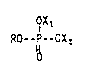Note: Descriptions are shown in the official language in which they were submitted.
P ~ /US9l/01622
W 0,~1/14693 ~ 2 ~ 7 8 ~ 4 2
NOVEL SURFACTANTS AND SURFACTANT COMPOSITIONS
TECHNICAL FIELD
The present invention relates to novel low skin irritation
surfactants and surfactant compositlons wh k h are high in foaming
properties containing, as an active ingredient, a diamine monoalkyl
phosphate salt.
BACKGROUND OF THE INVENTION
Soaps, detergents and abrasives have long been used to cleanse
the sktn on various portions of the body. An~onic surfactants such as
alkyl sulfates, polyoxyethylene alkyl sulfates, alkylbenzene sulfon-
ates and ~-olefin sulfonates have been widely used as the surfactant
component for many cleansers.
It is known that such anionic surfactants are adsorbed and
thereby remain on the s~in surface to cause dryness and scaling of the
epiderm1s, or sktn chapping and roughness, if they are used continual-
ly. Thus, skin troubles such as roughness of hands are apt to be
caused by the use of detergents. Skin roughntss can be a precursor of
more severe skin troubles such as eczema. Thus, there is an urgent
need to eliminate this dlsadvantage.
Nonionic surfactants, on the other hand, cause llttle or no skin
dryness or roughness. However, they do not h~ve the same advantageous
properties of foa~ing and detergency as anionic surfactants. It has,
therefore, not been suitable to incorpor-te nonionic surfactants into
cleansing compos1tions as the primary detergent component.
Phosphortc ~ctd esters have been known as anionic surfactants,
gen~rally as mono- and diesters, or mi%tures thereof. However, in
gener~l, thetr water solubilities and foaming properties are not very
goot.
European Patent No. 265 702 published May 4, 1988 discloses
transparent or semi-transptrent cosmetic composittons containing a
monoalkyl phosphate, water, oil and alcohol for skin treatment. The
oils and alcohols are used to obtain clarity. U.S. Patent 4,573,749
to McIntosh, issued June 28, 1988 discloses monoamine alkyl phosphates
having antimicrobial activity. U.S. Patent 4,290,904 to Poper et al.
issued September 22, 1981, U.K. Patent No. 1,513,053, published June
7, 1978 and European Patent 023 978, published February 18, 1981
disclose cosmetic and toiletry preparations containing alkoxylatea
w o 91/14693 2 Q 7 8 ~ ~ ~ 2 - P ~ /US9l~016 ~alkyl~ne dl~ ~nes. U.S. Patent ~,~76,043, ~,~76,0~ and 4,47 5 to
O'L~ntck, all issued October 9, 198~ and U.S. Patent 4,477,372 to
O'Len~ck, issued October 16, 198~ disclose substantially non-aqueous
surfactants containing an organic sulfate, mineral oil and an alkox~l-
ated amine. I' .leve~, none of these references disclose the use of adiamine alkyl phosphate surfactant.
Monoalkyl phosph~te salts h~ve also been disclosed as useful
surfactants in U.S. Patent 4,139,~85 to Imokawa et al., issued Febru-
ary 13, 19~9. However, these surfactants form turbid aqueous solu-
tions unless they contain other solvents (e.g., solubilizing agents).Further, the rheology of these salts limits the variety of formula-
tions which can be made with aesthet k ally pleasing characteristics.
It has been discovered that certain novel diamine monoalkyl
phosphate salts as well as certain transparent and stable surfactant
compositions containing these salts readily enable the preparation of
stable non-pressurized, aerated foams. These foams possess good
cleansing power and detergency, are mild and non-irritating, and leave
little, if any, residual film rem~ining on the cleansed surface of the
skin. These compositions are homo3eneous in nature, can be structured
to be wet or dry, st~ble or fastbretking, and are aesthetical7y suit-
able for use in a w1de varlety of personal care products.
One of the prtncip~l purposes of this invent~on is to describe
the preparation of an aqueous skin cleansing composition made from the
novel diamine monoalkyl phosphate salts of the present invention which
will prodùce ~ usable foa~ from a hand-held, squeezable foam dispens-
ing device, as well as from an aerosol device and further which will
not render the d~vtcc unusable by, for ex~mple, clogging the device.
Another ob~ect of this invention is to provide surfactants and
foa~-produclng surfactant compositions wh~ch produce relatively stable
or collapsible fou~s. A preferred embod1ment of th1s invention is to
provide sk1n cleansing foams of low density.
Another object of this invention is to provide surfactants and
foaming composit~ons w~th creamy lather, good skin feel and good
rinsability and which retains skin elasticity.
Another ob~ect of this invention is to provide a surfactant and
surfactant composition which is mild and non-irritating to skin and
eyes and which does not require the use of propellants, thereby
avoiding the danqer of explosion or corrosion of the containerO
W O 91/lq693 3 P¢~r/ussl/o~6~ ~8~4
Still another object of this invention is to provide a foam
produclng co~position such that if placed in a hand-held, foam dis-
pensing device having deformable walls, the amount of force required
to produce the foa~ is not excessive (i.e., less than about 15 psi)
and is readily usable by the average person.
These ob~ectives as well as others apparent to those skilled in
the art are obtained with the compounds and compositions described
herein.
SUMMARY OF THE IHVENTION
This invention comprises diamine monoalkyl phosphate salts having
low irritation effect to human skin of the formula:
OX~
RO--P--0% 2
o
wherein R is a hy~rophobic group or the condensation product of a
hydrophobic group wtth ethylene oxide, preferably R is alkyl or
alkenyl having an average of from about 10 to 18 carbon atoms, and
wherein Xl and X2 are independently selected from the group consisting
of hydro~en, alkall metal, ammonium, substituted ammonium (e.g.,
alkoxylated ammonium, alkylammonium, alkoxylated allphattc amines,
polyethoxylated amines) and alkylene diamine, provided that at least
one of X! and X is a tQtally hydroxyalkylated alkyl2r,e d~amine.
Also disclosed are compositions comprising these diamine monoalkyl
phosphate salts.
Sesquialkyl phosphate salts are not within the scope of the
present invent1on.
All parts, percentages and ratios used herein are by weight and
all measure~ents are at 25'C unless otherwise indicated.
DETAILED DESCRIPTION OF T~E INVENTION
The surfactant compounds of the present invention are of the
formula:
OX~
RO--P- -OX2
o
wherein R is a hydrophobic group or the condensation product of a
hydrophobic group with ethylene oxide, preferably R is alkyl or
alkenyl having an average of from about lO to 18 carbon atoms, and
wherein Xl and X2 are independently selected from the group consisting
of hydrogen, alkali metal, ammonium, substituted ammonium (e.g.,
W O 91/14693 2 Q 7 ~ 4 4 ~ 4- P~/US91/01622
alkoxyl~ted a~ontum, alkylammon~um, alkoxylated al1phatic ~lnes,
polyethoxyl~ted amines) and alkylene diamine, provided that at least
one of X1 and X2 is a totally hydroxy alkylatedalkylene diamine.
Monoalkyl phosphate salts in the present invention can be pre-
pared, for example, by a known process wherein a long chain al iphatic
alcohol (or the condensation product of a long chain aliphatic alcohol
with ethylene oxide) is reacted with a phosphatizing agent such as
phosphoric anhydride or phosphorus oxychloride. It is recognized that
the dialkyl phosphate can be by-produced by this process and which
possess poor water-solubility or foaming properties. Such dialkyl
phosphates have the formula:
OX3
RO--P--OR
a
wherein R is a hyd~ophobtc ~roup or the condensation product of a
hydrophob1c group with ethylene oxide, preferably R is alkyl or
alkenyl having an a~erage of from about lO to 18 carbon atoms, and
wherein X3 iS selected from the group consisting of hydrogen, al kal i
metal, ammonium, substituted ammonium (e.g., alkoxylated amm,onium,
alkylammonium, alkoxyl~ted aliphatic amines, polyethoxylated amines)
and alkylene diamine. Preferably, R is the condensation product of a
hydrophob~c group w~th from about 1 to about 10 moles and preferably
from about 1 to about 4 moles of ethylene oxide.
Preferably, the weight ratio of the diamine monoalkyl phosphate
salt to the dialkyl phosphate salt is from about 99:1 to about 70:30,
respectively, preferably from about 100:0 to about 9O:10, most prefer-
red is a r~t~o of sùbstantially 100:0. However, unlike monoamine,
alk~li, or a~monium phosphate salts, these diamine phosphates of the
diester are unlquely water-soluble and, therefore, will not interfere
as much ~ith the devlces and formulations described herein.
The surfactants of the present invention are particularly useful
in detergent products whlch are directly contacted with the skin for a
long time such as facial cleansers, shampoos, and solid synthetic
detergent toilet bars, because they have a characteristic, excellent
foaming power and skin roughness is not caused. The surfactants can
also be used as ingredients of dish-washing liquid detergents, powder
detergents and dentifrices and are particularly useful compositions
where a clear and non-turbid composition is desirable.
~ ~ ~ Pt~r/US9l/01622
W 0 91/14693 ~ 2 ~ 7 8 ~ ~ 2
The satur~ted and unsaturated hydroc~rbon groups having an
aver~ge carbon number of 10-18 (R) are straight chain, branched or
al~cyclic hydrocarbons such as decyl, undecyl, dodecyl, tridecyl,
tetradecyl, pentadecyl, hexadecyl, heptadecyl, and octadecyl grou~s
and correspondtng olefinicallY unsaturated groups. Those hydrocarbon
groups are contained in the compositions singly or in the form of a
combinat~on of several groups. 5aturated hydrocarbon groups of an
average carbon number of 10 to 14 and unsaturated hydrocarbon groups
of an average carbon number of 16 are particularly preferred. Also
preferred for use herein are the saturated or unsaturated hydrocarbon
groups wherein said hydrocarbon groups possess from about 1 to about
lO units and preferably from about l to about 4 units of ethylene
oxide per molecule.
The preferred alkali metals for Xl and %2 according to the
invent~on, are, for example, lithium, sodium and potassium.
The alkylammonium or substituted alkylammonium for Xl and X2
according to the inventlon, are cations produced from amines used for
neutralization of the corresponding phosphoric acids by salt formation
after the neutralizatlon step in the process for preparing monoalkyl
phosphate salts ~n the present invention. The corresponding amines
are prtmary, secondary and tertiary amines having alkyl qroups of 1 to
4 ~arbon atoms whieh ean be further substttuted, part kularly by
hydroxyl groups. As the amines, there can be mentioned, for example,
dtmethylmonoethanola~ine, methyldiethanolamine, trimethylamine,
trtethylamine, dibutylamine~ butyld~methylamine, monoethanolamine.
diethanolamine, triethanolamine, isopropyldimethylamine and isopropyl-
ethanol~ne. Preferred am~nes are monoethanolamine, diethanolamine
and triethanola~ine. A particularly preferred amine is triethanol-
a~ne. Other us~ful amines include arg~nine, lysine, mono-, di- or
triisopropanolamine, N,N-tris(hydroxymethyl)aminomethane, diglycol-
amine, glucamine, am~nomethylpropanol, and aminomethylpropanediol.
Also useful in the present invention are the copolymers derived from
the addition of ethylene oxide and propylene oxide available as
Tetronic~ and Tetronic R~ available from BASF Corporation and the
3~ polyethoxylated amines available as the Ethomeen~ series available
from Armak Corporation.
W o 91/14693 2 0 7 8 ~ ~ 2 6 P ~ IUS91/01622Th~ alkylene diamtne, according to the present invention ~ the
general for~ula:
RlR2N--R3--NR,R~,
in wh kh Rl, R2, R~ and R5 are independently hydrogen, an alkyl group
having 1 to 4 carbon atoms or a hydroxylalkyl or dihydroxyalkyl group
having l to 4 carbon atoms or R, and R5 jointly form a saturated 5 or
6 membered heterocycltc ring, which can be substltuted by an oxo- or
hydroxyalkyl group and R3 represents an alkylene or hydroxyalkylene
group having 2 to 4 carbon atoms, provided that at least one of R1,
R2, R3, R~ and R~ contain at least one hydroxy group.
These compounds are fully disclosed in U.K. Patent 1,513,053,
published June 7, 1978.
A part kular am~ne class that is useful herein is an N,N-tetrakis
(hydroxyalkyl) ethylene diamine having the formula:
I5 R R
~HO--CH--CH2]n ~ ,~CH2--CHOH]n
N--R~--N
[HO--cH--cH2]n ~ ~cH2--cHoH3n
R R
wherein R1 is alkylene having two to four carbon atoms, R is hydrogen
or an alkyl group having one to six carbon atoms and n is from one to
four. The foregoing diamine preferably has a mQlecUlar weight cf
under about 1700, preférably under about 1200, even more preferably
under about 800 and most preferably under about 500.
Although other examples w~ll also be given hereinafter, the
tetrakls (hydroxyalkyl) ethylene diamine is best exemplified by the
compound N,N,N',H'-tetrakis(2-hydroxypropyl)-ethylenedlamine, obtain-
able co~merc1ally under the trademark Quadrol or Neutrol ~E (both
ava11able from BASF ~yandotte Company).
Examples of diamine monoalkyl phosphate salts useful in the
present invention include, but are not limited to,
tetrahydroxypropylethylenediamine monococoylphosphate.
tetrahydroxypropylethylenediamine monolaurylphosphate,
tetrahydroxypropylethylenediamine (monoethoxylated)
monolaurylphosphate,
tetrahydroxypropylethylenediamine monoisostearylphosphate,
tetrahydroxypropylethylenediamine monostearylphosphate,
tetrahydroxypropylethylenediamine (monoethoxylated)
monostearylphosphate,
i` ` PCI/US91/01622
tetrakydt~x~.opylethylenediamine monomyrtstylphosph~te, 8 ~ ~ 2
tetrahyJ~oxy~ropylethylenediamine (monoethoxylated)
monomyristglphosphate,
tetrahydroxypropylethylenediamine monooleoylphosphate,
tetrahydroxypropylethylenediamine monopalmiltylphosphate,
tetrahydroxypropylethylenediamine (monoethoxylated)
monopalmiltylphosphate,
tetrahydroxypropylethylenediamine monocaprylphosphate,
tetrahydroxypropylethylenediamine (monoethoxylated)
monocaprylphosphtte,
tetrahydroxypropylethylenediamine monoundecylenylphosphate,
tetrahydroxypropylethylenediamine monotridecylenylphosphate,
tetrahydroxypropylethylened~amine mono(methylmyristyl)phosphate,
tetrahydroxypropylethylenediamine mono(isopropyllauryl)phosphate,
tetrahydroxypropylethylenediamine mono(isodecylneo~entyl)phosphate,
tetrahydroxypropylethylenediamine monocetylphosphate, and
tetrahydroxypropylethylenediamine mono(isopropylmyristyl)phosphate.
COMPOSITIONS
The compositions of the present invention compr~se:
(a~ from about 0.1% to about 9gX of one or more of a diamine
monoalkyl phosphate salt of the present invention; and
~b~ from abou~ 1.Q% to ahout 9g.~% o~ ~ detergent carrier.
Preferred compositions comprise a mixture of the diamine mono-
alkyl phosphate salts wherein R is selected from th~ group consisting
of C12 to C~ alkyl or alkenyl and C1~ to C1~ alkyl or alkenyl (or the
condensation porduct of these moieties with from about 1 to about 10
moles of ethylene ox1te) and wherein said salts are present in a ratio
of from about 80:20 to about 20:80, preferably from about 60:40 to
about ~0:60 and most preferably from about 55:45 to about 45:55,
respecttvely.
The compositions of the present invention can be administered
topically, i.e., by the direct laying on or spreading of the composi-
tion on epidermal or epithelial tissue. Such compositions can be
formulated as foams, lotions, creams, ointments, solutions, gels or
sol~ds. A highly preferred composition contains the monoalkyl phos-
phate salt in a soap màtrix (i.e., liquid and solid). These topical
compositions comprise a safe and effective amount, usually from about
0.1% to about 99%, and preferably from about 1% to about 10%, of the
W O 91/14693 2 ~ 7 ~ ~ 4 ~ 8 - P~/US91/016Z2
mono~lkyl phQsphate salt. Bar composit1Ons gener-lly conta ~ very
htgh levels i.e., above 80%, of the d~amine mono~lkyl phosphate salt.
Generally, the carrler is either or~anic in nature or a solution or an
aqueous emulsion and is capable of having the diamine monoalkyl
5 phosphate salt dispersed. dtssolved or suspended therein. The carrier
can include pharmaceutically-acceptable emollients, skin penetration
enhancers, coloring agents, fragrances, emulsifiers, thickening
agents, and solvents. A more detailed description of such forms
follows:
I0 I. Lotions.
The lot~ons can comprise a safe and effective amount of the
monoalkyl phosphate salt; from about 0.1% to about 25%, preferably
from about 3% to about 15%, of an emollient; the b~lance being water,
a C2 or C3 alcohol, or a mixture of water and the alcohol. Examples
I5 of suitable emollients are as follows:
I. Hydrocarbon oils and waxes. Examples are mineral oil,
petrolatum, paraffin, ceresin, ozokerite, microcrystalline wax,
polyethylene, and perhydrosqualane.
2. Sllicone oils, such as polydimethylsiloxanes, methylphenyl-
polysiloxanes, water-soluble and alcohol-soluble silicone~glycol
copolymers.
3. Trtglycer~de fats ant oils, such ~s those derived from
vegetable, antmal and marine sources. Examples include castor oil,
safflower oil, cotton seed oil, corn oil, olive oil, cod liver oil,
almond oil, avocado oil, palm oil, sesame oil, and soybean oil.
4. Acetoglyceride esters, such as acetylated monoglycerides.
5. Ethoxylated glycerides, such as ethoxylated glyceryl mono-
stearate.
6. Alkyl esters of fatty acids having I0 to 20 carbon atoms.
Methyl, lsopropyl and butyl esters of fatty acids are especially
useful herein. Examples include hexyl laurate, isohexyl laurate.
isohexyl palmltate, isopropyl palmitate, decyl oleate, isodecyl
oleate, hexadecyl stearate, decyl stearate, isopropyl isostearate,
diisopropyl adipate, diisohexyl adipate, dihexyldecyl adipate, diiso-
propyl sebacate, lauryl lactate, myristyl lactate, and cetyl lactate.
7. Alkenyl esters of fatty acids having I0 to 20 carbon atoms.Examples thereof include oleyl myristate, oleyl stearate, and oleyl
oleate.
. P ~ /US91/01622
W O 91/14693 9 2 ~ 2
8. Fatty ac1ds having 9 to 22 carbon atoms. Suitable examples
include pelargonic. laurtc, myristtc, palmttic, stearic, isostearic,
hyJ.o~ysteartc, oleic, linoleic, ricinoleic, arachtdonic, behenic, and
eructc actds.
9. Fatty alcohols having 10 to 22 carbon atoms. Lauryl, myris-
tyl, cetyl, hexadecyl, stearyl, isostearyl, hydroxystearyl, oleyl,
ricinoleyl, behenyl, erucyl, and 2-octyl dodecyl alcohols are examples
of suitable fatty alcohols.
10. Fatty alcohol ethers. Ethoxylated fatty alcohols of 10 to 20
carbon atoms include the lauryl, cetyl, stearyl, ~sostearyl, oleyl,
and cholesterol alcohols having attached thereto from 1 to 50 ethylene
oxide groups or 1 to 50 propylene oxide groups, or a mt%ture thereof.
11. Ether-esters such as fatty acid esters of ethoxylated fatty
alcohols.
12. Lanolin and tts dertvatives. Lanolin, lanolin oil, lanolin
wax, lanolin alcohQls~ lanolin fatty acids, isopropyl lanolate,
ethoxylated lanolin, ethoxylated lanolin alcohols, ethoxylated choles-
terol, propoxylated lanol~n alcohols, acetylated lanolin, acetylated
lanoltn alcohols, lanoltn alcohols linoleate, lanolin alcohols
rtcinoleate, acetate of lanolin alcohols rictnoleate, acetate of
ethoxylated alcohols-esters, hydrogenolysis of lanolin, ethoxylated
hyd?ogenated lanc~, ethoxy~ated sorbttol lanolin, and liquid and
semisoltd lanoltn absorption bases are illustrative of emollients
dertved from lanoltn.
13. Polyhydric alcohols and polyether derivatives. Propylene
glycol, dtpropylene glycol, polypropylene glycol (M.~. 2000-4000).
polyoxyethylene potyoxypropylene glycols, polyoxypropylene polyoxy-
ethylene glycols, glycerol, ethoxylated glycerol, propoxylated gly-
cerol, sorb~tol, ethoxylated sorbitol, hydroxypropyl sorbitol, poly-
ethylene glycol (M.~. 200-6000), methoxy polyethylene glycols, poly-
tethylene oxide~ homopolymers (M.W. 100,000-5,000,000), polyalkylene
glycols and derivatives, hexylene glycol (2-methyl-2,4-pentanediol),
1,3-butylene glycol, 1,2,6-hexanetriol, ethohexadiol USP (2-ethyl-
1,3-hexanediol), Cl5-CI~ vicinal glycol, and polyoxypropylene deriva-
tives of trimethylolpropane are examples thereof.
14. Polyhydric alcohol esters. Ethylene glycol mono- and
di-fatty acid esters, diethylene glycol mono- and di-fatty acid
esters, polyethylene glycol (M.~. 200-6000) mono- and di-fatty acid
esters, propylene glycol mono- and di-fatty acid esters, polypropylene
W o 91/14~93 2 0 7 ~ o- P ~ /US91/01622
glycol 2000 monooleate, polypropylene glycol 2000 monos rate,
ethoxylated propylene propylene glycol monostearate, glyceryl mono-
and d~-fatty acid esters, polyglycerol poly-fatty acid esters, eth-
oxylated gylceryl monostearate, 1,3-butylene glycol monosteara~e,
1,3-butylene glycol distearate, polyoxyethylene polyol fatty acid
ester, sorbitan fatty acid esters, and polyoxyethylene sorbitan fatty
acid esters are satisfactory polyhydric alcohol esters.
15. Wax esters, such as beeswax, spermaceti, myristyl myristate,
stearyl stearate.
16. Beeswax derivatives, e.g., polyoxyethylene sorbitol beeswax.
These are reaction products of beeswax with ethoxylated sorbitol of
varying ethylene oxide content, forming a mlxture of ether-esters.
1~. Vegetable waxes including carnauba and candelilla waxes.
18. Phospholipids, such as lecithin and derivatives.
19. Sterols. Cholesterol, cholesterol fatty acid esters are
examples thereof.
20. Amides, such as fatty acid amides, ethoxylated fatty acid
amides, solid fatty acid alkanolamides.
The lotions further comprise from about 1% to about 10%, prefera-
bly from about 2% to about 5%, of an emulsifier. The emulslfiers can
be nonionic, anionic or cattonic. Examples of satisfactory nonionic
emul~tfiers ~noiude ratty acid alcohols hav~ng 10 to 20 carbon atoms,
fatty alcohols hav~ng 10 to 20 carbon atoms condensed with 2 to 20
moles of ethylene oxide or propylene oxide, alkyl phenols with 6 to 12
carbon atoms in the alkyl chain condensed with 2 to 20 moles of
ethylene oxide, mono- and di-fatty acid esters of ethylene oxide,
mono- and di-fatty ac1d esters of ethylene glycol wherein the fatty
ac1d moiety cont~tns from 10 to 20 carbon atoms, diethylene glycol.
polyethylene glycols of molecular weight 200 to 6000, propylene
glycols of molecular weight 200 to 3000, glycerol, sorbitol, sorbitan,
polyoxyethylene sorbltol, polyoxyethylene sorbitan and hydrophilic wax
esters. Suitable anionic emulsifiers include the fatty acid soaps.
e.g. sodium, potassium and triethanolamine soaps, wherein the fatty
acid moiety contains from 10 to 20 carbon atoms. Other suitable
anionic emulsiflers include the akali metal, ammonium or substituted
ammonium alkyl sulfates, alkyl arylsulfonates, and alkyl ethoxy ether
sulfonates having 10 to 30 carbon atoms in the alkyl moiety. The
alkyl ethoxy ether sulfonates contain from 1 to 50 ethylene oxlde
PC~r/US91/01622
W O gl/14693 2078442
_ units. Ho~ever, it is recognized that certain anion1c emulsifiers can
result in a turbid formulatiOn~ and hence, anionic emulsifiers are
- less preferred for use herein.
The ba~an~e of the lotion is water or a C2 or C~ alcohol, or a
- 5 mixture of water and the alcohol. The lotions are formulated by
simply admtxing all of the components together. Preferably the
monoalkyl phosphate salt is dissolved in the mixture. Conventional
optional components can be included. One such additive is a thicken-
ing agent at a level from about ll~ to about 10% of the composition.
Examples of suitable thic~ening agents include: cross-linked carboxy-
polymethylene polymers (sufficiently neutralized), magnesium aluminum
si1lcate, car~oxymethylcellulose, hydroxyethylcellulose, acrylic acid
polymers (e.g., Acrysol~ ICS-I, ava~lable from Rohm ~ Haas Corpora
t~on), polyethylene glycols, gum tragacanth, gum kharaya, xanthan gums
and bentonite. Cation1c polymers, such as cationic guar gum, are not
pr~ferred for use herein.
2. Creams.
Compositions of the present invention also can be formulated in a
cream form. The crea~s comprise safe and effective amounts of the
monoalkyl phosphate salt; from about 0.1% to g5~/0, preferably from
about 10% to about 2~%, of an emollient; the balance being water. The
e~71~erts ~bove d~seribed can aiso be used in the cream compositions.
Optionally, the cre~m form contains a su1table emulsifier, as previ-
ously described. When an emulsifier ls included, it is is the compo-
sition at a level from about 3D/~ to about 50~/~, preferably from about 57.
to a~out 20~.
3. Solut1ons.
The co~poslt10ns of this invention can also be formulated as a
solut10n. The solution form comprises a safe and effective amount of
3Q the mano~lkyl phosphate salt, usually at least about O.Ol~/o up to about
50% and preferably about 0.1% to about lO~; the balance being water or
a suitable organic solvent. Suitable organic materials useful as the
solvent or a part of a solvent system are as follows: propylene
glycol, polyethylene glycol (M.~. 2~0-1000), polypropylene ~lycol
(M.~. 425-2025), butylene glycol, glycerine, sorbitol esters, l,2,6-
hexanetriol, ethanol, isopropanol, diethyl tartrate, butanediol, and
mixtures thereof.
t, ~
2 0 7 8 4 4 2 l2 PC~r~US91/016~
W O 91/14693
~These solutions can be applied to the skin as is~ or else can be
- for~ul~ted into, for ex~mple, sque~7e devices descrtbed below or as an
aerosol and sprayed onto the s~in from an aerosol container, or as a
mouth~ash composition and used as an oral rinse. The aerosol composi-
tions further comprise from about 25% to about 80%~ preferably from
about 30% to about 54~, of suitable propellants. Examples of such
propellants are the chlorinated, fluorinated and chlorofluorinated
lower molecular weight hydrocarbons. Nitrous oxide, carbon dioxide,
butane, and propane can also be used as propellant gases. These
propellants are used at a leYel sufficient to expel the contents of
the container.
Squeeze foamer packages are well known as exemplified by the disclosures of
the following patents: U.S. Pat. Nos. 3,709,437, Wright, issued Jan. 9, 1973; 3,937,364,
lSWright, issued Feb. 10, 1976; 4,022,351, Wright, issued May 10, 1977; 4,147,306,
Bennett, issued Apr. 3, 1979; 4,184,615, Wright, issued Jan. 22, 1980; 4,598,8~2, Rice,
issued July 8, 1986; and 4,615,467, Grogan et al., issued Oct. 7, 1986; and French Pat.
2,604,622, Verhulst, published Apr. 8, 1988.
The above containers (packages) do not use any propellant and are
therefore safe for the consumer and the enYironmænt. They create a
foam from almost any surfactant compos1tion. Altho~gh there is no
need to add foa~ boosters merely for the purpose of stabil izing the
foam, such materials can be desirable. In some compositions the use
of foam boosters can eYen be counterproductive since the foam has to
2~ break in order for the container to work properly. The composition is
placed in the cont~tner reservoir (plastic squee~e bottle~. Squee ing
the Containe! wtth the hand forces the composition through a foamer
head, or other foam producing means, where the composition is mixed
with air and then through a homogenizing means that makes the foam
more homogeneous and controls the consistency of the foam. The foam
is then discharged as a uniform, non-pressurizéd aerated foam.
The minimum pressure to activate the squeeze foamer is about 1
psig, typically from abo~t 2 psig to about 7 psig. The m i nimum
pressure is related to the size of the channels in the dispenser, the
viscosity of the composition, etc.
In general, the density of the foam should be between about 0.002
and about 0.25 g/cc, preferably between about 0.01 and about 0.12
g~cc, ànd more preferably between about 0.02 and about 0.07 g/cc.
W o 91/14693 2 0 7 8 4 4 2 -13- P ~ /US91/0162Z
The c~rrter liquld in a ~outh~sh is generally a mixture of
eth~nol and ~ater. The amount of ethanol is generally from about 5%
to a~out 6~X, preferably from about 5% to about 25X by weight of the
carrier. ~ater then constltutes the remainder of the carrier liquid
5 mixture. These ~outhwash compositions can also contain other optional
components such as emulsifying agents as preYiously descrtbed, flavor-
ing agents, sweeteners. and humectants. Other mouthwash formulations
and methods for making mouthwashes useful in the present invention are
dtsclosed in U.S. Patent 4,323,5~1 to Parran, issued April 6, lg82.
4. Gels.
Compositions herein can be formulated into a gel form by simply
admixing a suitable thickening agent to the previously described
solutton compositions. ~xamples of suitab~e thickening agents have
been preYiously described with respect to the lottons.
The gelled compositions comprise a safe and effective amount of
the monGalkyl phosphate salt, fro~ about 0.5X to about 2~%, prefera~ly
fro~ about lX to about l~X, of the thlc~ening agent; the balance being
water.
5. Solids.
The compositions of th~s invention can also be formulated in a
solid form1 etg.7 2 stic~-type c~pasit,on. Sucn compositions com-
prise a safe and effect he amount of the monoa1kyl phosphate salt and
from about 0.01% to about 9gX, preferably from about 60X to about 90%,
of the prev10usly described emollients. This composition can further
comprise from about O.lX to about 20X, preferably from about 5% to
about l~X, of a sultable thie~ening agent, and opttonally emulsifiers
and ~ater. Th~c~ening agents previously described with respect to
lotions ar~ als~ suitable herein.
6. So~ps.
The co~positions of this in~ention can also be formulated into a
liquid or solid (e.g., bar) soap matrix. Such compositions comprise a
safe and effectiYe amount of the monoalkyl phosphate salt ranging from
abaut 0.1% to about 99%; and from about 1% to about ggX of an excipi-
ent such as those previously described. Optionally, the soap contains
a suitable emulsifier as previously described. ~hen an emulsifier is
included, it is in the composition at a leYel from abuut 10% to about
5~X.
,:
.
PC~r/US9l/016Z~
w 0 9l/14693
7. OentifrtcPs. 207~442
The c~oosittons of thts inventlon can also be formulated as
dentifric~s. Such dentifrices, espectally toothpaste, comprise a safe
and effective a~ount of the monoalkyl phosphate salt ranging frcm
about 0.1% to about 20X by weight of the composition. Toothpaste
compositions conventic-ally contain abrasive materials, sudsing
agents, binders, humectants, flavoring and sweetening agents.
Suitable dent1frice compositions and the methods of their manufacture
useful in the present invention are fully set forth in U.S. Patent
3,535,4Zl to Briner et al., issued October 20, 1970.
-
8. Shampoos.
Composit10ns of this invention also can be formulated in ashampoo for~. The shampoos comprise a safe and effective amount of
lS the dia~ine monoalkyl phosphate salt ranging from about 0.1% to about
%; from about 5% to about 6~% of a synthetic surfactant; and the
balance ~te~. A secondary surfactant can also ~e utilized, however,
such secondary surfactant shoùld be neutralized by a diamine as
descrtbed above.
These shampoos can contain a variety of nonessential optional
components. Such conventional optional ingredients are we71 known to
those skilled in the art, e.g., preservatives, such as benzyl alcohol,
ethyl paraben, propy7 paraben and imidazolidinyl urea; cationic
surfactants, such as cetyl trimethyl a~on1u~ chloride, stearyldi-
methyl benzyl a~oniu~ chloride, and di(partially hydrogenated tallow)
dimethylam~oniu~ chloride; thickeners and viscosity modifiers such as
diethanolamide of a long-chain fatty acid (e.g. PE~ 3 lauramide),
block poly~srs of eth~lene oxide and propylene oxide, sodium chloride.
sodium sulfate, polyvinyl alcohol, and ethyl alcohol; pH adjustina
agents, such as citric acid, succinic acid, phosphoric acid, sodium
carbonate; perfu~es; dyes; and, sequestering agents, such as disodium
ethylenediamine tetraacetate. Such a~ents generally are used indivi-
dually at a level of from about 0.01% to about 1C%, preferablY from
about O.5% t~ ~bout 5.0% by weight of the composition.
'S Additional minor components can be added to the compositions o;
this invention in order to increase their attractiveness, versatility
and shelf-life. Perfumes and water soluble, pharmaceutically acce~t-
able dyes or food colors can be added to enhance the attractiveness of
W o 91/14693 2 0 7 8 4 4 2 -15- PCT/US~1/0162~
these co~posttions. Antifungal and anttmtcrobtal agents are useful in
preYenttng ~old or bacterial contamtnatton and in increastng the
- shelf-life of the composttlons. Conventional anttbacterial agents can
be included in the present compositions at levels of from about 0.1,.
to about 4%, prefe~ably from about 0.2X to about 1%. Typical antibac-
terial agents whtch are suitable for use herein are ~,4-di- and
3,~',5-tribromosaltcylanildes; 4,4'-dichloro-3-(trifluoromethyl)car-
banilide; 3,4,4'-trichlorocarbanilide; phenoxy ethanol or propanol;
chlorhexidene salts; hexamidine salts; Irgasan~ DP 300 (Triclosan);
salicylic acid; parachlorometaxylenol; Octopirox, and mixtures of
these materials. For general purposes skin cleansing compositions
having a pH range from about 5.0 to about 8.0 are desirable. rf
necessary, the pH of these composittons can be adjusted downward using
cttric or lacttc acid. For sktn cleansers whtch deal with more
sensittve skin surfaces, such as in vaginal and perianal cleansers, a
pH of about 6.; ts desirable. These and other minor modifications can
be made wtthout materlally altertng or departtng from the bastc
concept of this invention.
The nature of the fo~ produced determtnes the usefulness of the
present compostttons. In order for a foa~ to be useful as a skin
cleansing agent, tt must have a uniform consistency, good spreadabil-
ity, and good cle~nsing ability.
The foa~abtltty and wettabiltty characteristtcs are governed by
the surface tenston of the s~1n cleansing comp~s1tion. The surface
tenston for the composittans of thts invention vartes from about 20 to
70 dynes/cm. For general skin cleansing compositions a range of from
about 23 to about 50 dynes/cm is preferred. Liquid compositions
having a surface tenston in the lower portion of this range possess
gre~t~r spread1ng and better wettinq characteristics with increased
foa~blltty. Foa~tble composttions naving htgher surface tensions
gene~ally provide ~ore stable foams but are also more difficult to
cause to foam and re~uire more force to extrude the foam.
The foam-productng s~tn cleansing compositions of thts invention
are particularly advantageous in that they leave a minimum amount of
surfactant residue on the surface of the skin. This has been achievea
in part by utiltzing a low percentage of total surfactants in the s~in
cleansing composittons itself, and by preparing foams with unusuall~
low density. The present compositions provide foam densities provia~
' ,
16 2 0 ~ a PCT/~'S91/01622
w O 91/14693
good cleansing ab~tity and more importantly, leave a nesligible amount
of surfact~nt residue on the surface of the skin upon rinsing cr
flattening, thereby preserving skin elasticity, and reducing transepi-
- der~al moisture loss.
S The cleansing ability of these aerated foams is a direct function
of the cleansing ability of the surfactant solution itself which
produces the foam.
The following non-limiting examples illustrate embodiments of tne
subject invention wherein both essential and optional ingredients are
combined. It is to be understood that these examples are for illus-
trative purposes only and are not to be construed as limiting the
scope of the lnvention thereto.
EXAMPLE I
1 male of monolauryl phosphate acid is heated over a steam bath
to about 50-C until molten. 1 mole of ~,N,H',N'-tetrakis(2-hydroxy-
propyl)-ethylenediamine (available as Quadrol~ from BASF Corporation)
is heated in a separate beaker to 50~C until pourable.
The ~olten phosphate is slowly added to the H,H,N',N'-tetrakis(2-
hydroxypropyl)-ethylenediamine. The phosphate is added until a pH of
5.0 to 9.0 is reached (preferably 7.0). The resulting N,H,~',N'-
tetrakis(2-hydroxypropyl)-ethylenediamine monolaurylphosphate is then
cooled to for0 a clear. yellow solid.
This solid can be used as a solid bar or as a material to produce
liquid cream or gel product.
z5 Application of approximately O.S grams to the skin will provide a
foaming cleanser possessing good cleansing power and detergency, which
is ~ild and non-irrttating, and leaves little, if any, residual film
re~aintng on the cleansed surface o~ the skin.
Substantially similar results are obtained when the monolauryl
phosphate acid is replaced with an equivalent amount of monococoyl-
phosphate acid, monolaurylphosphate acid, monoisostearylphosphate
acid, monostearylphosphate acid, monomyristylphosphate acid, mono-
oleoylphosphate acid, monopalmitylphosphate acid, monocaprylphosphate
acid, monoundecylenylphosphate acid, monotridecylenylphosphate acid.
,5 mono(methylmyristyl)phosphate acid, mono(isopropyllauryl)phosphate
acid, mono(isodecylneopentyl)phosphate acid, monocetylphosphate aci~.
and mono(isopropylmyristyl)phosphate acid and mixtures thereof.
p~
W O 91/146932 0 7 ~ 4 4 2 -17- PC~r/US9l/0l62
EXAHP~E II
~A factal cleansing compositton for topical administration is
- prepared by combining the fol7Owing ingredients:
In~redtents Percent w/w
H N H H -tetrakis(2-hydroxypropyl)-
ethylenediamine monolaurylphosphate 2.500
Butylene Glycol 2.500
Methylparaben 0.250
Propylparaben 0.l50
iO - Potasstum coco(hydrolysed animal protein)l 0.032
Glycertn 2.000
Aloe Vera 0 500
Fragrance 0 050
Color 0.l70
Xanthan ~um2 o . oso
~ater q.s.
I Available as Lamepon~S from Henkel Corporation
2 Available as Keltrol~T from Kelco Corporatton
EXA~P~E III
20A facial cleansing cream composition for topical administration
is prepared by combtning the following ingredients:
Tn~redtents Per~ent w/w
H N N N -tetrakis(2-hydroxypropyl)-
ethylenediamine monolaurylphosphate 27.000
Laur1c actd 4 0OO
H H H H -tetrakis(2-hydroxypropyl)-
ethylenediamtnel 4 ooo
C~rboxyvinyl polymer2 0.200
Glycertne 8.000
Sorbttol 2.000
~ater q.s.
l Available as Quadrol from BASF-~yandotte
2 Available as Carbopol 941 from B.F. Goodrich Company
3~
W O 91/14693 -18- P ~ /US91/01622
= - - 207~44~ EYAMPLE IV
A facial cleansing composition for topical admtnistration is
prepared by combintng the following ingredients:
Inaredients Percent w/w
H,N,N',N'-tetrakis(2-hydroxypropyl)-
ethylenediamine monolaurylphosphate 3.000
Laurtc actd l.000
Pottssium coco(hydrolysed animal protein) 0.500
Hydroxyethyl cellulose z ooo
Methyl paraben O.lO0
Propyl paraben O.lO0
Glycerin 3.000
Fragrance 0.100
Color O.OOl
~ater q.s.
EXAMPLE V
A factal cleanstng gel composttton for toptcal administration is
prepared by combining the following ingredients:
Inaredtents Percent w/w
N,H,N',N'-tetrak1s(2-hydroxypropyl)-
ethylenediam1ne monolaurylphosphate lO.000
Acryltc acid polymer o.~oo
N,H,N',N'-tetrakis(2-hydroxypropyl)-
ethylened1amine 1.000
Glycertn 5 ooo
Lauric acid 2.000
~ater q.s.
",~ ,
WO 91/14693 1~ PCI`/US91/01622
~ FXAMPLE VI 2078~42
A facial shaving foam composition for topical administration is
prep~red by comblning the following ingredients:
In~re~ientsPercent w/w
N N N N -tetrakis(2-hydroxypropyl)-
ethylenediamine monolaurylphosphate 7.000
N N N N -tetrakis(2-hydroxypropyl)-
ethylenediamine monopalmityl phosphate 7.000
Lauric acid 1.500
Palmityl acid 1.500
N N N N -tetrakis(2-hydroxypropyl)-
ethylenediamine1.500
Fragrance 0.100
Aloe Vera 2.000
Water q.s.
EXAMPLE V~I
A faclal cleanstng composition for topical administration is
prepared by combining the followinq ingredients:
Inqredients Percent w/w
N N N N -tetrdkts(2-hydroxypropyl)-
ethylenediamine (1 mole ethoxylated )
monolaurylphosphate 2.500
Butylene Glycol 2.500
Methylparaben 0.250
Propylparaben 0.150
Potassiu~ coco(hydrolysed animal protein)'0.032
Glycerin 2.000
Aloe Vera 0.500
Fragrance 0.050
Color 0.170
Xanthan gum2 o.oso
Water q.s.
1 Available as Lamepon S from Henkel Corporation
2 Available as Keltrol T from Kelco Corporation
WHAT IS CLAIMED IS: