~7~?.~
-16-
a mixture of labeled assay reagent, for example
chitinase, and organism-containing solution is mixed and
then added to react with chitin attached to the solid
support. Chitin-containing organisms will compete with
S the immobilized chitin and thus reduce the degree of
signal produced by the label in a dose-related manner,
permitting a determination of the amount of chitin
present in the organisms in the sample.
Visualization of the binding of the assay
reagent to chitin present in the sample, may be
accomplished by directly labeling the chitinase directly
or indirectly with a substance capable of producing a
signal, for example, a radionuclide, enzyme or a
fluorescent agent, using known procedures. If an enzyme
label is employedl an enzyme is selected which when
reacted with its appropriate chromogenic reagent produces
a color or other visibly detectable signal. In those
instances where the enzyme substrate is to be used in
solution to contact the enzyme-labeled reagent bound to
the sample on the solid phase, a soluble enzy~e substrate
such as orthophenylenediamine (OPD) reactive with the
enzyme horseradish peroxidase (HRP) may be used.
In accordance with another method the sample or
specimen is applied manually to a solid phase such as a
glass slide to be visualized. A substrate such as
diaminobenzidine or 3-amino-9-ethyl-carbazole and the HRP
is used. The colored, insoluble reaction product from
the cleavage of the substrate by the enzyme will be
deposited at or near the location of the enzyme. Thus
when a fungal structure is coated with enzyme-labeled
chitinas2, the enzymatic reaction Sof the ~nzyme label)
will deposit the colored substrate around and on the
fungal structure and allow the fungus to be readily
detected by visual examination.
2~7~
-17-
Alternately, the chitin-binding enzyme assay
reagent may be indirectly labeled, attaching the signal-
producing label to an additional substance which binds to
the assay reagent. For example, where chitinase is used
to detect the chitin, an anti-chitinase antibody may be
conjugated with a label and bound to the chitinase for
reacting with the chitin. This assay may provide a more
sensitive assay for the detection o~ the organism because
more label can be bound per unit of assay ,eagent.
Biotin/avidin reagents may be used to
accomplish binding. In this case, biotin is covalently
bound to antibody, for example, anti-chitinase antibody.
The biotin-specific receptor protein avidin is conjugated
to a signal-generating enzyme, then reacted with biotin
to label the antibody. The labeled antibody is then used
in an assay to detect chitinase bound to chitin in the
sample being assayed.
SPECIFIC ASSAY EMBODIMENTS
The general or subgeneric embodiments of the
assay described herein are shown in Figures 1 and 2.
Although both of these subgeneric embodiments involve the
use of a chitin-binding enzyme such as chitinase, the
protocol as shown in Figure 1 involves binding ~he sample
to the substrate first whereas the protocol shown in
Figure 2 involves binding chitinase to the substratP
first. Regardless of which protocol is usPd, the chitin
within the sample is eventually attached to a label or
enzyme capable of generating a colorant thereby making it
possible to visually determine the presence or absence of
chitin within the sample. The subgeneric embodiment
sho~n within Figure 1 is essentially described in further
detail by the specific embodiments shown schematically
within Figures 3-~, described in detail below. The
2 ~
-18-
subgeneric embodiment shown within Figure 2 is further
described by reference to the specific schematic
representation shown within Figure 7. It should be noted
that although only one specific representation (Figure 7)
is given with respect to the subgeneric embodiment of
Figure 2, it is possible to rearrange the various
components as per Figures 3-6 to drive specific
embodiments of the subgeneric embodiment of Figure 2.
ASSAY OF FIGURE 3
A schematic of a particular embodiment of an
assay of the invention is shown in Figure 3. This assay
will be described in greater detail than the assays of
Figure 4-7, which will be described more briefly in that
specific information is given below with respect to
Figure 3 which can be applied to the assay shown in
Figures 4-7. In accordance with the assay of Figure 3, a
sample to be tested for the presence of chitin is placed
on the ~embrane surface such as the porous membrane
shown. The chitin in the sample is adhered to the
membrane in some manner. This can be done by using any of
the above-described methods. It is preferably done by
passing the sample through a permeable membrane which
acts as a filter and traps solids in the sample such as
the chitin.
once the sample is secured to a porous
memhrane, it is desirable to block other binding sites on
the membrane by the addition of an irrelevant protein
solution such as BSA. The use of such blocking reagents
can make any of the assay embodiments of the in~ention
more specific. Since the use of blocking proteins and
blocking reagents in connection with such assays is known
to those skilled in the art, the following description
~7~
will not repeat the step with respect to the use of
blocking reagents urther.
A chitin-binding enzyme, such as chitinase or
lysozyme, is added to the sample to be assayed under
conditions which allow the chitin-binding reagent to bind
to chitin present in the sample. The binding environment
may be controlled so that the enzymes can bind to ~he
chitin but not guickly degrade the chitin. Although some
enzymatic degradation nay occur, the system does not
depend on de~radation of the chitin to generate a signal
and detection ability is maintained even though the
chitin may be partially degraded. More specifically, the
system takes advantage of the enzyme's own ability to
specifically bind to the substrate chitin.
~ntibodies to the chitin-binding reagent (e.g.,
rabbit anti-chitinase antibody) can now be bound to the
chitin-chitinase complex in order to provide thP first
antigen-antibody reaction.
A second enzyme is conjugated to an anti-
antibodv, i.e., an antibody against the first antibody.For example, alkaline phosphatase is conjugated to goat
anti-rabbit IgG antibody. This is added to provide a
second antigen-antibody reaction. Finally, a reagent
(substrate) for the conjugated antibody is added to
generate a detection signal. Substrates for alkaline
phosphatase include bromo-chloro-indolyl phosphate and
nitro bluê tetrazolium in a buffer of aminoethyl
propanediol. However, other chromogenic signals can be
utilized Alternatively, the anti-chitinase-binding
antibody itself can be con~ugated to any label such as an
enzyme capable of generating a color in combination with
a chromogenic solution. Such enzymes include urease and
peroxidase.
2~76~2~
-2~-
ASSAY OF FIGURE 4
The schematic view of the assay shown in
Figure 4 includes a support in the form of a porous
membrane upon which the sample! which putatiYely contains
chitin is placed. The chitin-binding enzyme such as
chitinase is then added under conditions such that the
chitinase can bind to any chitin present. Ext~aneous and
unbound materials can then be washed away. A conjugate
is then added which conjugate is comprised of an enzyme
lo bound to rabbit anti-chitinase antibodies. If chitinase
is present in that it is bound to chitin, the antibodies
will bind to the chitinase. Unbound material is then
washed away. Thereafter, a chromogenic solution is added
to provide a color which can be visually detected.
ASSAY OF FIGURE 5
In Figure 5, the chitinase is bound to avidin
to form a conjugate. After this conjugate is allowed to
bind to the chitin due to the specificity of the
chitinase, a biotinylated enzyme is added which is
biotin-conjugated alkaline phosphatase. The biotinylated
enzyme will bind to the advidin and any bound material
can be detected at the addition of the chromogenic
solution.
ASSAY OF FIGURE 6
A very simple embodiment of an assay of the
invention is shown within Figure 6. In accordance with
this embodiment, the essential component is the conjugate
comprised of the chitinase bound to an enzyme such as
alkaline phosphatase. The chitinase will bind to any
chitin present on the support. After the binding,
washing takes place followed by the addition of a
2~7~
-2~.-
chromogenic solution which provides 2 color if the
alkaline phosphatase is present.
ASSAY OF FIGURE 7
The assay embodiment schematically shown within
Figure 7 is a specific embodiment of the subgeneric
method shown in figure 2. In accordance with ~his
embodiment, a chitin-binding enzyme such as chitinase is
first bound to the surface of a solid support, such as a
polystyrene support. It is not necessary to bind
chitinase to all of the available support surface.
Active sites which do not have chitinase bound thereto
can be protected by the addition of any suitable blocking
agent such as BSA. After the chitinase and blocking
agents are added, the sample which might contain chitin
is placed on the surface. If chitin is present in the
sample, it will bind to the immobilized chitinase. ~he
surface is then washed and more chitinase is added, which
will bind to any chitin attached to the surface
immobilized chitinase. After allowing the chitinase to
bind to the chitin, an anti-chitinase antibody is added
which binds to chitinase. After the antibody is allowed
to bind, washing is carried out followed by the addition
of a conjugate. This conjugate is comprised of an enzyme
such as alkaline phosphatase bound to an antibody which
binds to the chitinase antibody. For example, such an
anti-antibody might be an anti-IgG antibody such as a
goat anti-rabbit IgG antibody. After binding ~akes place
and washing has been carried out, a chromogenic solution
is added which generate a color after contacting the
enzyme such as the alkaline phosphatase.
Although Figure 7 provides the only specific
embodiment of the subgeneric embodiment of Figure 2,
other specific embodiments encompassed by the general
2~7~2~
-22-
concept of Figure 2 will be apparent to those skilled in
the art upon reading this disclosure and reviewing the
specific embodiments shown in Figures 3-6. All of the
embodiments include the use of a chitin-binding enzyme,
such as chitinase, in order to bind chitin. Different
layering techniques involving other reagents such as
antibodies and conjugates can be used in different forms
and manners in order to obtain different desired effects.
Various conjugates useful in connection with
the assays of the invention are also considered to be
aspects of the present invention. Different conjugates
can be formed using methodology known to ~hose skilled in
the art. With respect to the formation of specific
antibody to enzyme conjugates, reference is made to the
Sigma catalog relating to immunochemicals published in
1991 and to the various publications cited therein. Such
publications describe the formation of specific
conjugates such as enzyme/antibody conjugates, conjugates
formed between alkaline phosphatase and other enzymes or
antibodies, conjugates formed between peroxidase and
other enzymes or antibodies, as well as conjugates formed
between urease and other enzymes or antibodies. Specific
descriptions are given with respect to the formation of
biotin conjugates. The description contained within the
Sigma catalog, as well as the various publications cited
in that catalog, are incorporated her~in by reference.
An important aspect of applicants' invention
involves the anti-chitinase antibodies which are produced
in accordance with the methods described above. Various
conjugates formed using these anti-chitin antibodies are
also considered to be an important aspect of the present
invention. Such conjugates can include chitinaselother
enzyme antibodies which other enzymes are generally
enzymes capable of generatin~ a color when brought into
2 ~
contact with a chromogenic solution. Anti-chitinase
antibodies can also be conjugated to other antibodies
which in turn are conjugated to other enzymes which are
capable of generating a color upon con~act with a
chromogenic solution.
As will be apparent to those skilled in the art
to which the invention is addressed, the present
invention may be carried out by using techniques o~her
than those specifically discussed above without departing
lo from ~he spirit or essential characteristics of the
invention. The particular materials and processes
described above are therefore to be considerPd in all
respec~s as illustrative and not restrictive. For
example, labeling agents other than enzymes, such as
ra~ionuclides or fluorescing agents, may be used to
detect the reagent bound to chitin using procedures known
in the art. In addition, samples may be attached to a
substrate by other procedures, for example filtration of
a fluid sample and centrifugation of materials onto a
solid phase. The scope of the present invention is as
set forth in the appended claims rather than being
limited to the examples of the methods and procedures set
forth in the foregoing description.