Note: Descriptions are shown in the official language in which they were submitted.
2n~72l~
BACKGROUND OF THE INVENTION
I. FIELD OF THE INVENTION
This invention relates to a process and an apparatus for
reclaiming and/or concentrating waste aqueous solutions of gas
treating chemicals.
II. DISCUSSION OF THE PRIOR ART
Natural gas contains a number of acidic gaseous
components, such as hydrogen sulfide and carbon dioxide, as
well as water vapor, which should desirably be removed from
the gas before it is transported and/or used.
These components can be removed from natural gas, and
other similar gases if desired, by contacting the gas in
countercurrent flow with an aqueous solution of a gas treating
chemical, usually an alkanolamine such as monoethanolamine
(MEA), diethanolamine (DEA) or methyl diethanolamine, or a
glycol such as mono-, di- or tri-ethylene glycol, or sulfinol.
The solution of the gas treating chemical efficiently absorbs
the acid components or water from the natural gas.
Thereafter, the solution of the gas treating chemical is
regenerated by stripping the absorbed acid materials from the
solution so that the solution can be recirculated and re-used
for the treatment of further natural gas. This stripping
operation is normally brought about by flowing the solution
countercurrent through steam in a regenerator or stripper
apparatus.
Over a period of time, certain contaminants build up in
the gas treating solution subjected to repeated use in this
way to the extent that the efficiency of removal of the acidic
gaseous components is reduced or the gas treating solution
becomes too viscous to pump efficiently. When this occurs,
the solution is generally replaced by a fresh aqueous gas
treating chemical solution. However, this gives rise to two
2077218
disadvantages. Firstly, the resulting large quantities of
waste solution are difficult and expensive to dispose of
safely because of their content of toxic substances.
Secondly, the cost of fresh gas treating chemical is quite
high, so the overall cost efficiency of the process is
reduced. In view of this, attempts have been made to reclaim
contaminated (waste) aqueous solutions of gas treating
chemicals so that they can continue to be used.
The contaminants commonly found in waste solutions of gas
treating chemicals include products of the thermal degradation
of the gas treating chemical, heat-stable salts, asphaltenes,
light hydrocarbons, suspended solids or combinations thereof.
The degradation products are high boiling nitrogen and oxygen
compounds, and the heat-stable salts include sodium
thiosulfate, sodium thiocyanate and sodium sulfide. In order
to reclaim the waste solutions of gas treating chemicals, it
would therefore be necessary to remove all such impurities
from the solutions while leaving the gas treating chemical
substantially intact. One way to achieve this would be to
subject the waste solution to evaporation in order to liberate
water vapor and vaporized treating chemical, leaving the
contaminants in an unvaporized liquid residue. However, this
is made difficult by the fact that all of the gas treating
chemicals used for the treatment of natural gas are
susceptible to decomposition at elevated temperatures. In the
case of the alkanolamines, which are the preferred gas
treating chemicals, any attempt to separate contaminants from
the solutions by heating the solutions to temperatures above
about 400~F will result in significant thermal decomposition
of the gas treating chemical itself. While the vaporization
temperature of the waste solution can be reduced by carrying
out the evaporation under a vacuum, it is still difficult to
avoid heating parts of the solution to temperatures above
400~F if rapid recovery of chemical from the waste solution is
to be achieved.
Moreover, another problem that is encountered is that the
gas treating chemicals, and particularly the alkanolamines,
2077218
are corrosive and their corrosive activity increases with
temperature. The corrosion problem can be serious in those
parts of the equipment where temperatures are high and
residence times are long.
This corrosion problem has given rise to the conventional
wisdom that, when dealing with aqueous solutions of
alkanolamines and other gas treating chemicals (a) the
temperature should be limited to the range of 260~F to 300~F,
(b) the concentration of the alkanolamine should be limited to
30 weight percent, and (c) that stainless steel should be used
for critical parts of the apparatus, i.e. those parts of the
apparatus where the solution is heated and the residence times
are long. For example, in "Gas Purification" by Kohl and
Reisenfeld, 4th Edition, 198S, Gulf Publishing, page 121, it
is stated in connection with ethanolamine gas purification
that: "The authors concluded from the results of this study
that all vessels and exchanger shells in the treating plant
can be constructed of carbon steel. However, stainless steel,
type 304, is recommended for the hottest amine exchanger pass,
the boiler, the amine cooler, the amine reclaimer, and certain
sections of the piping."
However, it will be appreciated that the use of stainless
steel for the gas treating chemical reclaimer necessarily
significantly increases the capital cost of the apparatus
compared to similar apparatus made of carbon steel.
It is therefore difficult to design apparatus for
reclaiming waste aqueous solutions of gas treating chemicals
that can be operated both efficiently and at reasonable cost
for prolonged periods of time.
A further problem is that environmental considerations
are very important nowadays and gas treating chemicals, as
well as their contaminants and decomposition products, are
generally regarded as pollutants. It is therefore essential
to ensure that any effluent from the apparatus that is
intended to be discarded by dumping meets stringent anti-
pollution standards.
It was stated above that there is an alternative to
- 4 207721 8
reclaiming waste aqueous gas treating chemical solutions, i.e.
merely to discard them and to use fresh solutions, but that
this gives rise to disposal problems. One way of reducing
such problems would be to concentrate the waste solution by
evaporating off excess water and thus reducing the quantity of
hazardous material to be disposed of. Since, in such cases,
there would be no intention of re-using the gas treating
chemical, it might be thought that there would be no
disadvantage in subjecting the chemical to temperatures above
its decomposition point. This, however, is not the case
because the resulting decomposition products make the solution
viscous, and hence more difficult to pump, and because the
higher temperatures and concentration levels of the gas
treating chemical and decomposition products increase the risk
of serious corrosion of the apparatus.
OBJECTS OF THE INVENTION
In view of the problems outlined above, an object of the
present invention is to provide a process either for
reclaiming waste aqueous solutions of gas treating chemicals
or for concentrating such solutions in ways which avoid undue
thermal decomposition of the gas treating chemicals.
Another object of the invention is to provide processes
of this kind which can be carried out, if desired, in
apparatus made of carbon steel without undue corrosion of the
apparatus.
Yet another object of the invention is to provide
apparatus, desirably made of carbon steel, for carrying out
such processes.
SUMMARY OF THE INVENTION
The present invention makes it possible selectively to
concentrate waste aqueous solutions of chemicals, particularly
gas treating chemicals, or to reclaim such solutions for re-
use in such ways that undue decomposition of the chemical is
avoided and the production of corrosive components can be
minimized to such an extent that the process can be operated
in apparatus made of carbon steel without causing substantial
corrosion of the apparatus. Moreover, this can be achieved
20772 1 ~
while maintaining a satisfactory rate of treatment of the
- waste solution so that the process is commercially attractive.
These advantages are achieved by maintaining the
temperature of the chemical at all times below the
decomposition temperature of the chemical. This can be done
by partially vaporizing the solution under a high vacuum of at
least 16 inches of mercury, thereby producing a vapor and a
liquid residue, after heating the solution by mixing it with
partially recirculated liquid residue heated to a temperature
below the decomposition temperature of the chemical. The
heating of the liquid residue is preferably carried out by
conveying it through tubes passing through an internal space
in a heater heated by a burner producing combustion gases. To
avoid localized overheating of the liquid residue, the residue
is conveyed in a single pass through the internal space in a
direction generally co-current to the direction of flow of the
combustion gases and at such a velocity that even a thin film
of the liquid residue immediately adjacent to inner surfaces
of the tubes is not heated to a temperature above the
decomposition temperature of the chemical. Moreover, the
tubes carrying the hottest part of the liquid residue are
preferably shielded from radiant heat from the burner(s) in
order to reduce the risk of localized overheating even
further.
Whether the solution is merely concentrated or reclaimed
(purified) depends on its temperature and pressure during the
evaporation step. The pressure is governed by the vacuum
created in the apparatus and, while this does not vary
greatly, it is usually a little less when the solution is to
be concentrated than when it is to be reclaimed. The
temperature of the solution when it is subjected to
evaporation depends on the temperature of the feed solution,
the temperature of the heated liquid residue mixed with the
feed solution, and the ratio in which the heated liquid
residue and feed solution are mixed, since this mixing is the
only source of heating of the feed solution. If the
temperature produced in this way is above the vaporization
. .. ... . .. ..... . . . . .. ...... .. .
- 207721~3
temperature of both water and the chemical at the prevailing
- low pressure, the generated vapor contains both water vapor
and vaporized chemical. This vapour can be partially
condensed to separate a purified aqueous chemical solution
from residual water vapor and this solution is suitable for
re-use for gas treatment. If the temperature is below the
vaporization temperature of the chemical but above the
vaporization temperature of water, the generated vapor is
mainly water vapor and the remaining liquid residue is a
concentrated aqueous solution of the chemical containing the
impurities from the feed solution. This concentrated solution
is removed in part (the part not recycled to the heater for
heating the feed solution) and can be disposed of more easily
and economically than the feed solution itself because of its
reduced volume and increased concentration.
It is stated above that the process can be operated in
apparatus made of carbon steel without causing substantial
corrosion. By this, we mean that the rate of corrosion is low
enough that it does not become detrimental to the operational
lifetime of the apparatus.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures la, b and c combine to form a single schematic
flow diagram of a first embodiment of apparatus of the present
invention;
Figure 2 is an end view of a heater used in the apparatus
of Figure 1;
Figure 3 is a cross-sectional view taken along line III-
III of Figure 2;
Figure 4 is a side elevational view of a heater coil used
in the heater of Figures 2 and 3;
Figure 5 is an end view of the heater coil of Figure 4;
Figures 6(a) and 6(b) combine to form a single schematic
flow diagram of a second preferred embodiment of the apparatus
of the present invention;
Figure 7 is a vertical cross-section of a still used in
the apparatus of Figure 6;
Figure 8 is a cross-section of the still of Figure 7
taken on the line VIII-VIII of Figure 7;
.,, . ... ~ . , , . , . ~ ~
20772~8
Figure 9 is a cross-section of the still of Figure 7
taken on the line IX-IX of Figure 7;
Figure 10 is a vertical cross-section of a heater used in
the apparatus of Figure 6; and
Figure 11 is a cross-section of the heater of Figure 10
taken on the line XI-XI.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
For the sake of simplicity, the following detailed
description of preferred embodiments of the method and
apparatus is limited to the treatment of an aqueous
diethanolamine (DEA) solution. It will however be appreciated
that waste aqueous solutions of other gas treating chemicals
may be reclaimed or concentrated in essentially the same way.
FIRST EMBODIMENT (RECLAIMER)
The apparatus of the first embodiment of the invention
includes a gauge tank 1, a feed tank 2, a heater exchanger 3,
a separator 4, a mixer 53, a still 5, a heater 6, a condenser
7, a solvent cooler 8, a filter 9 and a water cooler 10.
A waste solution of DEA is fed into the apparatus under
pressure. The solution enters the gauge tank 1 through an
inlet pipe 12. The tank 1 operates at atmospheric pressure
and at a temperature of 40 - 75 ~F. The tank 1 is intended
to receive waste aqueous DEA feed solution from storage or
from regeneration apparatus and its purpose is to allow the
quantity of the solution to be measured using a liquid level
gauge (not shown). The levels in the tank 1 and the following
feed tank 2 are monitored. When the level in the gauge tank 1
nears the top of the tank, and the level in the feed tank 2 is
low enough to receive the entire contents of the tank 1, the
operator transfers the contents of the tank 1 to tank 2
through lines 13 and 14, pump 16 and valves 17, 18 and 19.
While tanks 1 and 2 are operated on a batch basis in this way,
the solution is fed from tank 2 on a continuous basis to the
remainder of the apparatus and the downstream process operates
continuously. If desired, tanks 1 and 2 may be dispensed with
and feed solution feed continuously to the remainder of the
apparatus.
A line 21 intersects the line 13 upstream of the pump 16
for carrying the contents of the line 13 to a drain header 23
207~218
and a receiver 24. A safety relief valve 25 is provided in
the line 21, and a drain valve 26 is provided in the header
23. An outlet duct 27 with a valve 28 is provided in the line
14 downstream of the valve 18, permitting the withdrawal of
samples for analysis.
During start-up operations, the valve 19 remains closed,
and a valve 29 in a pipe 30 is opened to feed solution to the
still 5. As noted above, feed from the tank 1 to the tank 2
is initiated manually when the operator observes that the
level in the tank 2 has fallen to a level where it can accept
fresh change of solution from the tank 1.
The tank 2 operates at atmospheric pressure and at a
temperature of 40 - 75~F. No pump is required for feeding
solution downstream from the tank 2, because the other
elements operate under a high vacuum of 16 to 28 inches of
mercury, and flow is caused by pressure differentials between
the tank 2 and such downstream elements. The feed tank 2
prevents surging, ensuring a constant flow rate in the
apparatus. Flow from the tank 2 passes through line 31 and a
one-way valve 32, and is controlled by a manually operated
valve 34. A bypass line 35 with a valve 36 extends from the
line 31 to the line 13 for draining the contents of the tank 2
through the lines 13 and 21, and the drain header 23. A line
38 with a safety relief valve 39 also connects the start-up
line 30 to the drain header 23. A bypass 41 with a valve 42
connects the line 30 to the line 38 for manually effecting
draining of the line 30 when the apparatus is shut down or the
line 30 is not in use. The line 31 can be connected to a
nitrogen purge header 43 by a line 44 and a valve 45.
Feed solution flowing from the tank 2 through the line 31
to the heat exchanger 3 enters the bottom of the latter. The
feed solution enters the tube side of the heat exchanger 3,
which includes a plurality of tubes 46 extending between inlet
and outlet chambers 47 and 48, respectively. During passage
through the tubes 46, the solution is heated by product vapors
flowing countercurrent through the shell or casing of the heat
exchanger 3. The product vapors flow from the still 5 via
line 49 to the top end of the heat exchanger 3, and are
discharged therefrom through pipe 50 to the separator 4.
2077218
The solution flowing through the tubes 46 is heated to
approximately 127~F and partially vaporized and is discharged
from the outlet chamber 48 through a line 51 and a valve 52 to
mixer 53. The vapors from the still 5 are cooled to
approximately 133~F during passage through the heat exchanger
3 and partially condensed. The controlled condensation of the
DEA solution, facilitates the removal of water and light
impurities from the solution. Moreover, the heat exchanger 3
recovers heat which otherwise would have to be added to the
system, and such heat would later have to be removed by water
cooling at additional expense. It should be noted, however,
that heat exchanger 3 could be entirely eliminated, if
desired, without changing the basic operation of this
embodiment of the invention since the preheating and partial
condensation thereby achieved are not essential process steps.
The valve 52, which is manually actuated, controls the degree
of condensation and product composition.
The partially vaporized feed solution from the heat
exchanger 3 flows through the line 51 and the valve 52 to the
mixer 53 where the solution is mixed with waste bottoms
(unvaporized liquid residue) from the still 5. Such liquid
residue is discharged through an outlet duct 60, a pump 61, a
one-way valve 62, a valve 64 and a line 66 to the heater 6. A
bypass 68 with a valve 69 therein is used in the event of a
shutdown to drain the coil 70 in the heater 6 into the still
5. A line 72 with a solenoid operated valve 73 is connected
to the line 66 and to the nitrogen purge header ~3. The valve
73 is normally closed during operation of the apparatus. In
the event of a power failure, a spring (not shown) in the
valve opens the latter to connect the line 66 to the purge
header 43, thus blowing the contents of the heater 6 into the
still 5. The liquid residue passes through the heater 6 and
are returned to the mixer 53 via a line 75.
During start-up, the still 5 is charged with solution
entering through the line 30, a one-way valve 76 and a control
valve 77. A hose connection 79 is provided in the line 30 for
admitting nitrogen for purging and flushing the system.
Caustic soda and anti-foaming agents, if desired, are added
to the recirculated liquid residue immediately prior to the
2~77~i8
mixer S3. The caustic soda and anti-foaming agent are fed
from containers 81 and 82 via lines 83 and 84, manually
operated valves 85 and 86 and lines 30 and 75 into the mixer
53.
The liquid residue entering the mixer 53 is approximately
5% to 15% DEA and less than 1% water, the remainder being
heavy impurities. The liquid residue is heated to no higher
than 360~F in the heater 6. The liquid residue flows to the
mixer 53, which is used to heat and thus complete the
vaporization of the DEA solution and vapors from the heat
exchanger 3. This is effected during an extremely short
residence time and with thorough mixing of the various
ingredients. The mixer 53 has a tangential liquid residue
entry to a main feed pipe. Mixing vanes or baffles in the
main pipe may be used to ensure thorough mixing of the
streams. The feed for the still 5 is changed quickly from a
state with the purified gas treating chemical partially
vaporized to a fully vaporized state in order to avoid
degradation. As noted above, caustic soda may be added to
diethanolamine feedstock to free the amine, which may be held
in a heat stable salt, and to raise the pH to above 8.0 for
reducing corrosion. An antifoaming agent may also be added as
required to eliminate foam in the still 5. The quantity of
heat and the resulting outlet temperature of the DEA from the
mixer 53 are controlled by adjusting the quantity of feed to
the process. This outlet temperature of the DEA from the
mixer is the most important single variable in the apparatus.
The still 5 receives the mixture produced in the mixer 53
through line 87, and separates such mixture into a vapor which
is returned to the heat exchanger 3 for partial condensation,
and liquid residue which constitutes waste. Essentially all
of the liquid residue is recirculated through the heater 6 and
returned to the mixer 53. The still 5 is designed to separate
vapor and liquid. The mixture from the mixer 53 is fed
through a "wiping" type entrance which promotes vapor/liquid
separation by centrifugal force followed by a low velocity
section with sufficient residence time that promotes good
vapor/liquid separation. The still 5 operates with a minimum
liquid level which is normally contained in a boot 88 beneath
2077218
11
the main body of the still casing.
The pump 61 is used to transfer liquid still reside to
the heater 6, and excess liquid residue through line 90,
one-way valve 91 and control valve 92 to waste drums (not
shown). A vent 93 to atmosphere containing a pressure
operated safety valve 94 is provided on the still 5. A hose
connection 95 is provided in the line 90 for purging of the
system. An outlet duct 96 with a valve 97 is also provided in
the line 90 permitting the removal of samples for analysis.
The pump 61 transfers liquid residue from the still 5 under
vacuum to the heater 6 and to disposal, both under pressure.
No control or discharge throttling is provided on the flow
through the pump 61 to ensure that maximum possible flow is
always maintained through the heater 6.
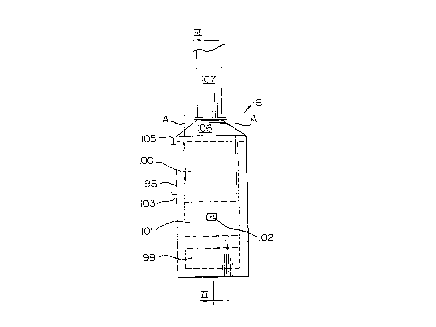
Referring to Figs. 2 and 3, the heater 6 includes a steel
casing 98 lined with refractory material defining a bottom
section or firebox 99 for receiving a burner (not shown) and a
top or coil section 100 for receiving the coil 70 (Figs. 4 and
5). The firebox 99 and the coil section 100 are separated by
a neck portion 101, so that there is no line of sight
visibility between the flame in the firebox 99 and the coil
70. Sight ports 102 are provided in the firebox 99 and in the
neck portion 101 of the casing. A coil inlet opening 103,
i.e. and inlet to the coil 70, is provided above the inclined
wall 104 of the coil section 100, and a coil outlet 105 is
provided near the upwardly tapering top wall 106 of the
casing. A hinged stack 107 carries flue gases from the casing
98. The stack 107 is located at the end of the casing
opposite to the neck portion 101 so that hot gases must flow
across the coil 70 before being exhausted from the heater 6.
The tube bundle defining the coil 70 fills most of the
coil section 100 of the casing 98. The area of the tube
bundle is delineated by broken lines A in Figs. 2 and 3. The
coil 70 (Figs. 4 and 5) defines a serpentine path through the
top section 100 of the casing 98 from a bottom inlet end 108
to a top outlet end 109. The coil 70 includes a plurality of
straight sections 110 interconnected at the ends by 180~
return bends 111. At the end of each horizontal row of
sections 110 a 180~ return bend 112 rises to the next
20~7218
12
superjacent row of sections 110.
The heater 6 heats the liquid residue in a one-pass flow
from an inlet temperature of 330~F to an outlet temperature of
360~F. There is no other heat source for such liquid reside.
At the heater outlet, less than 1% of the feed is vaporized.
Thus, the walls of the heater tubes are continuously covered
with liquid. The heater firebox 99 receives natural gas or
oil from a source thereof via line 114, solenoid valve 115 and
control valve 116. The firebox 99 also acts as a combustion
site for waste gases fed from the bottom of condenser 7 via
line 117, a vacuum pump 118, line 119, one-way valve 120,
solenoid- operated valve 121 and flame arrester 122. The
heater 6 provides the heat input for the apparatus and burns
waste gases from the vacuum pump 118, converting the gases to
less noxious substances. The products of combustion are
discharged via the stack 107.
The design and operation of the heater 6 are important in
order to prevent degradation of the DEA product. The use of a
vacuum in the still 5 permits vaporization of the DEA at a
temperature below that at which decomposition starts.
However, since the liquid residue contain DEA, care must be
taken to ensure that the liquid reside is not subjected to a
temperature of 400~F or more at any point in the heater 6. In
fact, this temperature limitation not only refers to the bulk
fluid temperature within the heater coils, i.e. the
temperature of the fluid at the centreline of the tubing, but
also the temperature immediately adjacent to the walls of the
tubing, ie. the temperature of a thin film of fluid adjacent
to the inner tube walls. Since the heat enters the fluid
through the tube walls, this latter thin film temperature is
always higher than the bulk fluid temperature, and care has to
be taken to ensure that the critical temperature is not
exceeded. This is achieved in the present invention by (a)
passing the liquid reside through the heater tubing at a
suitably high rate of flow (at least six feet per second and
more preferably 7 to 10) so that the residence time close to
any "hot spots" in the tubing is kept low, (b) passing the
liquid reside through the tubing of the heater in a single
pass to avoid possible problems of flow distribution between
2~72-i8
13
passes and to ensure that all parts of the tubing receive a
positive full flow of circulated material, which avoids points
at which the fluid may have a low velocity which may result in
decomposition, and (c) passing the liquid reside through the
tubing in a direction co-current to the combustion gases so
that the liquid residue at their coolest temperature enter the
tubing where the combustion gases are at their highest
temperature and then exits the tubing from the top of the
heater where the combustion gases are at their lowest
temperature. The liquid reside at their highest bulk
temperature are thus exposed only to the combustion gases at
their lowest temperature, further reducing the risk of
overheating by ensuring that the film temperature is raised by
only a minimum above the bulk temperature.
lS By taking these measures, not only can decomposition of
the DEA in the liquid residue be avoided, but the
corrosiveness of the liquid residue can be minimized, since
corrosiveness increases with temperature. It is therefore
possible to use inexpensive carbon steel for the heater tubes
rather than expensive stainless steel.
The quantity of bottom waste flowing to the heater 6 and
the velocity of waste liquid in the heater tubes also ensure
that outlet vaporization is less than 1~, and that the
residence time of waste liquid in the heater 6 is from 30 to
60 seconds. There is no direct heat transfer from the burner
flame to the waste liquid in the heater 6, heating being
effected by convection only. Thus, heat transfer is limited
to approximately 5,000 BTU/h/ft2. There is no direct flame
contact with the heater tubes.
The product vapor separated from the liquid reside in the
still 5 is returned to the product separator 4 (Fig. la) via
the line 49, the heat exchanger 3 and the line 50. Thus,
partially condensed DEA (the product) from the heat exchanger
3 is fed to the separator 4. The liquid, which is the
purified product, is separated from the vapor, which is
essentially all water vapor. The separated liquid is
discharged through a line 123 and pump 124 to a line 125 and
through a one-way valve 126, a control valve 127, a line 128
and a valve 129 for further treatment in the solvent cooler 8
20~7218
(Fig. lc) as described hereinafter in greater detail. The
line 50 is also connected to the line 125 through a
restriction orifice 131, which passes minimum flow for pump 21
through the line 125. Start-up condensate flows through the
valves 126 and 127, a valve 132 and a line 133 to product
water storage (not shown). A line 135 containing a safety
relief valve 136 connects the line 125 to the drain header 23.
The line 125 is also connected through a valve 137 downstream
of the valve 132 and the line 133 to the drain header 23. The
line 133 is also connected by a safety valve 139 to the drain
header 23. The water vapor discharged from the top of the
separator 4 is fed through line 140 to the top of the
condenser 7 (Fig. lc).
The product may be discharged without further treatment
or may be pumped to the cooler 8 under sufficient pressure to
deliver the product to the user. If the cooler is used, the
warm product at 130~F flows to the outer tube of the coaxial
tube, countercurrent flow cooler 8. The cooler 8 reduces the
temperature of the product to a level which is safer to handle
and deliver to the user. The cooled product then passes
through line 142 and, if desired, may be passed through valve
143 into the filter 9, or directly through a valve 145 to a
product discharge line 146. If the filter is used, the
product is discharged from the filter 9 through a line 148 and
a valve 149 to the line 146. Samples for analysis can be
removed from the line 146 through valve 150.
Warm vapor and non-condensibles from the separator 4
enter the top of the condenser 7 via line 140 at a temperature
of approximately 130~F. These substances flow through tubes
152, cooling to approximately 90~F and condensing. Cooling
water for the condenser 7 enters the bottom of the condenser
casing through a line 153, valve 154, line 156, pump 157 and
line 158 connected to a reservoir or tank 160. The tank 160,
lines lS6 and 158, and pump 157 also supply the cooler 8 via
valve 161 and line 162. ~ bypass 164 containing a valve 165
extends between the line 162 and the tank 160. Water passing
through the condenser 7 is discharged through a line 167 and
fed to the water cooler 10. The water cooler 10 includes a
fan 168 and an inlet tube 170 containing spray orifices. The
2~721-8
cooler 10 is connected by line 171 to the water tank 160. A
heater 173 in the tank 160 is used to prevent freezing during
start-up and shut down operations.
Non-condensible gases are separated from condensed water
in the bottom head 175 of the condenser 7, and flow through
line 117 to the vacuum pump 118 (Fig. lb). The condensed
water, after separation in the bottom head 175 flows through
line 177, condensate pump 178, line 179, one-way valve 180 and
control valve 182 to a discharge line 183, which is used to
discharge the condensate to disposal. A sample can be removed
from the discharge line 183 through valve 185 for analysis.
The line 179 is connected to the bottom head 175 of the
condenser 7 by a line 186 and a restriction orifice 187, which
ensures a minimum flow through the pump 178 at all times and
recirculation to suction.
During start-up, start-up condensate is passed through
line 133 from line 123 and the separator 4 through a valve 190
into the downstream end of the condenser system. A hose
connection 192 is provided in the line 133 for connecting such
line to a source of nitrogen for purging and flushing.
The condenser 7 cools and condenses water already removed
from the feed, and separates and removes non-condensible gases
from the condensate. The liquid residue head 175 acts as a
vapor/ liquid separator. Any air leaking into the vacuum
system or gases dissolved in the feed are present at the
outlet of the condenser as non-condensibles. The removal of
such air and gases through the line 106 is the mechanism by
which a high vacuum can be maintained in a major part of the
apparatus. In this connection, it will be noted that there is
a clear path by which to maintain a vacuum between the
condenser 7 via line 140 to the separator 4, via line 50 to
the heat exchanger 3, via line 49 to the still 5, via line 87
to the mixer 53, and via line 51 and the tubes 46 of the heat
exchanger 3.
The non-condensible gases from the condenser 7 flow to
the vacuum pump 118 where the gases are compressed from a
vacuum of approximately 28" of mercury to a slight positive
pressure above atmospheric. The discharge from the vacuum
pump 118 flows through the flame arrester 123 to a special
2077~8
16
burner in the heater 6 where the gases are completely burned
and discharged through the stack 107 to the atmosphere.
Briefly, during normal operation and following start-up
the process utilizing the above-described apparatus includes
the steps of charging the apparatus with chemical to be
reclaimed via the gauge tank 1 and the ~eed tank 2, preheatinq
the chemical in the heat exchanger 3, feeding the partially
vaporized chemical through line 51 to be mixed, if necessary,
with caustic soda and anti-foaming agent and then to the mixer
53 where it is mixed and further vaporized with heated liquid
residue from the still 5 and the heater 6, and mixture thus
produced in the still 5, returning the vapor from the still to
the heat exchanger 3, and separating reclaimed chemical
(product) in the separator 4. The residence time in the mixer
53, i.e. the mixing time is less than one second. An
important step in the process is the heating of the liquid
residue from the still in the heater 6, and using the thus
heated liquid residue to effect separation in the still 5 in a
high vacuum and at a temperature of less than 400~F to avoid
decomposition. There is no direct or other heating of the
still 5 or the contents thereof. It is worth noting that the
liquid residue from the still always contain 5 - 15~ of feed
chemical to ensure fluidity of such liquid residue.
As noted above, an advantage of the process is that it
can be carried out in apparatus made largely or wholly of
carbon steel rather than stainless steel with a large
consequent reduction of capital costs and without significant
corrosion. While certain pre-fabricated components such as
valve seats and mixer vanes would normally still be made of
stainless steel in the apparatus of the present invention,
inexpensive carbon steel could be used for all piping, heat
exchangers, condensers and, most importantly, for the still 5
and heater 6.
SECOND EMBODIMENT (CONCENTRATOR OR RECLAIMER)
A preferred second apparatus according to the present
invention is shown in Figs. 6 to 11. In these Figures, for
the sake of simplicity, elements similar or identical to those
in the previous figures have the same identifying reference
numerals.
2077~18
17
The apparatus shown in Figs. 6(a) and 6(b) is similar in
important ways to that shown in Figs. l(a), l(b) and l(c) but
differs in several material respects. Moreover, the apparatus
is capable of being used for concentrating solutions of waste
s gas treating chemicals as well as reclaiming them for re-use.
Solutions concentrated in this way cannot be re-used for
removing impurities from natural gas because they still
contain the impurities which made them ineffective for this
purpose in the first place, but the concentrated solutions can
10 be disposed of more easily and inexpensively than the dilute
waste aqueous solutions forming the feed to the apparatus.
The operation of the apparatus as a concentrator will be
described first.
FIRST CONCENTRATOR MODE OF OPERATION
A waste aqueous feed solution of the gas treating
chemical normally having a temperature of about 80~F is drawn
into the apparatus via inlet pipe 300 (Fig. 6(b)) by charge
pump 301 and is fed to mixer 53 via pipe 302, mixer 53 being a
static mixer having fixed internal vanes. Prior to entering
20 the mixer 53, however, the feed solution is mixed with heated
liquid from heater 6 at the junction of pipes 302 and 303.
The heated liquid is thoroughly mixed with the feed solution
in the mixer 53 and heats the feed solution without raising
its temperature above the decomposition temperature of the gas
25 treating chemical (400~F), as in the first embodiment
described earlier.
The heated mixed solution from the mixer 53 is conveyed
via pipe 304 to still 5. The still differs in design from
that shown in Figs. l(a), l(b) and l(c) and is more fully
30 described below with reference to Figs. 7 to 9. In the still
5, which operates under a vacuum of 16 to 28 inches of mercury
and preferably at 23-24 inches of mercury when the apparatus
is being operated as a concentrator, the mixed solution from
the mixer 53 is separated into a vapor component, which is
35 mainly water vapor, and a concentrated, impurity-containing,
solution of the gas treating chemical forming a liquid
residue. This differs from the separation taking place in
still 5 of the first embodiment where a vapor comprising water
and gas treating chemical is produced and separated from a
207~218
18
waste liquid residue containing some gas treating chemical and
impurities. The reason for this difference is that the
mixture from mixer 53 in this embodiment, when operated as a
concentrator, is at a temperature below the boiling point of
the gas treating chemical at the indicated pressure, but above
the boiling point of water. In the first embodiment, the
temperature was above the boiling point of both the gas
treating chemical and water at the prevailing pressure. The
temperature is of course governed by the respective
temperatures, pressures and rates of flow of the liquids
passing through pipes 302 and 303. Since the temperatures of
these liquids remain substantially constant, the temperature
of the mixture entering still 5 is controlled largely by the
position of valve 306 in pipe 302.
The unvaporized liquid residue of the gas treating
chemical exits the still 5 through pipe 307 and is circulated
by recirculation pump 308 to heater 6 via pipe 309 where it is
heated and delivered to pipe 303. The heater 6 may be the
same as the heater used in the first embodiment, but is
preferably a modified heater of the kind described in more
detail below with reference to Figs. 10 and 11.
As the process proceeds, the amount of liquid residue
from still 5 continuously increases and the excess is diverted
through pipe 400 according to the position of control valve
401, this valve being controlled automatically (as indicated
by the dashed line) according to the level of liquid residue
in the still 5 in order to keep the level between minimum and
maximum limits. The liquid residue passes through three-way
gate valve 404 to pipe 403 and from there to pipe 421. The
subsequent flow from pipe 421 will be explained more fully
later.
As in the first embodiment described earlier, caustic
soda solution (preferably a 20% solution) from tank 405 and a
solution of an anti-foaming agent from tank 406 may be added
to the mixture introduced into the still 5 from mixer 53, if
desired.
Vapor produced in still 5 is removed via pipe 410, which
communicates with the upper part of the still, and is conveyed
to a lower section of a wash column 415 containing a packing
- 19 207721 ~
medium 416, preferably Glitsch structured packing or
equivalent. Condensate (liquid water) is introduced into an
upper part of the wash column 415 from pipe 416 and trickles
downwardly through the packed medium 415a in contact with the
rising vapor from still 5. This creates a multi-stage
refluxing action between the condensate and the vapor, which
has the effect of condensing any small amount of gas treating
chemical vapor contained in the vapor from the still and
dissolving it in the solution draining to the bottom of the
wash column 415. Consequently, vapor emerging at the top of
the wash column is virtually pure water vapor, which then
exits the wash column 415 through pipe 417. The solution
collecting at the bottom of the wash column exits via pipe 418
and is pumped by pump 419 to cooler 420 via pipe 421, valve A
being open and valves B and C (in pipe 465) being closed. In
cooler 420, the solution is cooled by cooling water conveyed
to and from the cooler via pipes 422 and 423, respectively.
Prior to entering the cooler 420, however, the solution
is mixed with the excess liquid residue, i.e. concentrated gas
treating chemical solution, from pipe 403 as explained above.
The cooled solution leaving cooler 420 is discharged from
the apparatus via pipe 424 for disposal as partially
concentrated waste.
SECOND CONCENTRATOR MODE OF OPERATION
As an alternative to the above flow pattern, valve A may
be closed and valves B and C opened. This means that the
dilute solution from wash column 415 passes through pipe 418
to pipe 421, but is diverted via pipe 465 to mix with the
waste solution feed in pipe 302 and is thus recirculated to
the mixer 53 and still 5. Cooler 420 now receives only excess
liquid residue from still 5 via pipes 307, 309, 400, 403 and
the part of line 421 downstream of valve A. This allows an
even more concentrated solution to be discharged through pipe
424 without requiring higher temperatures in the still 5.
GENERAL CONCENTRATOR OPERATION IN BOTH MODES
The water vapor leaving the wash column 415 via pipe 417
passes through a cooler 425, supplied with cooling water via
pipes 426 and 427, to cool the vapor to a temperature below
its boiling point. The cooled vapor/condensate then passes to
~,
20772 1 ~
vapor/liquid separator 4 which collects the condensate in the
lower section of the separator, from where it is extracted by
condensate pump 428 via pipe 429. The condensate passes
through pipe 430 and is discharged from the apparatus via pipe
431 as virtually pure water which can be discharged without
risk of causing pollution. The discharge of the condensate is
automatically controlled by control valve 432 which is
operated automatically (as indicated by the dashed line)
according to the level of condensate in the separator 4 in
order to ensure that the condensate in separator 4 is always
maintained between minimum and maximum levels. However, pipe
416 communicates with pipe 430 so that some of the condensate
is diverted and delivered to wash column 415, as explained
above, according to the position of gate valve 433.
Gases remaining uncondensed in separator 4, which may
include uncondensed water vapor, decomposition gases or air
which has leaked into the apparatus, are withdrawn from the
top of the separator via pipes 435 and 436 under the action of
vacuum pump 437, which provides the vacuum for the entire
apparatus. Since the uncondensed gases may include noxious
substances, they are conveyed to heater 6 via pipe 438 for
destruction of any chemical components within the flames from
burners in the heater and are then discharged through heater
stack 439. A flame arrester 440 is provided in pipe 438 near
the heater 6 to prevent backdraft and explosion in the pipe.
The cooling water used for heat extraction in coolers 420
and 425 is conveyed from the coolers via pipes 423 and 427,
respectively, and pipe 449 to cooling tower 450 where the heat
is exchanged with cooling air circulated over cooling tubes by
fan 451. The cooled water is collected in surge tank 452 and
any shortfall is made up by water from pipe 453. The cooling
water from the surge tank is extracted by through pipe 454 by
cooling water pump 455 and delivered via pipe 456 to the
coolers 425 and 420 via pipes 426 and 422, respectively.
A fuel, such as natural gas or oil, is fed to heater 6
via pipe 460 for the operation of burners within the heater to
effect heating of the concentrated solution from the still 5
as explained above.
When the apparatus is operated as a concentrator, the
2077218
21
input waste aqueous solution of gas treating chemical is
separated into a concentrated, impurity-containing solution
discharged through pipe 424 and substantially pure water
discharged through pipe 431.
The pure water discharged from the apparatus meets
current standards regarding C.O.D. and B.O.D. and can thus be
discharged into a river, lake or sea without causing
pollution, and the concentrated solution can be disposed of as
a hazardous chemical.
RECLAIMER MODE OF OPERATION
When the apparatus of Figs. 6(a) and 6(b) is to be used
for reclaiming waste aqueous solutions of gas treating
chemicals rather than concentrating them in the manner
described above, the temperature of the liquids entering the
still 5 from the mixer 53 is raised above the boiling point of
both the gas treating chemical and water at the pressure
within the still (which is preferably decreased to a vacuum of
about 28 inches of mercury) by reducing the input rate of the
feed solution through pipes 300 and 302. The vapor leaving
the still via pipe 410 then contains both water and gas
treating chemical. In the wash column 415, the gas treating
chemical is separated from much of the remaining water vapor
and a fairly concentrated purified solution of gas treating
chemical emerges via pipe 418 and is pumped by pump 419 to
line 421 for cooling in cooler 420 and discharge through line
424.
The liquid residue in the still 5 contains impurities and
a small amount of gas treating chemical to decrease the
viscosity, as in the first embodiment described earlier.
Since the amount of liquid produced in the still is reduced,
only a small amount is removed through pipe 400. Valve 404 is
positioned so that all of this residue enters pipe 402 rather
than 403 and is discharged from the apparatus. The apparatus
functions in essentially the same way as the apparatus of
Figs. l(a), l(b) and l(c), except for the lack of preheating
of the feed solution, so further detailed description is
believed to be unnecessary.
As mentioned above, in the apparatus shown in Figs. 6(a)
and 6(b), the still 5 has been modified in design compared to
20~72~8
the still shown in Fig. 1, although the function remains
essentially the same. The modified still is shown in greater
detail in Figs. 7, 8 and 9.
The still 5, instead of being in the form of a
horizontally-arranged cylinder provided with a liquid
reservoir or boot 88, is in the form of a vertically-arranged
cylinder 560 provided with an inwardly tapering conical lower
section S61 providing an inwardly and downwardly tapering
inner conical surface area. The still has a tangential inlet
562 for receiving mixed feed solution and heated liquid from
mixer 53 via pipe 304. The still is also provided with a
vapor outlet 563 for connection to pipe 410 and a liquid
outlet 564 for connection to pipe 307.
The tangential arrangement of inlet 562 causes the mixed
feed solution and heated liquid from mixer 53 to follow a
generally spiral path around the inner surface of the
cylindrical and conical sections 560 and 561 as gravity
attracts the liquid towards the liquid residue outlet 564.
This helps to permit the mixture to separate quickly into
vapor and liquid components by ensuring that the mixture is
"wiped" by centrifugal force over a considerable area of the
inner still wall.
The conical lower section 561 collects the unvaporized
liquid residue around the liquid outlet 564 and, because of
the conical design, provides a minimum of liquid volume or
residence time within the still, thus minimizing the
possibility of degradation or settling of solids that may be
present, while allowing a sufficient workable liquid level for
control. Moreover, the tangential positioning of the inlet
562 and the conical design of the lower section 561 ensures
that the entering liquid flows at high velocities in a
circular and downward direction. This down-flowing liquid has
a flow pattern which ensures that any solids present are
washed down to the outlet 564 and thus avoid the potential
adhesion and accumulation of such solids on the internal walls
of the still.
At the extreme lower end of the conical section 561, a
vortex breaker element 565 is provided immediately ahead of
the outlet 564. This element is formed by a pair of flat
23 - 207721 ~
plates intersecting each other at right angles. These plates
prevent circular motion of the fluid adjacent to the outlet to
prevent gaseous components from being drawn into the outlet
tubing 307 which is the suction of the circulating pump 308.
Towards the upper end of the still 5, a semi-circular
baffle plate 566 projects from the inner cylindrical wall of
the still. This prevents the liquid in the mixture from inlet
562 from moving to the upper part of the still. Vapor outlet
563 is connected to an internal pipe 567 having an inlet 568
facing upper end wall 569 of the still. This arrangement
forces vapor separated from the mixture in the lower part of
the still to follow a reverse flow path at the inlet 568,
causing any liquid entrained in the vapor to impact and adhere
to the inner surface of the wall 569, thus effectively
removing the liquid from the vapor and allowing it to return
eventually to the lower outlet 564.
It should be noted that, although the still 5 is shown as
part of the apparatus of the second embodiment, it could also
be used in the apparatus of the first embodiment.
The preferred heater 6 of Fig. 6, which also could be
used in the apparatus of Fig. 1, if desired, is shown in
detail in Figs. 10 and 11. The heater 6 comprises a steel
casing 580 lined with refractory material, e.g. firebrick
backed with a castable refractory. As shown, the housing has
an enlarged body 581 and a narrow neck joined by a sloping
shoulder section 583. One or more, and preferably a pair of
burners 584 are positioned centrally within the lower part of
the body portion 581. These burners may be fired, for
example, by natural gas or oil.
Positioned around the walls of the enlarged portion 581
are interconnected tubes forming tube coils 585 and 586.
Further interconnected tubes are provided in the shoulder
region 583 and the neck region 582 above the burners 584 to
form a further coil 587.
Coil 585 receives liquid residue from still 5 via inlet
588 and moves upwardly through the tubes in the direction of
the arrows. The liquid then exits the heater via outlet 589
and transported by insulated tubing outside the heater to coil
586 via inlet 590 where again the liquid rises through the
2~772~8
24
coil in the direction of the arrows. The liquid again exits
the heater via outlet 591 and are transported outside the
heater to reenter via inlet 592 to the coil 587. The liquid
rises through the coil in the direction of the arrows and
finally exit the heater through outlet 593 for transportation
to the mixer 53.
As in the case of heater 6 described in connection with
Figs. 1 to 5, the heater shown in Figs. 10 and ll is the sole
heat source for the entire apparatus of Fig. 6, and it must
again provide heat in such a way that the liquid does not
undergo decomposition reactions, which degrade the gas
treating chemical, nor generate corrosive conditions which
might quickly corrode and destroy the carbon steel tubes used
to form coils 585, 586 and 587.
The tubes forming coils 585, 586 and 587 are of such a
diameter and total length that, given the capacity of the
still recirculation pump 61 and the burners 584, the liquid
flows through the tubes so quickly that temperatures in the
thin liquid film immediately adjacent to the tube walls do not
exceed 400~F, but the liquid residue nevertheless picks up
sufficient heat to reach a bulk (centre line) temperature at
outlet 593 of about 360~F. Advantageously, the fluid flow
through the tubes is at least seven feet per second.
It will also be appreciated that the liquid residue
passes completely through the heater in a single pass since
the tubing is not divided into two or more parallel flow paths
and later recombined. The one-pass flow ensures that there
can be no problem of flow distribution between parallel paths
and that all points of the tubes receive a positive full flow
of circulated liquid residue. This avoids the development of
points at which the liquid has a low velocity which could
result in overheating and thus decomposition and/or corrosion.
It will also be noted that the circulated liquid residue
flows generally co-current to the combustion gases (the latter
being indicated by arrow 595). This means that the inlet
liquid at its coolest enters the heater via inlet 588 in the
radiant combustion zone A of the heater indicated generally by
dotted line 596. The liquid residue heated in this way are
then circulated to coil 586 while still relatively cool again
-
25 20772 1 8
to be introduced into the radiant heating zone A. The heated
liquid residue then pass to coil 587 positioned fully within
the convection heating zone B, where heating is less rapid and
intense, and they finally exit the heater 6 at the top of the
heater via outlet 593 where the combustion gases are at their
lowest temperature. This arrangement ensures that the liquid
residue, when at its highest bulk temperature, is exposed only
to the lowest temperature combustion gases so that the maximum
tube wall film temperature is raised by a minimum above the
bulk temperature.
In order to reduce the potential for high heat transfer
and resulting high film temperature in those tubes at the
front of the entrance to the convection section which are
fully exposed to the radiant transfer from the combustion
zone, a heat resistant baffle 300 (made, for example, of
stainless steel) is provided beneath those tubes to prevent
them from receiving the full direct radiation from the visible
flame.
These features make it possible to construct the tubes of
coils 585, 586 and 587 from carbon steel, as stated, because
the temperature of the liquid residue never gets so high that
corrosion becomes a serious problem.
The use of finned tubes for the top three rows of tubes
in the convection section B (as shown) provides the benefits
of obtaining high energy efficiency while reducing the exit
temperature of the combustion gases to a level where the use
of carbon steel without refractory lining for the combustion
gas breaching and stack (not shown) becomes both practical and
safe.
It will be appreciated that the apparatus of Fig. 6 to
11, and especially the still 5 and heater 6, may be made
substantially entirely of carbon steel and that it may be used
either to reclaim waste aqueous gas treating solutions or to
concentrate them for more economical disposal while producing,
as a by-product, water of such purity that it can be discarded
in any convenient manner.