Note: Descriptions are shown in the official language in which they were submitted.
2076240
PH/5- 1 8761/A
Novel herbicides
The present invention relates to novel herbicidally active benzothiazolone derivatives, to
processes for the preparation thereof, to compositions comprising them as active ingre-
dients and to the use thereof for controlling weeds, especially selectively in crops of useful
plants.
Herbicidally active benzothiazolone derivatives are known, for example, from US Patent
4 786 310. Novel herbicidally active benzothiazolone derivatives have now been found.
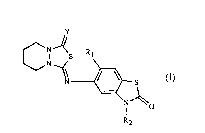
The benzothiazolone derivatives according to the invention have the formula I
~~ ~ s (I)
I ~o
R2
wherein
Y is oxygen or sulfur;
R1 is hydrogen or fluorine; and
R2 is hydrogen, C1-C6alkyl, C2-C4alkenyl, or C2-C6alkynyl; halo-substituted Cl-C6alkyl,
C2-C4aLl~enyl, or C3-C6alkynyl; C1-C4aL~coxy-C1-C4alkyl, Cl-C4aLlcoxy-Cl-C2alkoxy-
C1-C2alkyl, 1-phenylpropen-3-yl, cyano- or C3-C6cycloalkyl-substituted C1-C6alkyl;
carboxy-Cl-C4alkyl9 Cl-C6alkoxycarbonyl-CI-C4alkyl, halo-CI-C6aLlcoxycarbonyl-Cl-C4-
aLkyl, C1-C4aLkoxy-C1-C2aL~coxycarbonyl-CI-C4alkyl, Cl-C6alkoxycarbonyl-CI-C2alkoxy-
carbonyl-Cl-C4alkyl, C3-C6cycloaLkyl-Cl-C2aLkoxycarbonyl-Cl-C4alkyl, Cl-C5aLkyl-
aminocarbonyl-Cl-C4aL~cyl, di-Cl-Csalkylaminocarbonyl-CI-C4alkyl, C3-C6cycloaLkyl,
Cl-C4aLkylthio-CI-C~,aLkyl, benzyl or halo-substituted benzyl, Cl-C4alkylsulfonyl, C3-C6-
alkenyloxy-Cl-C4aLIcyl, Cl-Cgalkylcarbonyl, C1-C4alkYI-COO C
20762~0
Cl-G4alkyl-COC~Cs ~ ~C , Cl-C4alkylthiocarbonyl-Cl-C4alkyl, or
the group
Il
C1 - C4alkyl - S - I - CH - O - C - Cl - C4alkyl
o R3
R3 is hydrogen or Cl-C4aL~cyl; and
R4 is hydrogen or Cl-C4aL~yl.
The aL~cyl groups appearing in the definition of substituent R2 may be straight-chain or
branched and are, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, iso-
butyl, tert-butyl, pentyl or the isomeric pentyls, hexyl or the isomeric hexyls.
Haloalkyl is, for example, fluoromethyl, difluoromethyl, trifluoromethyl, chloromethyl,
dichloromethyl, trichloromethyl, 2,2,2-trifluoroethyl, 2-fluoroethyl, 2-chloroethyl, 2,2,2-
trichloroethyl and 7-chloroheptyl; preferably trichloromethyl, difluorochloromethyl, tri-
fluoromethyl and dichlorofluoromethyl.
Alkoxy is, for example, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, isobutoxy, sec-
butoxy and tert-butoxy; preferably methoxy and ethoxy.
In the above definitioDs, halogen is to be understood as being fluorine, chlorine, bromine
and iodine, preferably fluorine, chlorine and bromine.
Cl-C4alkylsulfonyl is, for example, methylsulfonyl, ethylsulfonyl, n-propylsulfonyl,
isopropylsulfonyl, n-butylsulfonyl, sec-butylsulfonyl, isobutylsulfonyl and tert-butyl-
sulfonyl.
The C2-C4aL~cenyl radicals may be in the Z-fo~n (cis) or in the E-form (trans) and may be
straight-chain or branched. ALIcenyl radicals having a chain length of two or three carbon
atoms are preferred. Examples of C2-C4alkenyl radicals are: vinyl, allyl, methallyl,
20762~0
1-methylvinyl and but-2-en- l-yl. Vinyl and allyl are preferred. In halo-substituted C2-C4-
alkenyl radicals the individual meanings of halogen are fluorine, chlorine, bromine and
iodine. Preferred h~logen atoms that may occur as substituents of C2-C4aL~cenyl radicals
are fluorine and chlorine. Preferred halo-substituted C2-C4alkenyl radicals are those
having a ehain length of two or three carbon atoms. Espeeially preferred C2-C4aLkenyl
radicals mono- to tri-substituted by halogen are 1-ehlorovinyl, 2-ehlorovinyl, 3-fluoroallyl
and 4,4,4-trifluorobut-2-en-1-yl. l-Chlorovinyl and 2-ehlorovinyl are very espeeially
preferred.
AL~cylthio is, for exarnple, methylthio, ethylthio, propylthio, isopropylthio, n-butylthio,
isobutylthio, see-butylthio and tert-butylthio, preferably methylthio and ethylthio.
The C2-C6aL~ynyl radicals may be straight-ehain or branched. ALIcynyl radicals having a
chain length of 2 or 3 carbon atoms are preferred. C2-C6aL~cynyl radicals are, for example,
ethynyl, propargyl, 1-propynyl, 3-butynyl, 1-methylpropargyl, 3-pentynyl and 3-hexynyl,
with ethynyl and propargyl being especially preferred.
The C3-C6eyeloalkyl groups appearing in the definition of substituent R2 may be substi-
tuted or unsubstituted and inelude, for example, eyelopropyl, 2-fluoroeyelopropyl, 2,4-
difluoroeyelopropyl, 2-ehloroeyelopropyl, 2,3-dichloroeyclopropyl, 2-methylcyclopropyl,
2-methylthioeyelopropyl, 2,3-dimethyleyelopropyl, 2-methoxyeyelopropyl, eyclobutyl,
cyclopent~yl, 3-methyleyclopentyl, 3,4-dimethoxycyclopentyl, cyelohexyl, 3-fluoroeyclo-
hexyl, 4-methyleyelohexyl and 4-methylthioeyclohexyl.
Of the compounds of formula I preference is given to those wherein Rl is fluorine, with Y
preferably being oxygen.
Special preference is given to those compounds of formula I wherein R2 is hydrogen,
Cl-C4aLIcyl, C2-C4aLIcenyl, or C2-C4alkynyl; halo-substituted Cl-C4alkyl, C2-C4alkenyl, or
C3-C4alkynyl; C1-C4aLIcoxy-Cl-C4alkyl, Cl-C4alkoxy-C1-C2alkoxy-C1-C2aL~cyl, 1-phenyl-
propen-3-yl, cyano- or C3-C6cyeloaL~cyl-substituted Cl-C4alkyl, benzyl or halo-substituted
benzyl, C3-C6alkenyloxy-Cl-C4aL~cyl, Cl-C8aL~ylcarbonyl, C -C4alkyl-COC~O
20762~0
C1-C4alkyl-CO~C~ ~ Cl-C4aLIcylthiocarbonyl-Cl-C4aL~cyl or
the group
Il
Cl - C4alkyl - S - I - CH - O - C- Cl - C4alkyl
o R3
R3 is hydrogen or Cl-C4alkyl; and
R4 is hydrogen or Cl-C4alkyl.
Special interest is accorded also to compounds of formula I wherein R2 is hydrogen,
Cl-C4alkyl, C2-C4alkenyl, or C2-C4alkynyl; halo-substituted Cl-C4alkyl, C2-C4alkenyl, or
C3-C4alkynyl; or Cl-C2alkXY-C,-C2alkYI
Very special preference is given to those compounds of forrnula I wherein R2 is hydrogen,
Cl-C4alkyl, C2-C4alkenyl, fluoro-substituted Cl-C4alkyl or C2-C4alkenyl.
In a further prominent group of compounds of formula I, R2 is C3-C6cycloalkyl, C3-C6-
cycloaLI~yl-substituted Cl-C6alkyl; Cl-C4alkylthio-Cl-C4aLkyl, benzyl, or halo-substituted
benzyl, Cl-C4alkylsulfonyl, C3-C6alkenyloxy-Cl-C4alkyl, Cl-C8alkylcarbonyl,
C1-C alkyl-COC~O C1-C4alkyl-CO~ RJ
alkylthiocarbonyl-Cl-C4alkyl, or the group
Il
Cl - C4alkyl - S - C - CH - O - C - Cl - C4alkyl
o R3
R3 is hydrogen or Cl-C4aL~cyl; and
20762~0
R4 is hydrogen or Cl-C4allcyl;
special prominence being given to those compounds wherein Y is oxygen, Rl is fluorine
and R3 is hydrogen or methyl.
In a very especially preferred group of compounds of formula I, Y is oxygen, R1 is
~luorine and R2 is Cl-C3alkyl, CH2F, CH2CH2F, CH2CH2CH2F, CHF2, allyl, propargyl,
1-methyl-3-propynyl, methoxycarbonylmethyl, ethoxycarbonylmethyl, isopropoxy-
carbonylmethyl, 1-(methoxycarbonyl)-ethyl, 1-(ethoxycarbonyl)-ethyl or 1-(isopropoxy-
carbonyl)-ethyl.
A preferred individual compound within the scope of formula I is:
~-[3-(1 -methylethyl)-6-fluoro-2(3H)-benzothiazolon-5-yl]-8-thia-1,6-diazabicyclo[4.3.0]-
nonan-7-one (Compound No. 1.001).
In a further, especially preferred sub-group of compounds of formula I,R2 is halo-substituted Cl-C6alkyl, C2-C4alkenyl, or C3-C6alkynyl; Cl-C4alkoxy-CI-C4-
alkyl, Cl-C4alkoxy-CI-C2alkoxy-C~-C2alkyl, cyano- or C3-C6cycloalkyl-substitutedCl-C6alkyl; carboxy-CI-C4alkyl, C~-C6alkoxycarbonyl-C~-C4alkyl, Cl-C4alkoxy-Cl-C2-
alkoxycarbonyl-Cl-C4alkyl, C~-C6alkoxycarbonyl-C~-C2alkoxycarbonyl-C~-C4alkyl,
C3-C6cycloalkyl-CI-C2alkoxycarbonyl-Cl-C4alkyl, Cl-Csalkylaminocarbonyl-CI-C4alkyl,
di-Cl-Csalkylarninocarbonyl-Cl-C4alkyl, C3-C6cycloalkyl, Cl-C4aLkylthio-CI-C4alkyl,
C3-c6alkenyloxy-cl-c4aL~cyls C -C alkyl-COc~o
C1-C4alkyl-CO~S , ~ , C1-C4alkylthiocarbonyl-C1-C4alkyl, or
the group
Il
Cl - C4alkyl - S - I - I ~ - O - C - Cl - C4alkyl
o R3
R1 preferably being fluorine and Y being oxygen.
2076240
- 6 -
Of that group special preference is given to those compounds wherein
R~ is Cl-C4alkoxy-CI-C4aLlcyl, Cl-C4alkoxy-CI-C2alkoxy-Cl-C2alkyl, cyano-substituted
Cl-C6alkyl; carboxy-CI-C4alkyl, Cl-C6alkoxycarbonyl-Cl-C4alkyl, Cl-C4alkoxy-Cl-C2-
alkoxycarbonyl-CI-C4alkyl, Cl-C6alkoxycarbonyl-CI-C2alkoxycarbonyl-CI-C4alkyl,
C3-C6cycloalkyl-CI-C2alkoxycarbonyl-CI-C4alkyl, Cl-Csalkylaminocarbonyl-Cl-C4alkyl,
di-Cl-C5alkylarninocarbonyl-CI-C4alkyl or C3-C6cycloaIkyl, but more especially Cl-C6-
alkoxycarbonyl-CI-C4alkyl or Cl-C4alkoxy-CI-C2alkoxycarbonyl-CI-C4alkyl.
The compounds of formula I are prepared by converting an isothiocyanate of formula II
Rl
S=C--N ~ S
\=< ,~ (II),
NI O
R2
wherein the substituents Rl and R2 are as defined for formula I, with a hexahydro-
pyridazine of formula III
,~ NH
~NH (III)
into a compound of forrnula IV
Rl
N C NH~ S
CNH ~ (IV)
R2
which is then reacted with a compound of formula V
CYCl2 (V),
20762~0
wherein Y is oxygen or sulfur, in the presence of a base.
The reaction of the isothiocyanate of formula II with the hexahydropyridazine offormula m is advantageously carried out in an inert solvent at temperatures of from -5C
up to the boiling temperature of the solvent, especially from 0 to +50C, preferably at
room temperature. Suitable solvents for this reaction are, for example, toluene, xylene,
ethyl acetate and acetonitrile.
The reaction of the compound of formula IV with the compound of formula V is advanta-
geously carried out in an inert solvent at low temperatures, preferably at from 0 to +50C,
especially at from 0 to +15C. Suitable bases for this reaction are, for example, pyridine,
triethyla nine and N,N-dimethylaniline. Suitable solvents are, for example, 1,2-dichloro-
ethane, dichloromethane and toluene.
The hexahydropyridazine of formula III used as starting material for the compounds of
formula I according to the invention is known from EP-A-0 304 920. The isothiocyanates
of formula II and the preparation thereof are described in US Patent 4 828 605.
The processes given above can be carried out analogously to processes known in the
literature. The reaction conditions preferred in those processes, such as temperature,
molar ratios of the starting materials, reaction procedure, solvent, any reagents which may
be necessary, such as acids, basçs, water-binding agents etc., are familiar to the person
skilled in the art~
The compounds of formula I are used in unmodified form, as obtainable from the
synthesis, or, preferably, together with the adjuvants customarily employed in formulation
technology and are therefore formulated in known manner e.g. into emulsifiable concen-
trates, directly sprayable or dilutable solutions, dilute emulsions, wettable powders,
sohlble powders, dusts, granules, and also encapsulations in e.g. polymer substances. As
with the nature of the compositions, the methods of application, such as spraying,
atomising, dusting, wetting, scattering or pouring, are chosen in accordance with the
intended obiectives and the prevailing circumstances.
The formulations, i.e. the compositions, preparations or mixtures comprising thecompound (active ingredient) of formula I and, where appropriate, one or more solid or
liquid adjuvants, are p;epared in known manner, e.g. by homogeneously mixing andlor
2076240
grinding the active ingredients with extenders, e.g. solvents, solid carriers and, where
appropriate, surface-active compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the fractions containing 8 to 12
carbon atoms, such as mixtures of alkylbenzenes, e.g. xylene mixtures or alkylated
naphthalenes; aliphatic and cycloaliphatic hydrocarbons, such as paraffins, cyclohexane or
tetrahydronaphthalene; alcohols, such as ethanol, propanol or butanol; glycols and their
ethers and esters, such as propylene glycol or dipropylene glycol ether; ketones, such as
cyclohexanone, isophorone or diacetone alcohol; strongly polar solvents, such as N-
methyl-2-pyrrolidone, dimethyl sulfoxide or water; vegetable oils and esters thereof, such
as rape oil, castor oil or soybean oil; and, where appropriate, also silicone oils.
The solid carriers used, e.g. for dusts and dispersible powders, are normally natural
mineral fillers, such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed silicic acid or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous
types, for example pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent
carriers are, for example, calcite or sand. In addition, a great number of pregranulated
materials of inorganic or organic nature can be used, e.g. especially dolomite or pulverised
plant residues.
Depending upon the nature of the compound of formula I to be forrnulated, suitable
surface-active compounds are non-ionic, cationic andlor anionic surfactants having good
emulsifying, dispersing and wetting properties. The term "surfactants" will also be under-
stood as comprising mixtures of surfactants.
Both s~called water-soluble soaps and water-soluble synthetic surface-active compounds
are suitable anionic surfactants.
Suitable soaps are the al~ali metal salts, alkaline earth metal salts or unsubstituted or
substituted ammonium salts of higher fatty acids (C10-C22), e.g. the sodium or potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be obtained e.g.
from coconut oil or tallow oil. Mention may also be made of fatty acid methyltaurin salts.
More frequently, however, so called synthetic surfactants are used, especially fatty alcohol
sulfonates, fatty alcohol sulfates, sulfonated benzimidazole derivatives or alkylaryl
207~240
sulfonates.
The fatty alcohol sulfonates or sulfates are usually in the form of alkali metal salts,
aikaline earth metal salts or unsubstituted or substituted ammonium salts and contain a
C8-C22alkyl radical, which also includes the alkyl moiety of acyl radicals, e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecyl sulfate or of a mixture of fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the salts of
sulfated and sulfonated fatty alcohoUethylene oxide adducts. The sulfonated benz-
imidazole derivatives preferably contain two sulfonic acid groups and one fatty acid
radical containing 8 to 22 carbon atoms. Examples of alkylarylsulfonates are the sodium,
calcium or triethanolamine salts of dodecylbenzenesulfonic acid, dibutylnaphthalene-
sulfonic acid, or of a condensate of naphthalenesulfonic acid and formaldehyde.
Also suitable are corresponding phosphates, e.g. salts of the phosphoric acid ester of an
adduct of p-nonylphenol with 4 to 14 mol of ethylene oxide, or phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic or cyclo-
aliphatic alcohols, saturated or unsaturatcd fatty acids and alkylphenols, said derivatives
containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic) hydro-
carbon moiety and 6 to 18 carbon atoms in the aL~yl moiety of the alkylphenols.
Further suitable non-ionic surfactants are the water-soluble adducts of polyethylene oxide
with polypropylene glycol, ethylenediarninopolypropylene glycol and aLIcylpolypropylene
glycol containing 1 to 10 carbon atoms in the alkyl chain, which adducts contain 20 to 250
ethylene glycol ether groups and 10 to 100 propylene glycol ether groups. These
compounds usually contain l to 5 ethylene glycol units per propylene glycol unit.
Representative exarnples of non-ionic surfactants are nonylphenol polyethoxyethanols,
castor oil polyglycol ethers, polypropylene/polyethylene oxide adducts, tributylphenoxy-
polyethoxyethanol, polyethylene glycol and octylphenoxypolyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are
also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which contain, as
N-substituent, at least one C8-C22alkyl radical and, as further substituents, unsubstituted or
20762~0
- 10-
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethylammonium
chloride or benzyldi~2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in formulation technology are described inter alia in
the following publications:
- "Mc Cutcheon's Detergents and Emulsifiers Annual", MC Publishing Corp., Glen Rock, New Jersey, 1988.
- M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-m, Chemical Publishing Co.,
New York, 1980-1981.
- Dr. Helmut Stache "Tensid-Taschenbuch" (Surfactant Handbook), Carl Hanser Verlag,
Munich/Vienna 1981.
The pesticidal compositions usually comprise 0.1 to 99 %, preferably 0.1 to 95 %, of a
compound of formula I, 1 to 99 % of a solid or liquid adjuvant, and 0 to 25 %, preferably
0.1 to 25 %, of a surfactant.
Whereas commercial products will preferably be formulated as concentrates, the end user
will normally employ dilute formulations.
The compositions may also comprise further auxiliaries, such as stabilisers, e.g. vegetable
oils or epoxidised vegetable oils (epoxidised coconut oil, rape oil or soybean oil), anti-
foams, e.~. silicone oil, preservatives, viscosity regulators, binders and tackifiers, as well
as fertilisers or other active ingredients for obtaining special effects.
Preferred formulations have especially the following composition (throughout,
percentages are by weight)
Emulsifiable concentrates:
active ingredient: 1 to 90 %, preferably 5 to 20 %
surface-active agent: 1 to 30 %, preferably 10 to 20 %
liquid carrier: S to 94 %, preferably 70 to 85 %
Dusts:
active ingredient: 0.1 to 10 %, preferably 0.1 to 1 %
20762~0
solid carrier: 99.9 to 90 %, preferably 99.9 to 99 %
Suspension concentrates:
active ingredient: S to 75 %, preferably 10 to 50 %
water 94 to 24 %, preferably 88 to 30 %
surface-active agent: 1 to 40 %, preferably 2 to 30 %
Wettable powders:
active ingredient: 0.5 to 90 %, preferably 1 to 80 %
surface-active agent: 0.5 to 20 %, preferably 1 to 15 %
solid carrier S to 95 %, preferably 15 to 90 %
Granules:
active ingredient: 0.5 to 30 %, preferably 3 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %
The compounds of fonnula I are generally used successfully at rates of application of from
0.001 to 4 kg~a, especially from 0.005 to 1 kg/ha. The concentration required to achieve
the desired effect can be determined by experiment. It is dependent upon t'ne type of
action, the stage of development of the cultivated plant and of the weed, and also upon the
application (place, time, method) and, in dependence on those parameters, can vary within
wide limits.
When used at relatively low rates of application, the compounds of formula I are distin-
guished by growth-inhibiting and herbicidal properties, which render them excellently
suitable for use in crops of useful plants, especially in cereals, cotton, soybeans, rape,
maiæ and rice.
The invention relates also to herbicidal compositions comprising a novel compound of
formula I, and to methods of inhibiting plant growth.
2076240
Preparation Examples:
Example Pl: Preparation of 2-[6-fluoro-3-(1-methylethyl)-2-benzothiazolon-5-yl-amino-
thiocarbonyl] -hexahydropyridazine:
CN C NH~ S
NH I ~0
CH(cH3)2
At a temperature of +5C, a solution of 10.8 g of 6-fluoro-3-(1-methylethyl)-2-benzo-
thiazolon-5-yl-isothiocyanate in 150 ml of ethanol is added dropwise to a solution of
3.6 ml of hexahydropyridazine in 50 ml of ethanol. After being stirred for 12 hours at
room temperature, the reaction mixture is concentrated by evaporation. Purification by
means of silica gel chromatography (ethyl acetate/n-hexane 1:9) yields 6.9 g of 2-[6-
fluoro-3-(1-methylethyl)-2-benzothiazolon-5-yl-aminothiocarbonyl]-hexahydropyridazine
having a melting point of 198-199C.
Example P2: Preparation of 9-[3-(1-methylethyl)-~fluoro-2(3H)-benzothiazolon-5-yl]-8-
thia-l,~diazabicyclo[4.3.0]nonan-7-one (Compound No. 1.001):
CNI ~S F
S ( 1.001 )
I ~o
CH(CH3)2
At a temperature of +5C, 7 ml of a 20 % toluene solution of phosgene is added dropwise
to a solution of 4.4 g of 2-[6-fluoro-3-(1-methylethyl)-2-benzothiazolon-5-yl-arninothio-
carbonyl]-hexahydropyridazine prepared according to Example Pl. After being stirred for
one hour, the reaction mixture is poured into ice-water. After separation of the organic
phase and drying over sodium sulfate, the solution is concentrated by evaporation.
Chromatographic purification over silica gel (ethyl acetate/n-hexane 1:1) yields 2.9 g of
20762~0
9-[3-(1-methylethyl)-6-fluoro 2(3H)-benzothiazolon-5-yl]-8-thia-1,6-diazabicyclo[4.3.0]-
nonan-7-one (Compound No. 1.001) having a melting point of 132-133C.
The compounds of Table 1 can be prepared analogously to the above Example and the
preparation processes mentioned in the description:
2076240
- 14-
T~ble 1: Compounds of formula I:
--(N ~ S (I)
N~
O
Comp. Rl Y R2 phys. data
No.
-
1.001 F O (CH3)2CH m.p. 132-133C
1.002 F O H
1.003 F O CH3
1.004 F O C2H5
1.005 F O C3H7
1.006 F O C4Hg
1.007 F O iso-C4Hg
1.008 F O sec-C4Hg
1.009 F O tert-C4Hg
1.010 F O CsHIl
1.011 F O CH3-CH2-CH(CH3)-CH2
1.012 F O CH3-CH2-CH2-CH(CH3)
1.013 F O (C2Hs)2CH
1.014 F O CH3-CH(CH3)-CH(CH3)
1.015 F O CH3-C(CH3)2-CH2
1.016 F O C6Hl3
1.017 F O (C2Hs)2-cH-cH2
1.018 F O (CH3)3-C-CH2-CH2
1.019 F O (CH3~3-C-CH(CH3)
1.020 F O CH3-CH2-CH2-CH2-CH(CH3)
1.021 F O CH3-CH2-CH2-CH(C2Hs)
1.022 F O CH3-CH2-CH2-CH(CH3)-CH2
20762~ 0
- 15 -
Table I (continuation)
Comp. Rl Y R2 phys. data
No.
1.023 F O CH3-CH2-CH2-C(CH3)2
CH3 - CH2 - CH - CH(CH3)2
1.024 F O
1.025 F O CH3-CH2-CH(CH3)-CH2-cH2
1.026 F O (C2Hs)2(CH3)C
1.027 F O (CH3)2-CH-CH2-CH2-CH2
1.028 F O (CH3)2-CH-CH2-CH(CH3)
1.029 F O CH2=CH
1.030 F O CH2=CH-CH2
1.031 F O CH3-CH=CH-CH2
1.032 F O CH2=C(CH3)-CH2
1.033 F O C6Hs-CH=CH-CH2
1.034 F O HC_C-CH2
1.035 F O CH3-C-C-CH2
1.036 F O HC_C-CH(CH3)
1.037 F O FCH2
1.038 F O F2C~I2
1.039 F O FCH2CH2
1.040 F O CF3CH2
1.041 F O FcH2cH2cH2
1.042 F O ClCH2
1.043 F O BrCH2
1.044 F O Cl3C
1.045 F O F3C
1.046 F O ClCH2CH2
1.047 F O BrCH2CH2
1.048 F O CF3CF3
Cl CH2--
1.049 F O >=<
H Cl
H CH2 -
1.050 F O ~<CI
20762~0
- 16-
Table I (continuation)
Comp. Rl Y R2 phys. data
H CH2--
1.051 F O H>=<CI
H CH2--
1.052 F O CH2CI >=<H
Cl CH2--
1.053 F CH3 H
Cl CH2--
1.054 F O H~=<H
Br CH2--
1.055 F O H>=<Br
H CH2--
1.056 F O B>=<Br
H CH2--
1.057 F O H>=<sr
H CH2--
1.058 F O CH2Br >=<H
Br CH2--
1.059 F CH3 H
Br CH2--
1.060 F O H>=<H
1.061 F O IC=-C-CH2
1.062 F O CH3-O-CH2
1.063 F O C2Hs-O-CH2
20762~0
- 17 -
T~ble 1 (continu~tion)
Comp. Rl Y R2 phys. d~ta
No.
1.064 F O C3H7-O-CH2
1.065 F O C4H9-O-CH2
1.066 F O CH3-O-CH2-CH2
1.067 F O C2H5-O-CH2-CH2
1.068 F O CH3-O-CH2-CH(CH3)
1.069 F O CH3-O-CH2-O-CH2
1.070 F O CH3-O-CH2-CH2-O-CH2
1.071 F O C2Hs-O-CH2-O-CH2
1.072 F O CH3-O-CH2-O-CH2-CH2
1.073 F O C2H5-O-CH2-O-CH2-CH2
1.074 F O C2H5-O-CH2-CH2-O-CH2
1.075 P O C2Hs-O-CH2-CH2-O-CH2-CH2
1.076 F O C6H5-CH=CH-CH2
1.077 F O NC-CH2
1.078 F O NC-CH2-CH2
1.079 F O NC-CH(CH3) m.p. 77-78C
1.080 F O HOOC-CH2
1.081 F O HOOC-CH2-CH2
1.082 F O HOOC-CH(CH3)
1.083 F O HOOC-CH(C2Hs)
1.084 F O HOOC-CH2-CH2-CH2-CH2
1.085 F O CH3OOC-CH2
1.086 F O C2HsOOC-CH2
1.087 F O (CH3)2CH-OOC-CH2
1.088 F O CsHI lOOC-CH2
1.089 F O CH3OOC-CH2-CH2
1.090 F O C2H5OOC-CH2-CH2
1.091 F O (CH3)2CH-OOC-CH2-CH2
1.092 F O CH300C-CH(CH3)
1.093 F O C2H5OOC-CH(CH3)
1.094 F O ClCH2CH2OOC-CH2
1.095 F O FCH2CH200C-CH2
20762~
- 18-
T~ble I (continu;ltion)
Comp. Rl Y R2 phys. d~ta
No.
1.096 F O FCH2CH2CH2OOC-CH2
1.097 F O CF3CH2OOC-CH2
1.098 F O CH3O-CH2-CH2OOC-CH2
1.099 F O C2HsOOC-CH(CH3)-OOC-CH2
1.100 F O ~OOC-CH2
CH3
N C CH2
1.101 F O C4Hg / O
1.102 F O CH3-S-CH2-CH(CH3)
1.103 F O C2Hs-S-CH2-CH(CH3) resinous
1.104 F O CH3-s-cH2-cH2
1.105 F O C3H7-S-CH2-CH(CH3)
1.106 F O (CH3)2CH-S-CH2-CH(CH3)
1.107 F O C4Hg-S-CH2-CH(CH3)
1.108 F O CsH1l-S-CH2-CH(CH3)
1.109 F O C6H5-CH2
c
1.110 F O ~
~=~ CH2
1 111 F O CH2=CH-CH2-O-CH2
1.112 F O CH3-CO
1.113 F O O~ooc-cH2
1.114 F O ~ OOC-
1.115 F O C3H7-S-CO-CH2
1.116 F O CH3-S-CO-CH(CH3)-OOC-CH2
o~ CH2
1.117 F O
~H3
20762~0
Table 1 (continuation)
Comp. Rl Y R2 phys. data
No.
1.118 F O CH3-SO2
1.119 F O C2Hs-S2
1.120 F O D--CH(CH3)
1.121 F S CH3-CH(CH3)
1.122 F S CH2=CH-CH2
1.123 F S HC--C-CH2
1.124 F S CH2F
1.125 F S C2HsF
1.126 H O CH3
1.127 H O C2H5
1.128 H O (CH3)2-CH
1.129 H O CH2=CH-CH2
1.130 H O CH-C-CH2
1.131 H O CH2=c(cH3)-cH2
1.132 H O CH2F-CH2
1.133 H O CH2F
1.134 H O CH2F-CH2-CH2
1.135 H O CH3-OOC-CH2
1.13~ H O C2Hs-ooc-cH2
1.137 H O CsH1l-OOC-CH2
1.13~ H O CH3-OOC-CH(CH3)
1.139 H O C2Hs-OOC-CH(CH3)
1.140 H O CH2F-CH2-CH2-OOc-cH2
1.141 F O CHFCl-CF2
1.142 F O CF2CI
1.143 F O CF2H-CF2
1.144 F O CHCl=CCl
1.145 F O ~ C2Hs
CN
20762~o
-2Q-
Tablel(continuation)
Comp. Rl Y R2 phys.data
No.
1.146 F O - CH-COO- C3H
CH3
1.147 F o - CH - COO-C3H7(i) resinous
CH3
1.148 F O - CH- COO-C4H
CH3
/ H3
1.149 F o - CH-COO- CH resinous
CH3 C2Hs
/CH3
1.150 F O - CH-COO-CH2- CH n9 1.5819
CH3 CH3
CIH3
1.151 F O - CH- CCK}- C - CH3
CH3 CH3
1.152 . O - CH- COO - CsTI11 nD9 1.5612
CH3
1.153 F O - CH- COO- CH2- CH- CH2- CH3
._T T
CH3 ~3
1.154 F O - CH- COO- CH- CH2- CH2- CH3
~H3 CH3
CH2- CH3
.155 F O - CH- COO- CH
CH3 CH2- CH3
-21-
Table 1 (continuation)
<IMG>
20762~0
-22-
Tablel(continuation)
Comp. Rl Y R2 phys.data
No.
CIH3
1.165 F O - CH- COO- C - CH2-CH2-CH3
CH3 CH3
CH3
1.166 F O - CH- COO-CH- CH
I
CH3 CH2 CH3
CH3
1.167 F O - CH-COO-CH2-CH2-CH- CH2- CH3
CH3 CH3
CH3
1.168 F O - CH-COO- C - CH2- CH3
CH3 CH2- CH3
/CH3
1.169 F O - CH-COO- CHr CH2-CH2-CH
CH3 CH3
/CH3
1.170 F O CH-COO - CH - CH2-CH
IH3 CH3 CH3
1.171 F O CH-COO- CH2-CH2-O - CH3 m.p.:50-54GC
CH3
1.172 F O - CH-COO- CH2- CH2- O-CH2-CH3 m.pnllO-113C
CH3
2076240
- 23 -
Table 1 (continuation)
Comp. Rl Y R2 phys. data
No.
1.173 F O --CH--COO--CH2- CH2--O--C3H7
I
CH3
/CH3
1.174 F O --CH--COO--CH2- CH2--O-- CH m.p.: 84-86C
CH3 CH3
1.17~ F O --CH--COO--CH2- CH2-- C4H9
I
CH3
/CH3
1.176 F O --CH--COO--CH--CH2--O--CH3
I
CH3
1.177 F O --CH--COO--CH--CH2--O--CH2CH3
CH3 CH3
1.178 F O --CH--COO--CH2-CH2--Cl
I
CH3
1.179 F O --CH--COO--CH2- CH2--F
I
c~3
1.180 F O --CH--COO--CH2- CH2--CH2F
I
CH3
1.181 F O --CH--COO--CH2- CF3
I
CH3
2076240
- 24 -
Table 1 (continuation)
Comp. Rl Y R2 phys. data
1.182 F O ~ COO--CH2- COO-CH3
I
CH3
1.183 F O --CH--COO--CH2- COO-C2Hs
CH3
CH3
I
1.184 F O --CH--COO--CH--COO-CH3
I
CH3
1.185 F O --CH--COO--CH--COO-C2Hs
CH3 CH3
/CH3
1.186 F O --CH--COO--CH--COO-CH
I
CH3 CH3 CH3
1.187 F O --CH- COO- CH~
CH3 CH3
1.188 F O --(~I--C NH--CH3
11
CH3 O
1.189 F O --CH--C NH--C2Hs
11
CH3 O
1.190 F O --CH--C-NH - C3H7
11
CH3 O
20762~0
T~ble 1 (continuation)
Nolnp. Rl Y R2 phys. data
CH3
1.191 F O --CH--COO--NH--CH
CH3 CH3
1.192 F O --CH--COO--NH--C4Hg
CH3
CH3
1.193 F O --CH-COO-N
CH
CH3 3
1.194 F O --CH--CO~\S
CH3 V
CH3
1.195 F O --C--COO-H
CH3
Cl H3
1.196 F O --C--COO--CH3
(~H3
CH3
1.197 F O --C CO~ C2H5
CH3
2076240
- 26-
Table 1 (continuation)
CNomp. Rl Y R2 phys. data
ICH3
1.198 F O --C COO--C3H7
CH3
ICH3
1.199 F O --C--COO-C3H7(;
CH3
Ci H3
1.200 F O --C--COO-C4Hg
CH3
ICH3 /CH3
1.201 F O --C--COO--CH
CH3 C2Hs
Cl H3 /CH3
1.202 F o --Cl--COO- CH2--CH
CH3 CH3
ICH3 CH3
1.203 F O --C--COO--C--CH3
CH3 CH3
Cl H3
1.204 F O --C--COO--CsH
CH3
2076240
Table I (con~inua~ion)
Comp. Rl Y R2 phys. data
No.
Cl H3
1.205 F Q --C--COO--CH2--CH--CH2--CH3
CH3 CH3
ICH3
1.206 F O --C--COO--CH--CH2- CH2- CH3
CH3 CH3
Cl H3 /CH2--CH3
1.207 F O --C--COO--CH
CH3 CH2--CH3
Cl H3
1.208 F O --C--COO- CH--CH--CH3
CH3 CH3 CH3
ICH3 Cj H3
1.209 F O --C--COO--CH2--C--CH3
CH3 CH3
Cl H3
1.210 F O --C--COO--C6HI3
CH3
2076240
Table 1 (continuation~
Comp. Rl Y R2 phys~ data
No.
Cl H3 CH2-CH3
1.211 F O --C--COO-CH2--CH
CH3 CH2-CH3
ICH3 CH3
1.212 F O --C--COO--CH2 CH2--C--CH3
CH3 CH3
Cl H3 /CH3
1.213 F O --C--COO--CH--C--CH3
CH3 CH3 CH3
Cl H3
1.214 F O --C--COO--CH--CH2- CH2--CH2--CH3
CH3 CH3
CH3
1.215 F O --C--COO--CH--CH2- CH2--CH3
CH3 C2H5
Cl H3
1.216 F O --C--COO--CH2- CH--CH2- CH2- CH3
CH3 CH3
2076240
- 29 -
Table 1 (continu;~tion)
Comp. Rl Y R2 phys. d~ta
No.
Cl H3 Cl H3
1.217 F O --C--COO- C--CH2- CH2- CH3
CH3 CH3
Cl H3 /CH3
1.218 F O --C--COO-CH--CH
I
CH3 CH2 CH3
CH3
CH3
I
1.219 F O --C--COO- CH2- CH2- CH--CH2--CH3
CH3 CH3
CH3 l H3
1.220 F O --C--COO--C--CH2--CH3
CH3 CH2--CH3
l H3 /CH3
.221 F O --C--COO--CH2- CH2- CHr CH
CH3 CH3
CH3 CH3
.222 F O --C--COO--CH--CH2~H
CH3 CH3 CH3
20762~o
- 30 -
Table 1 (continuation)
Nomp. Rl Y R2 phys. data
Cl H3
1.223 F O --C--COO--CH2- CH2- 0--CH3
CH3
CH3
1.224 F O --C--COO--CH2--CH2- 0 - CH2- CH3
CH3
CH3
1.225 F O --C--COO--CH2--CH2--0--C3H7
CH3
CH3 / H3
1.226 F O --C--COO--CH2- CH2--O CH
CH3 CH3
Cl H3
1.227 F O --C--COO--CH2- CH2--0--C4Hg
CH3
CH3 CH3
1.228 F O --C--COO--CH--CH2~ 0--CH3
CH3
207624~
Table 1 (con~inua~ion)
Comp. Rl Y R2 phys. dat~
No.
Cl H3
1.229 F O --C--COO--CH--CH2--O--CH2CH3
CH3 CH3
CH3
1.230 F O --C--COO--CH2- CH2--Cl
CH3
CH3
1.231 F O --f co~ CH2- CH2--F
CH3
CH3
1.232 F O --C--COO--CH2- CH2--CH2F
CH3
CH3
1.233 F O --C--COO--CH2- CF3
CH3
I H3
1.234 F O --C ~ CO~ CH2- COO-CH3
CH3
2076240
Table I (continuation)
Nomp. Rl Y R2 phys. data
CH3
1 235 F O --C--COO--CH2--COO-C2H5
CH3
CH3 CH3
1 236 F O --C--COO--CH--COO-CH3
CH3
1 3
1 237 F O --C--COO--CH--COO-C2H5
CH3 CH3
CI H3 CH3
1 238 F O --C--CO~ CH--COO- CH
CH3 IH3 CH3
CH3
1 349 F O --C--COO--CH~
CH3 CH3
CH3
1240 F O --C--C--NH--CH3
CH3 O
20762~0
- 33 -
Table 1 (continuation)
Comp. Rl Y R2 phys. data
1 3
1.241 F O --C--C NH--C2Hs
CH3 0
CH3
1.242 F O --C C-NH C3H7
CII3 0
1 3 ~ H3
1.243 F O --C--COO--NH--CH
CH3 CH3
CH3
1.244 F O --C--COO--NH--C4H9
CH3
l H3 / H3
1.245 F O --C~OO-N
CH3 CH3
CH3 /\
1.246 F O --C COO~ S
CH3
20762~0
- 34 -
Table 1 (continua~ion)
Comp. Rl Y R2 phys. data
No.
1.247 F O --CH--CH2--COO-H
CH3
1.248 F O --CH--CH2--COO--CH3
I
CH3
1.249 F O --CH--CH2--COO--C2H5
CH3
1.250 F O --CH--CH2--COO--C3H7
I
CH3
1.251 F O --CH--CH2--COO-C3H7 (;
CH3
1.252 F O --CH--CH2- COO-C4Hg (n
CH3
/CH3
1.253 F O --CH- CH2- COO- CH
CH3 C2Hs
/CH3
1.254 F O --CH- CH2- COO- CH2--CH
CH3 CH3
ICH3
1.255 F O --CH--CH2--COO- C - CH3
CH3 CH3
20762~0
Table I (continuation)
Nomp. Rl Y R2 phys. data
-
1.256 F O --CH--CH2--COO--CsH~
CH3
1.257 F O --CH--CH2--COO- CH2- CH- CH2- CH3
CH3 CH3
1.258 F O --CH--CH2--COO--CH--CHr CH2- CH3
CH3 CH3
/CH2--CH3
1.259 F O --CH--CH2--COO- CH
CH3 CH2--CH3
1.260 F O --CH- CH2- CO~ CH--CH--CH3
CH3 CH3 CH3
Cl H3
1.261 F O --CH--CH2--CO~ CH2- C--CH3
CH3 CH3
1.262 F O --CH--CH2--COO- C6H~3
CH3
CH2-CH3
1.263 F O --CH~ CH2- COO-CH2--CH
CH3 CH2-CH3
20762~0
- 36 -
Table 1 (continuation)
Cornp. Rl Y R2 phys. data
/ H3
1.264 F o - CH CH2- COO - CH2 - CH2- C - CH3
CH3 CH3
CH3
1.265 F O - CH - CH2- COO - CH- C - CH3
CH3 CH3 CH3
1.266 F O - CH- CH2- COO- CH- CHr CH2- CH2- CH3
CH3 CH3
1.267 F O - CH-CH2- CO0-CH- CH2-CHr CH3
CH3 C2H5
1.268 F O - CH- CH2- COO- CH2-CH- CH2-CH2-CH3
CH3 CH3
CH3
1.269 F O - CH- CH2- COO- C - CH2-CH2-CH3
CH3 CH3
CH3
1.270 F O - CH - CH2- COC~ CH- CH
CH3 CH2 CH3
CH3
2076240
Table 1 (continua~ion)
Comp. Rl Y R2 phys. data
No.
1.271 F O --CH- CH2- COO- CH2- CH2- CH- CH2--CH3
CH3 CH3
/CH3
1.272 F O --CH--CH2--COO- C--CH2--CH3
CH3 CH2--CH3
CH3
1.273 F O --CH--CH2--COO--CH2- CH2- CH2- CH
CH3 CH3
/CH3
1.274 F O --CH--CH2 CO~ CH--CH2--CH
CH3 CH3 CH3
1.275 F O --CH--CH2--CO~ CH2- CH2- 0--CH3
CH3
1.276 F O --CH- CH2- COO--CH2- CH2- 0 - CH2--CH3
CH3
1.277 F O ~ CH--CH2--COO--CH2- CH2--O--C3H7
CH3
/CH3
1.278 F O --CH--CH2--COO CH2--CH2--O CH
CH3 CH3
20762~0
Table_ (continuation)
Comp. Rl Y R2 phys. data
No.
1.279 F O --CH--CH2--COO--CH2--CH2-- C
I
CH3
/CH3
1.280 F O --CH CH2--COO--CH--CH2--O--CH3
I
CH3
1.281 F O --CH--CH2 COO--CH--CH2--O--CH2CH3
CH3 CH3
1.282 F O --CH--CH2--COO--CH2--CH2--Cl
I
CH3
1.283 F O --CH--CH2--CO~ CH2--CH2--F
I
CH3
1.284 F O --CH--CH2--CO~ CH2 CH2--CH2F
I
CH3
1.285 F O --CH--CH2--COO--CH2- CF3
I
CH3
1.286 F O --CH--CH2--CO~ CH2- COO-CH3
I
CH3
1.287 F O --CH--CH2--CO~ CH2--COO-C2Hs
CH3
2~762~0
- 39-
Table 1 (con~inuation)
Comp. Rl Y R2 phys. data
CH3
1.288 F O - CH - CH2- COO-CH- COO-CH3
CH3
1.289 F O - CH- CH2- COO-CH- COO-C2Hs
CH3 CH3
~CH3
1.290 F O - CH - CH2- COO-CH- COC~CH
I
C~3 CH3 CH3
1.291 F O - CH- CH2- COO-CH
CH3 CH3
1.292 F O - CH-CH2- C NH- CH3
CH3 O
1.293 F O -CH- CH2 C NH- C2H5
CH3 O
1.294 F O - CH-CH2- C-NH C3H7
CH3 O
/ H3
1.295 F O - CH- CH2 COO-NH- CH
CH3 CH3
2~762~0
- 40 -
Table 1 (continuation)
Comp. Rl Y R2 phys. data
No.
1.296 FO --CH--CH2 COO--NH--C4H9
CH3
/CH3
1.297 F O --CH--CH2--COO-N
CH3 CH3
1.298 F O ~ CH2 CIX~ S
1.299 F O --CH--COO-H
C2Hs
1.300 F O --CH--COO--CH3 m.p. 149-151C
C2Hs
1.301 F O --CH--COO--C2Hs
C2Hs
1.302 F O --C~--COO--C3H7 nD19 1.5692
C2Hs
1.303 F O --CH--COO-C3H7(;
C2Hs
1.304 F O --CH--COO-C4H
C2Hs
2076240
- 41 -
Table 1 (continua~ion)
Nomp. Rl Y R2 phys. data
. . . _ . . _
~CH3
1.305 F O - CH-COO- CH resinous
C2H5 C2Hs
/CH3
1.306 F O - CH-COO- CH2 - CH resinous
C2H5 CH3
ICH3
.307 F O - CH-COO-C - CH3
C2H5 CH3
.308 F O - CH-COO - CsH
C2Hs
.3~ F O - CH- COO- CH2-CH- CH2-CH3
C2Hs CH3
.310 F O -CH- COO- CH- CH2-CH2-CH3
C2Hs CH3
~CH2- CH3
1.311 F O - CH-COO-CH
l2HS CH2- CH3
.312 F O - CH-COO-CH-CH- CH3
C2Hs CH3 CH3
2076240
-42-
Tablel(continua~on)
Comp. Rl Y R2 phys.data
CH3
1.313 F O - CH-COO- CH2- C - CH3
C2Hs CH3
1.314 F O - CH- COO- C6HI3
C2H5
CH2-CH3
1.31~ F O - CH-COO-CH2- CH
C2Hs CH2-CH3
CH3
1.316 F O - CH- COO-- CH2 CH2- C - CH3
C2Hs CH3
1.317 F O - CH- COO - CH - C - CH3
C2Hs C~I3 CH3
1.318 F O - CH-COO- CH- CH2- CH2- CH2- CH3
C2Hs CH3
1.319 F O - CH-COO- CH - CH2- CH2- CH3
C2Hs C2Hs
1.320 F O - CH- COO- CH2-CH- CH2-CH2- CH3
C2Hs CH3
2076240
-43-
Tablel(continuation)
Comp. Rl Y R2 phys.data
No.
CH3
1.321 F O - CH-COO-C - CH2-CH2-CH3
C2Hs CH3
/CH3
1.322 F O - CH-COO-CH- CH
C2H5 CH2 CH3
CH3
1.323 F O - CH-COO-CH2-CH2-CH-CH2- CH3
C2Hs CH3
1 ~3
.324 F O - CH-COO- C - CH2-CH3
C2Hs CH2- CH3
/CH3
.325 F O -CH-COO-CH2-CH2-CH2-CH
C2H5 \CH3
/CH3.326 F O - CH-COO- CH- CH2-CH
C2Hs CH3 CH3.327 F O - CH-COO-CH2-CH2-O - CH3
C2Hs
2~762~0
- 44 -
Table 1 ~continuation)
Comp. Rl Y R2 phys. data
No.
1.328 F O --CH--COO- CH2--CH2- O - CH2- CH3
C2Hs
1.329 F O ~ CH--COO--CH2- CH2--O - C3H7
I
C2H5
/CH3
1.330 F O --CH--COO--CH2- CH2--O--CH
C2Hs CH3
1.331 F O --CH--CO~ CH2--CH2--O--C4Hg
I
C2Hs
/CH3
1.332 F O --CH--COO--CH--CH2--O--CH3
I
C2Hs
1.333 F O --CH--CO~ CH--CH2--O--CH2CH3
C2H5 CH3
1.334 F O --CH--COO--CH2- CH2--C1
I
C2H5
1.335 P O ~ CH--COO--CHr CH2--F
I
C2Hs
20762~0
- 45 -
Table I (continuation)
Nomp. Rl Y R2 phys. data
-
1-336 F O --CH--COO--CH2- CH2--CH2F
C2Hs
1.337 F O --CH--COO--CH2- CF3
I
C2Hs
1.338 F O --CH--COO--CH2- COO-CH3
I
C2Hs
1.339 F O --CH--COO--CH2- COO-C2Hs
C2Hs
CH3
I
1.340 F O --CH- COO--CH--COO-CH3
C2Hs
1.341 F O --CH- COO--CH--COO-C2Hs
C2Hs CH3
/c~3
1.342 F O --CH--COO--CH--CO~ CH
I
C2Hs CH3 CH3
1.343 F O --CH- COO- CH~
C2Hs CH3
20762~0
- 46 -
Table 1 (continuation)
Comp. Rl Y R2 phys. data
1.344 F O --CH--C NH--CH3
C2Hs O
1.345 F O --CH--C--NH--C2H5
C2Hs O
1.326 F O --CH--C-NH C3H7
C2H5 0
/CH3
1.347 F O --CH--COO--NH--CH
C2H5 CH3
1.348 F O --CH--COO--NH--C4Hg
C2H5
/CH3
1.349 F O --CH-COO-N
C2H5 CH3
1.350 F O --CH--CO~¢S
C2H5
1.351 F O
1.352 F O
2076240
- 47 -
Table 1 (continua~on)
Comp. Rl Y R2 phys. data
No.
1.353 F O
1.354 F O
1.355 H O
1.356 H O
1.357 H O
1.358 H O ~
1.359 F O - CH - COO - CH3 m.p.143-145C
CH2CH2CH3
20762~0
- 48 -
Forrnulation Examples for liquid active ingredients of forrnula I (throu~hout~ percentages
are bY wei~ht)
1. Emulsifiable concentrates a) b) c)
a compound of Table 1 25 % 40 % 50 %
calcium dodecylben~enesulfonate 5 % 8 % 6 %
castor oil polyethylene glycol
ether (36 mol of ethylene oxide)5 % - -
tributylphenol polyethylene glycol
ether (30 mol of ethylene oxide) - 12 % 4 %
cyclohexanone - 15 % 20 %
xylene mixture 65 % 25 % 20 %
Emulsions of any desired concentration can be produced from such concentrates bydilution with water.
2. Solutions a) b) c) d)
a compound of Table 1 80 % 10 % 5 %95 %
propylene glycol monomethyl ether 20 %
polyethylene glycol
(mol. wt. 410) - 70 %
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oil - - 1% 5 %
benzine (boiling range 160-190C) - - 94 %
The solutions are suitable for application in the form of micro-drops.
3. Granules a) b~ c) d)
a compound of Table 1 5 % 10 % 8 %21 %
kaolin 94 % - 79 %54 %
highly dispersed silicic acid 1 % - 13 % 7 %
attapulgite - 90 % - 18 %
The active ingredient is dissolved in methylene chloride, the solution is sprayed onto the
carrier, and the solvent is subsequently evaporated off in vacuo.
2076240
^ 49 -
4. Dusts a) b)
a compound of Table 1 2 % 5 %
highlydispersed silicicacid 1% S %
talcum 97 %
kaolin - 90 %
Ready-for-use dusts are obtained by intimately mixing the carriers with the active
ingredient.
Formulation Examples for solid active ingredients of formula I (throughout, percentages
are bv weight)
5. Wettablepowders a) b) c)
acompoundofTable 1 25 % 50 %75 %
sodium lignosulfonate 5 % 5 %
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 %10 %
octylphenol polyethylene glycol
ether (7-8 mol of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 %10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the mixture is
thoroughly ground in a suitable mill, affording wettable powders which can be diluted
with water to give suspensions of the desired concentration.
6. Emulsifiable concentrate
a compound of Table 1 10 %
octylphenol polyethylene glycol ether
(4-5 mol of ethylene oxide) 3 %
calcium dodecylbenzenesulfonate 3 %
castor oil polyglycol ether
(36 mol of e;hylene oxide) 4 %
cyclohexanone 30 %
xylene mixture SO %
2076240
- 50 -
Emulsions of any required concentration can be obtained from this concentrate by dilution
with water.
7. Dusts a) b)
acompoundofTable I 5 %8 %
talcum 95 %
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the active ingredient with the carrier and
grinding the mixture in a suitable mill.
8. Extruder ~ranules
a compound of Table 1 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient is mixed and ground with the adjuvants, and the mixture ismoistened with water. The mixture is extruded and then dried in a stream of air.
9. Coated ~ranules
a compound of Table 1 3 %
polyethylçne glycol (mol. wt. 200) 3 %
kaolin 94 %
The finely ground active ingredient is uniformly applied, in a mixer, to the kaolin
moistened with polyethylene glycol. Non-dusty coated granules are obtained in this
manner.
10. Suspension concentrate
a compound of Table 1 40 %
propylene glycol 10 %
nonylphenol polyethylene glycol ether
(15 mol of ethylene oxide) 6 %
sodium lignosul~onate 10 %
2076240
- 51 -
carboxymethylcellulose 1 %
silicone oil in the forrn of a 75%
aqueous emulsion 1 %
water 32 %
The finely ground active ingredient is intimately mixed with the adjuvants, giving a
suspension concentrate from which suspensions of any desired concentration can be
obtained by dilution with water.
The eompounds of formula I are used in unmodified form or, preferably, in the form of
compositions together with the adjuvants eustomarily employed in formulation technology
and are therefore formulated in known manner e.g. into emulsifiable concentrates, directly
sprayable or dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granules, and also encapsulations in e.g. polymer substances. As with the nature of
the eompositions, the methods of applieation, such as spraying, atomising, dusting,
scattering or pouring, are ehosen in accordance with the intended objectives ancl the
. . .
prevallmg Clrcumstances.
2076240
- 52 -
Biolo~ical Examples:
Example B 1: Pre~mer~ence herbicidal action
In a greenhouse, immediately after the test plants have been sown in seed trays, the
surface of the soil is treated with an aqueous spray mixture corresponding to a rate of
application of 4 kg of active ingredient/hectare. The seed trays are kept in the greenhouse
at 22 - 25C and 50 - 70 % relative humidity.
The herbicidal action is evaluated after 3 weeks using a scale of nine ratings (1 = total
damage, 9 = no damage) in comparison with an untreated control group.
Ratings from 1 to 4 (especially from 1 to 3) indicate a good to very good herbicidal action.
Ratings from 6 to 9 (especially from 7 to 9) indicate good tolerance (especially in
cultivated plants).
In this test, the compounds of Table 1 exhibit pronounced herbicidal activity. For
example, compound 1.001 exhibits the following herbicidal action against the tested
weeds:
Table B1: Pre-emer ence herbicidal action:
Test plant: Avena Sinapis Setaria Stellaria
Compound No.
1.001
Example B2: Pre-emer~ence herbicidal action
In a greenhouse, immediately after the test plants have been sown in seed trays, the
surface of the soil is treated with an aqueous spray mixture corresponding to a rate of
application of 2 kg of active ingredient/hectare. The seed trays are kept in the greenhouse
at 22 - 25C and 50 - 70 % relative humidity.
The herbicidal action is evaluated after 3 weeks using a scale of nine ratings ( 1 = total
darnage, 9 = no damage) in comparison with an untreated control group.
Ratings from 1 to 4 (especially from 1 to 3) indicate a good to very good herbicidal action.
2076240
- 53 -
Ratings from 6 to 9 (especially from 7 to 9) indicate good tolerance (especially in
cultivated plants).
In this test, the compounds of Table 1 exhibit pronounced herbicidal activity. Examples of
good herbicidal activity are given in Table B2:
Table B2: Pre-emergence herbicidal action:
Test plant: Avena Sinapis Setaria Stellaria
ComPound No.
1.008 1 3
1.034
1.036
1.092
1.093 2
1.146 3
1.301 2 2 1 2
1.303 3
Example B3: Post-emer~ence herbicidal action (contact herbicide)
A number of weeds, both monocotyledonous and dicotyledonous, are sprayed post-
emergence (in the 4- to 6-leaf stage) with an aqueous active ingredient dispersion at a rate
of 4 kg of active ingredient/hectare and kept at 24-26C and 45-60 'rO relative humidity.
The herbicidal action is evaluated 15 days after treatment using a scale of nine ratings (1 =
total damage, 9 = no damage) in comparison with an untreated control group.
Ratings from 1 to 4 (especially from 1 to 3) indicate a good to very good herbicidal action.
Ratings from 6 to 9 (especially from 7 to 9) indicate good tolerance (especially in
cultivated plants).
In this test, the compounds of Table 1 exhibit pronounced herbicidal activity. For
example, compound 1.001 exhibits the following herbicidal action against the tested
weeds:
20762~o
- 54 -
Table B3. Post-emer ence herbicidal action:
Test plant: Avena Setaria Lolium Solanu~ Sinapis Stellaria
Compound No.
1.001 1 2
Example B4: Post-emer~ence herbicidal action (contact herbicide)
A number of weeds, both monocotyledonous and dicotyledonous, are sprayed post-
emergence (in the 4- to 6-leaf stage) with an aqueous active ingredient dispersion at a rate
of 2 kg of active ingredient/hectare and kept at 24-26C and 45-60 % relative humidity.
The herbicidal action is evaluated 15 days after treatment using a scale of nine ratings (1 =
total damage, 9 = no damage) in comparison with an untreated control group.
Ratings from 1 to 4 (especially from 1 to 3) indicate a good to very good herbicidal action.
Ratings from 6 to 9 (especially from 7 to 9) indicate good tolerance (especially in
cultivated plants).
In this test, the compounds of Table 1 exhibit pronounced herbicidal activity. Exarnples of
good herbicidal achvity are given in Table B4:
Table B4: Post-emer ence herbicidal action:
Test plant: Avena Sinapis Setaria Stellaria
ComPo-und No.
1.008 2 4
1.034 1 2
1.036
1.092 1 2
1.093 1 2
1.146 2
1.301 2 2
1.303 3 1 1 2
Example B5: Herbicidal action in wild rice (paddy)
The weeds Echinochloa crus galli and Monocharia vag., which occur in water, are sown in
plastic beakers (surface area: 60 cm2, volume: 500 ml). After sowing, the beakers are
20762~o
- ss -
filled with water up to the surface of the soil. 3 days after sowing, the water level is
increased to slightly above the soil surface (3-5 mm). Application is effected 3 days after
sowing by spraying the beakers with the test compounds. The dosage corresponds to an
amount of active ingredient of 4 kg a.i. per hectare. The beakers are then kept in a green-
house under optirnum growth conditions for rice weeds, i.e. 25-30C and high humidity.
The tests are evaluated 3 weeks after application. The compounds of Table 1 damage the
weeds.