Note: Descriptions are shown in the official language in which they were submitted.
~~ .i
CA 02078811 2002-12-11
30517-88 , r
The im~ention relates to new sulphonylaminocarbonyl-
triazoliaones having two substituents bonded via oxygen,
to a plurality of processes and new intermediates for
their preparation, and to their use as herbicides.
~ It-has been disclosed that certain substituted sulphonyl-
aminocarbonyltriazolinones .such as, for example, 2-
(2-chloro-phenylsulphonylaminocarbonyl)-4,5-dimethyl-2,4-
dihydro-3H,1,.2,4-triazol-3-one,. have_ herbicidal .
properties (cf. 8P-A 341,489; cf. also 8P-A 422,469, EP-A
425,948 and EP-A 431,291). However, the action of these
compounds is not satisfactory fn all respects.
Sulphonylaminocarbonyltriazolinones having one
substituent which is bonded via oxygen (in the 5
position) are the subject of -a previous, but non-prior
published Patent Application (cf. DE-A 41 107 95)..
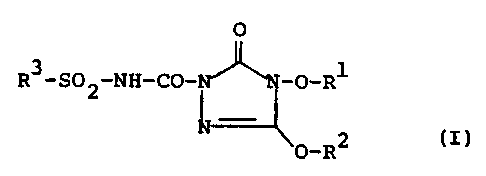
There have now been found the new sulphonylaminocarbonyl-
triazolinones having two substituents bonded via oxygen
of the general formula (I)
O
2~ g3~go2~NH_Op~H~N~O~R1 . (I)
~RZ
- 1 -
in which
R1 represents hydrogen or an optionally substituted
radical from the series comprising alkyl, alkenyl,
alkinyl, cycloalkyl, cycloalkenyl and aralkyl,
RZ represents an optionally substituted radical from
the series comprising alkyl, alkenyl, alkinyl,
cycloalkyl, cycloalkenyl and aralkyl, and
R3 represents an optionally substituted radical from
the series comprising alkyl, aralkyl, aryl and
heteroaryl,
and salts of compounds of the formula (I).
The new sulphonylaminocarbonyltriazolinones having two
substituents bonded via oxygen of the general foranula ( I )
are obtained when
(a) triazolinones of the general formula (II)
o
H-N~N-O-RI (II)
~_R2
in which
R1 and RZ have the abovementioned meanings,
Le A 28 679 - 2 -
207~~~.~
are reacted with sulphony:L isocyanates of the
general formula (III)
R3-SO2-N=C=0 ( I I I )
in which
R3 has the abovementioned meaning,
if appropriate in the presence of a diluent, or when
(b) triazolinone derivatives of the general formula (IV)
O
Z-CO--O-R1 (IV)
_R2.
in which
i0 R1 and RZ have the abovementioned meanings and
Z represents halogen, alkoxy, aralkoxy or
aryloxy,
are reacted with sulphonamides of the general
formula (V)
R3-S02-NHz ( V )
in which
Le A 28 679 - 3 -
2~7881.~
R' has the abovementioned meaning,
if appropriate in the pxesence of an acid acceptor
and if appropriate in the presence of a diluent, or
when
(c) triazolinones of the general formula (II)
o
,IiN~N-0-R1 ( I I
N--~ _ RZ
in which
R1 and RZ have the abovementioned meanings,
are reacted with sulphonamide derivatives of the
general foxmula (VI)
R3-SOz-NHf-CO-Z ( VI )
in which
R3 has the abovementioned meaning and
Z represents halogen, alkoxy, aralkoxy or
aryloxy,
if appropriate in the presence of an acid acceptor
and if appropriate in the presence of a diluent,
and, if appropriate, salts are formed with the compounds
Le A 28 679 - 4 -
of the formula (I) prepared by process (a), (b) or (c),
using customary methods.
The new sulphonylaminocarbonyltriazolinones having two
substituents bonded via oxygen of the formula (I) and
their salts are distinguished by a powerful herbicidal
activity.
Surprisingly, the new compounds of the formula (I) show
a considerably bettex herbicidal action than the compound
2-(2-chlorophenylsulphonylaminocarbonyl)-4,5-dimethyl-
2,4-dihydro-3H-1,2,4-triazol-3-one, which is known and
which has a similar structure.
The invention preferably relates to compounds of the
formula (I) in which
R1 represents hydrogen, or represents C1-C6-alkyl which
is optionally substituted by fluorine, chlorine,
bromine, cyano, C1-C4-alkoxy, C1-C,-alkylcarbonyl or
C1-Cd-alkoxy-carbonyl, or represents C3-C6-alkenyl or
C3-C6-alkinyl, each of which is optionally sub-
stituted by fluorine, chlorine and/or bromine, or
represents C3-C,-cycloalkyl or Cd-C,-cycloalkenyl,
each of which is optionally substituted by fluorine,
chlorine, bromine and/or C1-C,,-alkyl, or represents
phenyl-C1-C3-alkyl which is optionally substituted by
fluorine, chlorine, bromine, cyano, nitro, C1-C4-
alkyl, trifluoromethyl, C1-C4-alkoxy and/or
C1-Ce-alkoxy-carbonyl,
Le A 28 679 - 5 -
~fl~88~ ~.
R2 represents C1-C6-alkyl which is optionally sub-
stituted by fluorine, chlorine, bromine, cyano,
C,-C6-cycloalkyl, C1-C6-alkoxy or C1-C,-alkoxy-
carbonyl, or represents C3-C6-alkenyl or C,-C6-
alkinyl, each of which is optionally substituted by
fluorine, chlorine and/or bromine, or represents
C3-C6-cycloalkyl which is optionally substituted by
fluorine, chlorine, bromine and/or C~-C4-alkyl, or
represents cyclohexenyl, or represents phenyl-C1-C3-
alkyl which is optionally substituted by fluorine,
chlorine, bromine, cyano, vitro, Cl-Cd-alkyl, tri-
fluoromethyl, C1-CQ-alkoxy and/or C1-C4-alkoxy-
carbonyl, and
R' represents the group
R5
R4
where
R' and R5 axe identical or different and represent
hydrogen, fluorine, chlorine, bromine, iodine,
vitro, Cl-C6-alkyl (which is optionally sub-
stituted by fluorine, chlorine, bromine, cyano,
carboxyl, C1-C,-alkoxycarbonyl,
C1-C,-alkyl amino-carbonyl , di- ( C,-C,-
alkyl)amino-carbonyl, hydroxyl, C1-C,-alkoxy,
formyloxy, Cz-C,-alkyl-carbonyloxy, C,,-C~-
he A 28 679 - 6 -
2~~8~i~.
alkoxy-carbonyloxy, Cl-CQ-alkylamino-
carbonyloxy, C1-Ca-alkylthio, C1-C,-
alkylsulphinyl, C1-Ca-alkylsulphonyl, di-(C1-C'-
alkyl)-aminosulphonyl, C3-C6-cycloalkyl or
phenyl), or represents C:2-C6-alkenyl (which
is
optionally substituted by fluorine, chlorine,
bromine, cyano, C1-Ca-alkoxy-carbonyl, carboxyl
or phenyl), or represents Cz-C6-alkinyl (which
is optionally substituted by fluorine,
chlorine, bromine, cyano, C1-C,-alkoxy-
carbonyl, carboxyl or phenyl), or represents
C1-Cd-alkoxy (which is optionally substituted
by fluorine, chlorine, bromine, cyano,
carboxyl, C,-Ca-alkoxy-carbonyl, C1-C,-alkoxy,
C1-C,-alkylthio, C1-C,-alkylsulphinyl or C1-C,-
alkylsulphonyl ) , or represents Cl-C,-alkylthio
(which is optionally substituted by fluorine,
chlorine, bromine, cyano, carboxyl, Cl-C,-
alkoxy-carbonyl, C1-C,-alkylthio, C1-C,-
alkylsulphinyl or C1-C,-alkylsulphonyl), or
represents C3-C6-alkenyloxy (which is
optionally substituted by fluorine, chlorine,
bromine, cyano or C1-C,-alkoxy-carbonyl), or
represents CZ-C6-alkenylthio (which is
optionally substituted by fluorine, chlorine,
bromine, cyano, vitro, C1-C~-alkylthio or Cl-Ca-
alkoxycarbonyl), C3-C6-alkinyloxy, C3-C6-
alkinylthio, or the radical -S(0)p-R6 where
p represents the numbers 1 or 2 and
Le A 28 679 - 7 -
R6 represents ci-ca-alkyl (which is optionally
substituted by fluorine, chlorine, bromine,
cyano or C1-Ca-alkoxy-carbonyl ) , C,-C6-alkenyl,
C3-C6-alkinyl, C1-Ca-all;oxy, C1-Ca-alkoxy-C1-Ca-
alkylamino, C,-Ca-alkylamino, di-(C1-Ca-alkyl)-
amino, phenyl, or the radical -NHOR', where
R' represents C,-C,Z-alkyl (which is option-
ally substituted by fluorine, chlorine,
cyano, C1-Ca-alkoxy, C,-Ca-alkylthio, Cl-Ca-
alkylsulphinyl, C,-Ca-alkylsulphonyl,
C1-Ca-alkyl-carbonyl, C,-Ca-alkoxy-
carbonyl, C1-Ca-alkylamino-carbonyl or di-
(C1-Ca-alkyl)-amino-carbonyl), or
represents C,-C6-alkenyl (which is
optionally substituted by fluorine,
chlorine or bromine), C3-C6-alkinyl, C,-C6-
cycloalkyl, C3-C6-cycloalkyl-Cl-Cz-alkyl,
phenyl-Cl-CZ-alkyl (which is optionally
substituted by fluorine, chlorine, nitro,
cyano, C1-Ca-alkyl, C1-Ca-alkoxy or C1-Ca-
alkoxy-carbonyl), or represents
benzhydryl, or represents phenyl (which
is optionally substituted by fluorine,
chlorine, vitro, cyano, C1-Ca-alkyl,
trifluoromethyl, C1-Ca-alkoxy, C1-CZ-
fluoroalkoxy, C1-Ca-alkylthio,
t r i f 1 a o r o m a t h y 1 t h i o o r
Cl-Ca-alkoxy-carbonyl),
be A 28 679 - 8 -
20~8~~.~.
R° and/or R5 furthermore represent phenyl or phenoxy,
or represent C1-C,-alk;ylcarbonylamino, C1-Cd-
alkoxy-carbonylamino, C,-C4-alkylamino-
carbonyl-amino, di-(C1-C,-alkyl)-amino-carbonyl-
amino, or represent the radical -CO-Re, where
Re represents C1-C6-alkyl, C1-C6-alkoxy, C,-C6-
cycloalkoxy, C3-C6-alkenyloxy, C~-CQ-alkyl-
thio, C,-C,-alkylamino, C1-C,-alkoxyamino,
Cl-Cg-alkoxy-Cl-C,-alkyl-amino or di- ( C,-C,-
alkyl)-amino (each of which is optionally
substituted by fluorine and/or chlorine),
R° and R5 furthermore represent trimethylsilyl,
thiazolinyl, C~-Ca-alkylsulphonyloxy, di-(C1-C,-
alkyl)-aminosulphonylamino or represents the
radical -CH=N-R9 where
Rg represents C1-C6-alkyl which is optionally
substituted by fluorine, chlorine, cyano,
carboxyl, C1-C,-alkoxy, C1-Cd-alkylthio,
C1-Ca-alkylsulphinyl or C1-Ca-alkyl-
sulphonyl, or represents benzyl which is
optionally substituted by fluorine or
chlorine, or represents C3-C6-alkenyl or
C3-C6-alkinyl, each of which is optionally
substituted by fluorine or chlorine, or
represents phenyl which is optionally
substituted by fluorine, chlorine,
bromine, C1-Ca-alkyl, C1-Cd-alkoxy,
Le A 28 679 - 9 -
~~~881~
trifluoromethyl, trifluoromethoxy or
trifluoromethylthio, or represents Cl-C6-
alkoxy, C3-C6-alkenoxy, C3-C6-alkinoxy or
benzyloxy, each of which is optionally
substituted by fluorine and/or chlorine,
or represents amino, C1-C,-alkylamino, di-
( C1-C4-alkyl ) -amino, phenylamino, C1-C4-
alkyl-carbonyl-amino, Cz-Ca-alkoxy-
carbonylamino, C1-C4-alkyl-sulphonylamino,
or represents phenylsulphonylamino which
is optionally substituted by fluorine,
chlorine, bromine or methyl,
furthermore
R3 represents the radical
R12
-~ -.
R10 R11
where
R1° represents hydrogen or C1-C4-alkyl,
R'1 and R12 are identical or different and represent
hydrogen, fluorine, chlorine, bromin~, vitro,
cyano, C1-C,-alkyl (which is optionally sub-
stituted by fluorine and/or chlorine), C1-C,-
alkoxy (which is optionally substituted by
Le A 28 679 - 1p -
~078~~.~
fluorine and/or chlorine), carboxyl, C1-Ca-
alkoxy-carbonyl, dimethylaminocarbonyl,
C 1 - C d - a 1 k y 1 s a 1 p h o n y 1 o r
di-(C1-Ca-alkyl)-aminosulphonyl;
furthermore
R3 represents the radical
X13 ~ I ~ 19
v
where
R1' and R" are identical or different and represent
hydrogen, fluorine, chlorine, bromine, nitro,
cyano, C1-Ca-alkyl (which is optionally sub-
stituted by fluorine and/or chlorine) or C1-C,-
alkoxy (which is optionally substituted by
fluorine and/or chlorine);
I5 furthermore
R3 represents the radical
X15
R16
Le A 28 679 - 11 -
~0'~88~.~.
where
R1~ and R16 are identical or different and represent
hydrogen, fluorine, chlorine, bromine, nitro,
cyano, C1-Ca-alkyl (which is optionally sub-
stituted by flu orine and/or chlorine), C1-C,-
alkoxy (which is optionally substituted by
fluorine and/or chlorine), or represent C1-Cd-
alkylthio, C1-C4-alkylsulphinyl or C1-Ca-
alkylsul phonyl (which are optionally
substituted by fluorine and/or chlorine), or
represent aminosulphonyl, mono-(C1-C4-alkyl)-
aminosulphonyl, or represent di-(C1-Ca-alkyl)-
aminosulphonyl or C1-Cd-alkoxycarbonyl or
dimethylaminocarbonyl;
furthermore
R3 represents the radical
R17 ~ ( R18
_NNJ~~Jw
where
R1' and RlB are identical or different and represent
hydrogen, fluorine, chlorine, bromine, Cl-C4-
alkyl (which is optionally substituted by
fluorine and/or bromine), C1-C6-alkoxy (which
Le A 28 679 - 12 -
2~7881~
is optionally substituted by fluorine and/or
chlorine), or represent C1-Ca-alkylthio, C1-C4-
alkylsulphinyl or C1-C"-alkylsulphonyl (which
are optionally substita;dted by fluorine and/or
chlorine), or represent di-(C1-C4-alkyl)-amino-
sulphonyl;
furthermore
R3 represents the radical
R~~
__~~R20
A
where
R'9 and R2° are identical or different and represent
hydrogen, fluorine, chlorine, bromine, cyano,
vitro, C1-Ca-alkyl (which is optionally sub-
stituted by fluorine and/or chlorine), C,-Cd-
alkoxy (which is optionally 'substituted by
fluorine and/or chlorine), C1-CQ-alkylthio,
C1-C4-alkylsulphinyl or Cl-Cd-alkylsulphonyl
(which is optionally substituted by fluorine
and/or chlorine), di-(Cl-Ca-alkyl)-amino-
sulphonyl, C1-C,-alkoxy-carbonyl or dimethyl-
aminocarbonyl, and
Le A 28 679 - 13 -
2(~°~8~~,~.
A represents oxygen, sulphur or the group N-Z1,
where
Zz represents hydrogen, C1-C4-alkyl (which is
optionally substituted by fluorine,
chlorine, bromine or cyano), C3-C6-cyclo
alkyl, benzyl, phenyl (which is
optionally substituted by fluorine,
chlorine, bromine or vitro),
C~-C,-alkylcarbonyl, C,-Cd-alkoxy-carbonyl
or di-(C1-Cd-alkyl)-aminocarbonyl;
furthermore
R' represents the radical
R21
~22
~J ~~1
where
RZ1 and R22 are identical or different and represent
hydrogen, C1-C,-alkyl, halogen, C1-C4-alkoxy-
carbonyl, C1-C,-alkoxy or C1-C,-halogenoalkoxy,
Y1 represents sulphur or the group N-Ra', where
RZ3 represents hydrogen or C1-C,-alkyl;
Le A 28 679 - 14 -
~f~~~8~.~.
furthermore
R' represents the radical
R25
PIN
R25
124
where
RZ° represents hydrogen, C1-C,-alkyl, benzyl,
pyridyl, quinolinyl or phenyl,
RZ5 represents hydrogen, halogen, cyano, vitro,
C1-Cd-alkyl (which is optionally substituted by
fluorine and/or chlorine), C1-C4-alkoxy (which
1~D is optionally substituted by fluorine and/or
chlorine), dioxolanyl or C1-C,~-alkoxy-carbonyl,
and
R26 represents hydrogen, halogen or C1-C,-alkyl;
furthermore
R' represents one of the groups mentioned below,
Le A 28 679 - 15 -
2~7~~1~.
I H3 I I
~N-C~I~i9 : M'~~ CH~CP'3
O
'11
0
Furthermore, the invention preferably relates to sodium
salts, potassium salts, magnesium salts, calcium salts,
ammonium salts, Cl-G,-alkyl-ammonium salts, di-(C1-C,-
alkyl ) .-ammonium salts, tri- ( C1-C,-alkyl ) -ammonium salts ,
CS- or C6-cycloalkyl-ammonium salts and di- ( Cl-CZ-alkyl ) -
benzyl-ammonium salts of compounds of the formula (I) in
which R1, RZ and R3 have the meanings given above as being
preferred.
In particular, the invention relates to compounds of the
formula (I) in which
R1 represents hydrogen, or represents C1-C,-alkyl which
is optionally substituted, by fluorine, cyano,
methoxy or ethoxy, or represents allyl, or re-
presents C3-C6-cycloalkyl, or'represents benzyl,
RZ represents C1-Co-alkyl which is optionally
Le A 28 679 - 16 -
20'~88~.~.
substituted by fluorine and/or chlorine, methoxy or
ethoxy, or represents C3-Cs--alkenyl which is option-
ally substituted by fluorine and/or chlorine, or
represents C3-C6-cycloalkyl_, or represents benzyl
which is optionally substituted by fluorine, chlor-
ine and/or methyl, and
R' represents the group
R5
R~
where
R° represents fluorine, chlorine, bromine, methyl,
trifluoromethyl, methoxy, difluoromethoxy,
trifluoromethoxy, 2-chloro-ethoxy, 2-methoxy-
ethoxy, Cl-C3-alkylthio, C1-C3-alkylsulphinyl,
C1-C3-alkylsulphonyl, dimethylaminosulphonyl,
diethylaminosulphonyl, N-methoxy-N-methylamino-
sulphonyl, methoxyaminosulphonyl, phenyl,
phenoxy or C1-C3-alkoxy-carbonyl, and
R5 represents hydrogen, fluorine, chlorine or
bromine;
furthermore
R' represents the radical
Le A 28 679 - 17
~0?~~~ ~
R11
R12
-C
R10
where
R1° represents hydrogen,
R11 represents fluorine, chlorine, bromine,
methyl, methoxy, difluoromethoxy, tri-
fluoromethoxy, ethoxy, methoxycarbonyl,
ethoxycarbonyl, methylsulphonyl or di-
methylaminosulphonyl and
R12 represents hydrogen;
furthermore
R3 represents the radical
RO-
A
where R represents C1-C4-alkyl, or represents the
radical
Le A 28 679 - 18 -
,i..i i I
CA 02078811 2002-12-11
30517-88 ,
O
RO~
CH3
where R' represents CI-C~-alkyl.
More preferably, Rl is other than hydrogen.
Examples of the compounds according to the invention are
listed in Table 1' below - ~cf. - also the
Preparation Examples.
0
R3_802_NN_CO_~~O_R1
2 (I)
-R
_ 1g -
Table 1: Examples of the compounds of the formula (I)
R1 Rz R3
F
CH3
Cl
~ CH3
COOC~HS
CH3 CzHs
OCHF~
CH3 CHz-CH=CHz
SOZId t CIi3 ) 2
CH3 CH3
COOCH3
CH3 CH3
2
OOCZH~
CH3 CZHS
CH3
Le A 28 679 - 20 -
Z~"~8~~.?
Table 1: (continuation)
Rz Rz Rs
CH3 CzHS
OOCFi3
a
CH3 CZHS
a
CzHS CzHS
CzH5 C3H7
OGP'3
--~a CH3 M _
2
OCHZCHZG1
CzHS
~ CH(CH3)z i ( t9NtGH~)~
Le A 28 679 - 21 -
~07881~.
Table 1: (continuation)
R~ R' R'
CH3 CH ( CH3 ) z ~~~502NH2
SCH(CH3)2
S CH3 CHz-CH=CHZ
502CH3
C2H5 CH3
F
C~HS CH3
5i(CH3)3
CzHs CzHs
OCF3
CzHs C3H~ ~H' .
Z
CH3 CzHS i I ON ( CH3 ) 2
Ze A 28 679 - 22 -
207~~~.~
Table 1: (Continuation)
R' RZ R'
~ Br
CH2--( ) CHs
OCH~CHZ-OCH~
~ CzHs
CH3
i
CHI
S02NfCH~)2
CZHg
OCHFZ
C3H~-n H _
2
COOCH(CH~)a
CH3 CZHS
Cl
Le A 28 679 - 23 -
20'~~$~ ~
Tablo 1: (continuation)
R1 Rz R3
COOC2H6
CH3 CH3 \
F~CtIO
CH3 CzHS
CH3 S-CHz-C=CH
N
0-CH2-CF3
CH3 CzHs
OOCF~3
CzHS
i '
r
OOCHS
CH3
s
Le A 28 679 - 24 -
2~'~881.~
Table 1: (continuation)
~1 ~2 R3
1
CH3 CH3 I
CH3 C3H., r I ON ( CH3 ) 2
ti3C w
CZH~ CZHS J I
0
0CH3
C00CH3
CHZ-CH=CHZ C2H5
H -
2
r
i I
CHZ-CH=CHa CH3 N~ I
CHI
COOCt~i~
CZHS CH3
50~NCIi~ t 0CH3
14 CZHS C2H5
Le A 28 679 - 25 -
2~'~~8~.~
Table 1: (continuation)
R1 Rz R3
CH3
C3H~ CHz-CH=CHz
OCH~
CH3 CH3
C1
CzHs \
CH3
Hr
-~. CZHS
CH3
C1
CzHs CzHS
CHI
CH3 CzHj
SO2
Le A 28 679 - 26 -
20'~~8~ ~.
Table 1: (continuation)
R1 Rz R3
OCHFZ
CH3 C3H~ ~H
2
C3H., CH3
OOCH3
l
OC~1~
C3H~ CH3
OCHZCHZ-C1
C3H~ CzHs
F
C3H~ CzHs
CH3 CZHS _
0
~'3
CH3 CH3
Le A 28 679 - 27 -
Table 1: (continuation)
RI R2 R3
r
CZHS CH3 v s
v
CH3 CzHS .~ I SOaNH2
~\~SOZNH~
CzHa CzHS
-0 CH3 a I S02NH2
CH3 CzHs
-CF2-CP'~C1
OCH2CH2-C1
CzHS CH3
Le A 28 679 - 28 -
~0~~~~ ~.
If, for example, 2,6-difluoro-phenylsulphonyl isocyanate
and 5-ethoxy-4-methoxy-2,4-dihydro-3H-1,2,4-triazol-3-one
are used as starting substances, the course of the
reaction in process (a) according to the invention can be
outlined by the following equation:
F 0
02-NaC=0 ~ H'~N~NiOCH3 >
F ~C2H5
F 0
X02-NH-CO'~.N~Ni0CH3
F CZHS
Tf, for example, 2-methylthio-benzenesulphonamide and
2-chlorocarbonyl-4-ethoxy-5-propyloxy-2,4-dihydro-3H-
1,2,4-triazol-3-one are used as starting substances, the
course of the reaction in process (b) according to the
invention can be outlined by the following equation:
Le A 28 679 - 29 -
scHS o
~SOZ-NHZ + C1-CO"~H~N~'OC2H5 >
-HC1
C3H'
SCH3 0
~OZ-NH-CO~N~N~'OC2H5
~C~H'
If, for example, N-methoxycarbonyl-2-methoxy-benzene-
sulphonamide and 5-methoxy-4-difluoromethoxy-2,4-dihydro-
3H-1,2,4-triazol-3-one are used as starting substances,
the course of the reaction in process ( c ) according to
the invention can be outlined by the following equation:
0
OCH3
~ H~N~N~'OCHF2
(, .l-S02-NH-COOCH3 + ~~
~CHS
OCH3 0
> is ,t-SOZ-NH-CO~N~OCHF2
°HOCH3
N~~OCH3
Le A 28 679 - 30 -
20'~8~1~
Formula (II) provides a general definition of the tri-
azolinones to be used as starting substances in
processes (a) and (c) according to the invention for the
preparation of compounds of the formula (I).
In formula (II), R1 and RZ preferably, or in particular,
have those meanings which have already been mentioned
above in connection with the description of the compounds
of the formula (I) according to the invention as being
preferred, or particularly preferred, for R1 and R2.
Examples of the starting substances of the formula (TI)
are listed in Table 2 below.
Le A 28 679 - 31 -
0
HN~N-0-R1 (II)
~_R2
Table 2: E~tamples of the starting substances of
the formula (II)
R1 R~
H CH,
CH3 CH3
C2H5 CH,
C3H, CH3
l~ CH ( CH3 ) CH3
Ca CH3
CH3 C2H5
CH, C,H,
CH3 CH ( CH3 ) z
CH3 CH2-CH=CHI
CH3 CFi2
CH3 CH2-C=CH
C2H5 C2H5
2~ C3H, CaHS
I;e A 28 679 - 32
20'~~8~.~.
Table 2: (continuation
R1 Ra
CzHs
CHa-CH=CHz CzHs
C3H~
CHa-CH=CHa
Calls CH ( CH3 ) a
C3H~ CH ( CH3 ) z
CHa-CH=CHz C3H~
Calls C3H~
Calls -CHa-C=CH
C3H~ C3H~
Calls
H C,H,
H CH ( CH3 ) a
H -CHa-CH=CHz
H -CHa-C--=CH
H -CH2
Le A 28 679 - 33 -
With the exception of the compounds 4-hydroxy-5-methoxy-
and 4-hydroxy-5-ethoxy-2,4-dihydro-3H-1,2,4-triazol-3-one
[cf. Arch. Pharm. (Weinheim) Vol. 301, p. 827-$29
(1968)], the starting substances of the formula (II) were
hitherto unknown from the literature and are, as new
substances, also a subject of the present patent applica-
tion.
The compounds of the formula (II) are obtained when
4-hydroxy-semicarbazide, of the formula (VII),
H2N-NH-CO-NH-OH (VII)
is reacted with orthocarbonates of the general
formula (VIII)
C(ORZ)4 (VIII)
in which
Rz has the abovementioned meaning,
if appropriate in the presence of a diluent such as, for
example, methanol, ethanol or propanol, at temperatures
between 0°C and 150°C and, if appropriate, the resulting
compounds of the general foranula (IIa)
0
-OH ~ ( I I a
_~
in which
Le A 28 679 - 34 _
2~l'~8821
RZ has the abovementioned meaning,
are reacted with alkylating agents of the general
formula (IX)
R1-X (IX)
in which
X represents chlorine, bromine, iodine, methyl-
sulphonyloxy, trifluoromethylsulphonyloxy, phenyl-
sulphonyloxy, tolylsulphonyloxy, or one of the
groups -0-CO-OR1 or -0-SOZ-OR1 and
Rx has the abovementioned meaning with the exception of
hydrogen,
if appropriate in the presence of a diluent such as, for
example, methanol, ethanol or propanol, and if appro-
priate in the presence of an acid binder such as, for
example, sodium hydroxide, potassium carbonate or tri-
ethylamine, at temperatures between -30°C and +100°C (cf.
the Preparation Examples).
Formula (III) provides a general definition of the
sulphonyl isocyanates furthermore to be used as starting
substances in process (a) according to the invention for
the preparation of compounds of the formula (I).
In formula (III), R' preferably, or in particular, has
the meaning which has already been mentioned above in
connection with the description of the compounds of the
Le A 28 679 - 35
20°~~~1~
formula (I) according to the invention as being pre-
ferred, or particularly preferred, for R3.
Examples of the starting substances of the formula (III)
which may be mentioned ares
2-fluoro-, 2-chloro-, 2-bromo-, 2-methyl-, 2-methoxy-,
2-trifluoromethyl-, 2-difluoro-methoxy-, 2-trifluoro-
methoxy-, 2-methylthio-, 2-ethylthio-, 2-propylthio-, 2-
methylsulphinyl-, 2-methylsulphonyl-, 2-dimethylamino-
sulphonyl-, 2-diethylaminosulphonyl-, 2-(N-methoxy-N-
methyl)-aminosulphonyl-, 2-phenyl-, 2-phenoxy-, 2-
methoxycarbonyl-, 2-ethoxycarbonyl-, 2-propoxycarbonyl-
and 2-isopropoxycarbonyl-phenylsulphonylisocyanate, 2-
fluorp-, 2-chloro-, 2-difluoromethoxy-, 2-trifluorome-
thoxy-, 2-methoxycarbonyl- and 2-ethoxycarbonyl-benzyl-
sulphonyl isocyanate, 2-methoxycarbonyl-3-thienyl-
sulphonyl isocyanate, 4-methoxycarbonyl- and 4-ethoxy-
carbonyl-1-methyl-pyrazol-5-yl-sulphonyl isocyanate.
The sulphonyl isocyanates of the formula (TII) are known
and/or can be prepared by processes known per se (cf.
US Patent Specification 4,127,405, 4,169,719, 4,371,391;
EP-A 7,687, 13,480, 21,641, 23,141, 23,422, 30,139,
35,893, 44,808, 44,809, 48,143, 51,466, 64,322, 70,041,
173,312).
Process (a) according to the invention for the
preparation of the new compounds of the formula (I) is
preferably carried out using diluents. Diluents which are
suitable for this purpose are virtually all inert organic
Le A 28 679 - 36 -
~n~~~l~-
solvents, These preferably include aliphatic and
aromatic, optionally halogenated hydrocarbons such as
pentane, hexane, heptane, cyclohexane, petroleum ether,
benzine, ligroin, benzene, toluene, xylene, methylene
chloride, ethylene chloride, chloroform, carbon tetra-
chloride, chlorobenzene and o-dichlorobenzene, ethers
such as diethyl ether and dibutyl ether, glycol dimethyl
ether and diglycol dimethyl ether, tetrahydrofuran and
dioxane, ketones such as acetone, methyl ethyl ketone,
methyl isopropyl ketone and methyl isobutyl ketone,
esters such as methyl acetate and ethyl acetate, nitriles
such as, for example, acetonitrile and propionitrile,
amides such as, for example, dimethylformamide, dimethyl-
acetamide and N-methyl-pyrrolidone, and also dimethyl
sulphoxide, tetramethylene sulphone and hexamethyl-
phosphoric triamide.
When carrying out process (a) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
temperatures between 0 °C and 150 °C, preferably at
temperatures between 10 °C and 80 °C.
Process (a) according to the invention is generally
carried out under atmospheric pressure.
For carrying out process (a) according to the invention,
between 1 and 3 moles, preferably between 1 and 2 moles,
of sulphonyl isocyanate of the formula (III) is generally
employed per mole of triazolinone of the formula (II).
Le A 28 679 - 37 -
~o~s~~~
The reactants can be combined in any desired sequence.
The reaction mixture is stirred until the reaction is
complete, and the product is isolated by filtration with
suction. In another processing variant, the mixture is
concentrated, and the crude produces, which remains in the
residue, is brought to crystallisation using a suitable
solvent such as, for example, diethyl ether. The product
of the formula (I), which is obtained in crystalline form
in this process, is isolated by filtration with suction.
1.0 Formula (IV) provides a general definition of the tri-
azolinone derivatives to be used as starting substances
in process (b) according to the invention for the prepar-
ation of compounds of the formula (I).
In formula (IV), R' and R~ preferably, or in particular,
have the meanings which have already been mentioned above
in connection with the description of the compounds of
the formula (I) according to the invention as being
preferred, or particularly preferred, for R1 and R2, and
Z preferably represents chlorine, C,-C4-alkoxy, benzyl-
oxy or phenoxy, in particular methoxy or phenoxy.
Rxamples of the starting substances of the formula (IV)
which are possible are the compounds of the formula (IV)
which are to be prepared with the compounds of the
formula (IT) which are listed in Table 2 and phosgene,
methyl chloroformate, benzyl chloroformate, phenyl
chloroformate or diphenyl carbonate.
Le A 28 679 - 38 -
~0~~~1~.
The starting substances of the formula (IV) were hitherto
unknown and, as new substances, also a subject of the
present invention.
The new triazolinone derivatives of the formula (IV) are
obtained when triazolinones of the general formula (II)
0
xx~rr-o-Ri t y a )
-Rz
in which
R1 and R2 have the abovementioned meanings,
are reacted with carbonic acid derivatives of the general
formula (X)
Z-CO-Zq tX)
in which
Z has the abovementioned meaning and
Z1 represents a leaving group such as chlorine, meth-
oxy, benzyloxy or phenoxy,
if appropriate in the presence of a diluent such as, for
example, tetrahydrofuran, and if appropriate in the
presence of an acid acceptor such as, for example, sodium
hydride or potassium tent-butylate, at temperatures
between -20°C and +100°C.
Le A 2~ 679 - 39 -
Formula (V) provides a general definition of the sulphon-
amides furthermore to be used as starting substances in
process (b) according to the invention fox the prepar-
ation of compounds of the formula (I).
In formula (V), R3 preferably, or in particular, has the
meaning which has already been mentioned above in connec-
tion with the description of the compounds of the
formula (I) according to the invention as being pre-
ferred, or particularly preferred, for R~.
Examples of the starting substances of the formula (V)
which may be mentioned are:
2-fluoro-, 2-chloro-, 2-bromo-, 2-methyl-, 2-methoxy-, 2-
trifluoromethyl-, 2-difluoro-methoxy-, 2-trifluoro-
methoxy-, 2-methylthia-, 2-ethylthio-, 2-propylthio-,
2-methylsulphinyl-, 2-methylsulphonyl-, 2-dimethylamino-
sulphonyl-, 2-diethylaminosulphonyl-, 2-(N-methoxy-N-
methyl)-aminosulphonyl-, 2-phenyl-, 2-phenoxy-, 2-
methoxycarbonyl, 2-ethoxycarbonyl-, 2-propoxycarbonyl-
and 2-isopropoxycarbonyl-benzenesulphonamide, 2-fluoro-,
2-chloro-, 2-difluoromethoxy-, 2-trifluoromethoxy-,
2-methoxycarbonyl- and 2-ethoxycarbonyl-phenylmethane-
sulphonamide, 2-methoxycarbonyl-3-thiophenesulphonamide,
4-methoxycarbonyl- and 4-ethoxycarbonyl-1-methyl-
pyrazole-5-sulphonamide.
The sulphonamides of the formula (V) are known andlar can
be prepared by processes known per se (cf. US Patent
Specification 4,127,405, 4,169,719, 4,371,391;
Le A 28 679 - 40 -
~~'~8g~.~
EP-A 7,687, 13,480, 21,641, 2:3,141, 23,422, 30,139,
35,893, 44,808, 44,809, 48,143, 51,466, 64,322, 70,041,
173,312).
Process (b) according to the inaention for the
preparation of the new compounds of the formula (I) is
preferably carried out using dilu~ents. Diluents which are
suitab~.e fox this purpose are all inert organic solvents,
for example those which are indicated above in
process (a) according to the invention.
Acid acceptors which can be employed in process (b)
according to the invention are all acid binders which can
customarily be used for reactions of this type. The
following are preferably suitable: alkali metal hyd-
roxides such as, for example, sodium hydroxide and
potassium hydroxide, alkaline earth metal hydroxides such
as, for example, calcium hydroxide, alkali metal car-
bonates and alkali alcoholates and metal such as sodium
carbonate potassium carbonate, sodium tert-butylate and
potassium tert-butylate, further aliphatic, aromatic or
heterocyclic amines, for example triethylamine, tri-
methylamine, dimethylaniline, dimethylbenzylamine,
pyridine, 1,5-diazabicyclo-[4,3,0]-non-5-ene (DBN), 1,8-
diazabicyclo-[5,4,0]-undec-7-ene (DBU) and 1,4-diazabi-
cyclo-[2,2,2]-octane (DABCO).
When carrying out process (b) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
1,e A 28 679 - 41 -
20~88:1~.
temperatures between 0 °C and 100 °C, preferably at
temperatures between 10 °C and 60 °C.
Process (b) according to the invention is generally
carried out under atmospheric pressure. However, it is
also possible to carry out the process under increased or
reduced pressure.
For carrying out process (b) according to the invention,
the starting substances required in each case are gener-
ally employed in approximately equimolar amounts.
However, it is also possible to use one of the two com-
ponents employed in each case in a larger excess. The
reactions are generally carried out in a suitable diluent
in the presence of an acid acceptor, and the reaction
mixture is stirred for several hours at the temperature
required in each case. Working-up in process (b)
according to the invention is carried out in each case by
customary methods.
The triazolinones of the formula (II) which are to be
used as starting substances in process (c) according to
the invention for the preparation of compounds of the
formula (I) have already been described as starting
substances for process (a) according to the invention.
Formula (VI) provides a general definition of the sul-
phonamide derivatives furthermore to be used as starting
substances in process (c) according to the invention for
the preparation of compounds of the foranula (I).
Le A 28 679 - 42 -
~0'~~8~
In formula (VI), R' and Z preferably, or in particular,
have those meanings which have already been mentioned
above in connection with the description of the compounds
of the formula (I) and (IV), respectively, according to
the invention as being preferred, or particularly pre-
ferred, for R3 and Z.
Process (c) according to the invention is preferably
carried out using diluents. Substances which are suitable
for this purpose are the same organic solvents which have
been mentioned above in connection with the description
of process (a) according to the invention.
If appropriate, process (c) is carried out in the pres-
ence of an acid acceptor. Substances which are suitable
foz~ this purpose are the same acid binders which have
been mentioned above in connection with the description
of process (b) according to the invention.
When carrying out process (c) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
temperatures between 0 °C and 100 °C, preferably at
temperatures between 10 °C and 60 °C.
Process (c) according to the invention is generally
carried out under atmospheric pressure. However, it is
also possible to carry out the process under increased or
reduced pressure.
Le A 28 679 - 43 -
20"~ 8~~.~.
For carrying out process (c) according to the invention,
the starting substances required in each case are gener-
ally employed in approximately equimolar amounts. How-
ever, it is also possible to use one of the two com-
ponents employed in each case in a larger excess. The
reactions are generally carried out in a suitable diluent
in the presence of an acid acceptor, and the reaction
mixture is stirred for several hours at the temperature
required in each case. Working-up in process (c)
according to the invention is carried out in each case by
customary methods.
To convert the compounds of the formula (I) into salts,
they axe stirred with suitable salt formers such as, for
example, sodium hydroxide, sodium methylate or sodium
athylate or potassium hydroxide, potassium methylate or
potassium ethylate, ammonia, isopropylamine, dibutylamine
or triethylamine, in suitable diluents such as, for
example, water, methanol or ethanol. The salts can be
isolated in the form of crystalline products, if ap
propriate after concentration.
The active compounds according to the invention can be
used as defoliants, desiccants, agents for destroying
broad-leaved plants and, especially, as weed-killers. By
weeds, in the broadest sense, are to be understood all
plants which grow in locations where they are undesired.
Whether the substances according to the invention act as
total or selective herbicides depends essentially on the
amount used.
he A 28 679 - 44 -
20'~~~1:~
The active compounds according to the invention can be
used, for example, in connection with the following
plants:
Dicotyledon weeds of the qenera_ Sinapis, Lepidium,
Galium, Stellaria, Matricaria, Anthemis, Galinsoga,
Chenopodium, Urtica, Senecio, .Amaranthus, Portulaca,
Xanthium, Convolwlus, Ipomoea, Polygonum, Sesbania,
Ambrosia, Cirsium, Carduus, Sonchus, Solarium, Rorippa,
Rotala, Lindernia, Lamium, Veronica, Abutilon, Emex,
Datura, Viola, Galeopsis, Papaver, Centaurea, Trifolium,
Ranunculus and Taraxacum.
Dicotyledon cultures of the genera: Gossypium, Glycine,
Beta, Daucus, Phaseolus, Fisum, Solarium, Linum, Ipomoea,
Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
Lactuca, Cucumis and Cucurbita.
Monocotyledon weeds of the genera: Echinochloa, Setaria,
Panicum, Digitaria, Phleum, Poa, Festuca, Eleusine,
Brachiaria, Lolium, Bromus, Avena, Cyperus, Sorghum,
Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria,
Eleocharis, Scirpus, Paspalum, Ischaemum, Sphenoclea,
Dactyloctenium, Agrostis, Alopecurus and Apera.
Monocot'rledon cultures of the uenera: Oryza, Zea,
Triticum, Hordeum, Avena, Secale, Sorghum, Panicum,
Saccharum, Ananas, Asparagus and Allium.
However, the use of the active compounds according to the
Le A 28 679 - 45 -
20'~8~~ ~.
invention is in no way restricted to these genera, but
also extends in the same manner to other plants.
The compounds are suitable, depending on the concen-
tration, for the total combating of weeds, for example on
industrial terrain and rail tracks, and on paths and
squares with or without tree plantings. Equally, the
compounds can be employed for combating weeds in
perennial cultures, fox example afforestations, decora-
tive tree plantings, orchards, vineyards, citrus groves,
nut orchards, banana plantations, coffee plantations, tea
plantations, rubber plantations, oil palm plantations,
cocoa plantations, soft fruit plantings and hopfields, in
lawns, turf and pasture-land, and for the selective
combating of weeds in annual cultures.
The active compounds can be converted into the customary
formulations, such as solutions, emulsions, wettable
powders, suspensions, powders, dusting agents, pastes,
soluble powders, granules, suspension-emulsion concen-
trates, natural and synthetic materials impregnated with
active compound, and very fine capsules in polymeric
substances.
These formulations are produced in a known manner, for
example by mixing the active compounds with extenders,
that is liquid solvents and/or solid carriers, optionally
with the use of surface-active agents, that is emulsify-
ing agents and/or dispersing agents and/or foam-forming
agents.
Le A 28 679 - 46
In the case of the use of water as an extender, organic
solvents can, for example, also be used as auxiliary
solvents. As liquid solvents, there are suitable in the
main: aromatics, such as xylene, toluene or alkyl-
s naphthalenes, chlorinated aromatics and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloro-
ethylenes or methylene chloride, aliphatic hydrocarbons,
such as cyclohexane or paraffins, for example petroleum
fractions, mineral and vegetable oils, alcohols, such as
butanol or glycol as well as their ethers and esters,
ketones, such as acetone, methyl ethyl ketone, methyl
isobutyl ketone or cyclohexanone, strongly polar sol-
vents, such as dimethylformamide and dimethyl sulphoxide,
as well as water.
As solid carriers there are suitable: for example
ammonium salts and ground natural minerals, such as kao-
lins, clays, talc, chalk, quartz, attapulgite, mont-
morillonite or diatomaceous earth, and ground synthetic
minerals, such as highly disperse silica, alumina and
silicates; as solid carriers for granules there are
suitable: for example crushed and fractionated natural
rocks such as calcite, marble, pumice, sepiolite and
dolomite, as well as synthetic granules of inorganic and
organic meals, and granules of organic material such as
sawdust, coconut shells, maize cobs and tobacco stalks;
as emulsifying and/or foam-forming agents there are
suitable: for example non-ionic and anionic emulsifiers,
such as polyoxyethylene fatty acid esters, poly-
oxyethylene fatty alcohol ethers, for example alkylaryl
Le A 28 679 - 47 -
20'~~8~.~.
polyglycol ethers, alkylsulphonates, alkyl sulphates,
arylsulphonates as well as albumen hydrolysis products;
as dispersing agents there are suitable: for example
lignin-sulphite waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and natural and
synthetic polymers in the form of powders, granules or
latexes, such as gum arabic, polyvinyl alcohol and
polyvinyl acetate, as well as natural phospholipids, such
as cephalins and lecithins, and synthetic phospholipids,
can be used in the formulations. Further additives can be
mineral and vegetable oils.
It is possible to use colorants such as inorganic pig-
ments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dye-
stuffs, and trace nutrients such as salts of iron,
manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1 and 95
per cent by weight of active compound, preferably between
0.5 and 90$.
For combating weeds, the active compounds according to
the invention, as such or in the form of their formu
lations, can also be used as mixtures with known herbi
cides, finished formulations or tank mixes being
possible.
Le A 28 679 - 48 -
20'~88~ ~
Suitable herbicides for the mixtures are known herbi-
cides, for example anilides such as, for example,
diflufenican and propanil; arylcarboxylic acids such as,
for example, dichloropicolinic: acid, dicamba and
picloram; aryloxyalkanoic acids such as, for example,
2,4 D, 2,4 DB, 2,4 DP, fluroxypyr, MCPA, MCPP and
triclopyr; aryloxy-phenoxy-alkanoates such as, for
example, diclofop-methyl, fenoxaprop-ethyl, fluazifop-
butyl, haloxyfop-methyl and quizalofop-ethyl; azinones
such as, for example, chloridazon and norflurazon;
carbamates such as, for example, chlorpropham,
desmedipham, phenmedipham and propham; chloroacetanilides
such as, for example, alachlor, acetochlor, butachlor,
metazachlor, metolachlor, pretilachlor and propachlor;
dinitroanilines such as, for example, oryzalin, pendi-
methalin and trifluralin; diphenyl ethers such as, for
example, acifluorfen, bifenox, fluoroglycofen, fomesafen,
halosafen, lactofen and oxyfluorfen; ureas such as, for
example, chlortoluron, diuron, fluometuron, isoproturon,
linuron and methabenzthiazuron; hydroxylamines such as,
for example, alloxydim, clethodim, cycloxydim, sethoxydim
and tralkoxydim; imidazolinones such as, for example,
imazethapyr, imazamethabenz, imazapyr and imazaquin;
nitrites such as, for example, bromoxynil, dichiobenil
and ioxynil;oxyacetamidessuch as, for example, mefenacet;
sulphonylureas such as, for example, amidosulfuron,
bensulfuron-methyl, chlorimuron-ethyl, chlorsulfuron,
cinosulfuron, metsulfuron-methyl, nicosulfuron, primi-
sulfuron, pyrazosulfuron-ethyl, thifensulfuron-methyl,
triasulfuron and tribenuron-methyl; thiocarbamates such
Le A 2S 679 - 49 -
207881
as, for example, butylate, cyc:Loate, di-allate, EPTC,
esprocarb, molinate, prosulfocarb, thiobencarb and tri-
allate; triazines such as, for example, atrazine,
cyanazine, simazine, simetryne, terbutryne and terbutyl-
azine; triazinones such as, for example, hexazinone,
metamitron and metribuzin; others such as, fox example,
aminotriazol, benfuresate, bentazone, cinmethylin,
clomazone, clopyralid, difenzoquat, dithiopyr, etho-
fumesate, fluorochloridone, glufosinate, glyphosate,
isoxaben, pyridate, quinchlorac, quinmerac, sulphosate
and tridiphane.
Mixtures with other known active compounds, such as
fungicides, insecticides, acaricides, nematicides, bird
repellents, plant nutrients and agents which improve soil
structure, are also possible.
The active compounds can be used as such, in the form of
their formulations or in the use forms prepared therefrom
by further dilution, such as ready-to-use solutions,
suspensions, emulsions, powders, pastes and granules.
They are used in the customary manner, for example by
watering, spraying, atomising or scattering.
The active compounds according to the invention can be
applied either before or after emergence of the plants.
They can also be incorporated into the soil before
sowing.
The amount of active compound used can vary within a
Le A 28 679 - 50 -
20~~8~.~.
substantial range. It depends essentially on the nature
of the desired effect. In general., the amounts used are
between 1 g and 10 kg of active compound per hectare of
soil surface, preferably between 5 g and 5 kg per ha.
The preparation and use of the active compounds according
to the invention can be seen from the following examples.
Preparation Examples:
Example 1
COOCH3 0
(, ,j-SOZ-NH-CO-N~Id-OH
~OCH~
(Process (a))
A mixture of 1.31 g (10 mmol) of 4-hydroxy-5-methoxy-
2,4-dihydro-3H-1,2,4-triazol-3-one, 2.89 g (I2 mmol) of
2-methoxycarbonyl-phenylsulphonyl isocyanate and 40 ml of
acetonitrile is stirred for 5 hours ~at 20°C and subse-
quently concentrated under a water pump vacuum. The
residue is stirred with diethyl ether, and the product
which has been obtained in crystalline form is isolated
by filtration with suction and recrystallised from
isopropanol.
Le A 28 679 - 51 -
20~881~
1.22 g (33 ~ of theory) of 4-hydroxy-5-methoxy-1-
(2-methoxycarbonyl-phenylsulphonylaminocarbonyl)-2,4-
dihydro-3H-1,2,4-triazol-3-one of melting point 180°C are
obtained.
Other compounds of the formula (T) which can be prepared
analogously to Example 1 and following the general
description of the preparation processes according to the
invention are, for example, those listed in Table 3
below.
R3-S0~-NH-CO-N~N-0-R1 ~I)
N--~ _ R2
Le A 28 679 - 52 -
~~7~~~~
Table 3: Preparation examples of the compounds of
the fornnula ( I )
Rx. R1 RZ R3 Melting
No. point (°C)
CO~CH~
2 CH, CH3 1 Z 9
C1
3 CH3 CH3 111
OCF3
4 CH3 CH3 109
CI
5 CH3 CH3 ~ 126
CH3
C1
6 H CH, 169
Le A 28 679 - 53 -
20'~~~~.~.
Table 3 cnntinuati0n
-
Ex. R1 RZ R' Melting
NO. point (~C)
OCF~
7 H CH3 161
C1
8 H CH3 ~ 119
CH3
OCHFZ
9 CH3 CH3 151
COZCH3
CH3 CZHS 12 7
F
11 CH3 CH3 15 B
CP'3
10 12 CH, CH3 12 4
CH3
13 CH3 CH3 71
Le A 28 679 - 54 -
2~'~~8~~
Table 3 continuation
-
Ex. R1 Rz R' Melting
No. point (C)
CZ
14 CH3C2H5 9 0
C1
CH3C2H5 0 91
CH3
OCF3
16 CH3CZHS 109
OCHF2
17 CH3CZHS 98
CFA
18 CH3CZHS 112
F
10 19 CH3CZHS 124
CH3
cH,cZHS s7
c
21 CH3CH3 ! 17 0
C1
Le A 28 679 - 55 -
Table 3 continuation
-
Ex. R' RZ R' Melting
NO. point ('C)
C1
22 CH,CZHS ~ 119
~
C1
2 3 CH3CZHS ( 115
~
S
OZCH3
C2
24 CH3CZHS ~ 104
H2-
C1
COZC2H5
25 cH,c2H5 ~ 115
26 H CH3 189
CH3
27 H CH3 171
C1
28 H CH, ~ 192
C1
Le A 28 679 - 56 -
Table 3 continuation
-
Ex. R1 RZ R3 Melting
NO. point (C)
COZC2Fi5
29 H CH3 162
CF3
30 H CH3 160
COZCZ~JS
31 CH, cH3 15 0
C02C3H~
32 CH3 CH, 98
CI
33 CH3 CH3 157
/
H2_
C1
OCF3
ZO 34 CH3 CH3 139
H2_
35 CH3 CH3 ~ 156
~
OZCFI3
Le A 28 679 - 57 -
20788~.~
Table 3 - continuation
Ex. R1 RZ R' Melting
No. point (°C)
I ~ OzCZHS
36 CH3 CH3 Id~~ 121
CFi3
~ZC2H5
3 7 H CH3 ~ 16
3
CNg
OCF~
38 H CH3 164
H _
2
OCFiF2
39 H CH, 158
Br
4 0 CH3 CH3 171
Br
41 H CH3 149
J
Le A 28 679 - 58 -
Table 3 continuation
-
Ex. R1 RZ R' Melting
No. point (C)
COZCH3
42 H CZHS 74
CH3
43 H CZHS 168
CFA
44 H CZHS 76
OCF3
45 H CZHS 97
Le A 28 679 - 59 -
20'~$8~.~.
Table 3 - continuation
Ex~ Melting
R1 R2 R3
No. point (°C)
0CF3
46 GH3 C2H5 ~-~ CH2- 118
Br
47 CH3 CH3 i ~ 124
NON
CH3
COOCH3
48 C2H5 CH3 ~a~ 106
C1
49 C2H5 CH3 ~-~ 122
OCF3
50 C2H5 CH3 ~-~ 113
CH3
51 C2N5 CH3 ~-~ 141
COOC3H7-n
52 C2H5 CH3 ~-~ 88
I,e A 28 679 - 60 -
20'~~81~.
Table - continuation
3
Melting
Ex. R1 R2 R3
No.
point (
C)
OCHF2
53 C2H5 CH3 ~-~ 118
CF3
54 C2H5 CH3 ~-~ 119
F
55 C2H5 CH3 ~-~ 153
Br
56 C2H5 CH3 ~-~ 128
C1
57 C2H5 CH3 ~ ~ CH2- 113
C1
58 C2H5 CH3 ~ ~ 126
g OOCH3
OCF3
59 C2N5 CH3 ~-~ H2- 57
Le A 28 679 - 61 -
~~~88~.1
Table 3 - continuation
Melting
Ex. RS R2 R3
point (°C)
No.
COOC2H5
60 C2H5 CH3 ~ I 144
NON
CH3
COOC3H7-n
61 CH3 C2H5 ~ 101
Br
b2 CH3 C2H5 ~'~ 87
COOC2H5
63 CH3 C2N5 ~ ~
63
NON
CH3
COOC3H7-n
64 C2H5 CH3 ~-~
125
x ~
OCHF2
65 C2H5 CH3 ~-~
105
x?
CF3
bb C2H5 CH3 ~-~
132
x ~
Le A 28 679 - 62 -
207~81.~
Table 3 - continuation
Melting
Ex. R1 R2 R3 '
point C)
(
No.
F
67 C2H5 CH3 ~-~ 168 x
)
Br
68 C2H5 CH3 ~-~ 85 0)
COOC2H5
69 CH3 CH3 ~-~ 126 x)
COOCH3
70 CH3 C2H5 ~o~ 52 x)
COOC2H5
71 CH3 C2H5 ~-~ 72 x
)
CF3
72 CH3 C2H5 ~ 90 x)
OCHF2
73 CH3 C2H5 ~-~ 92 x
)
Le A 28 679 - 63 -
207~~1~
Table 3 - continuation
Melting
Ex. R1 R2 R3
point C)
(
No.
COOCH3
74 CH3 CH3 ~~~ 167 x
)
OCFO
75 CH3 CH3 ~-~ 1 12 x
)
C1
76 CH2 CH3 7 ~ 75 x)
CH3
CF3
77 CH3 CH3 7-~ 9q x)
OCF3
78 CH3 C2H5 ~-~ 1 10 x
)
OCH3
79 CHO CH3 ~ ~ 131
H3C0
OCH3
80 CH3 CH3 ~-~ 114
Le A 28 679 - 64 -
2078~~..~
Table 3 - continuation
Melting
Ex. RS R2 R3 point ('C)
No.
OC2H5
81 CH3 CHS /p\ 148
COOC2H5
/
82 C2H5 CH3 ~\ 121
C1
83 C2H5 CH3 / \ 159
C1
SC2H5
/
84 C2H5 CH3 ~\ 146
COOC2H5
85 C2H5 CH3 /-\ 61 x)
C1
86 C2H5 CH3 / \ 66 x)
C1
SC2H5
87 C2H5 CH3 /-\ 54 x )
Le A 28 679 - 65 -
20°~~~~
Table 3 - continuation
Melting
Ex. R1 R2 R3 '
point (
C)
No.
S-CH3
88 CH3 CH3 ~-~ 110
SC2H5
89 CH3 CH3 ~~~ 119
SO-C2H5
90 CH3 CH3 ~a~ 157
S02-C2HS
91 CH3 CH3 ~-~ 204
SO-CH3
92 CH3 CH3 ~~~ 130
S02-CH3
93 CH3 CH3 ~-~ 169
COOCH3
~
94 CH3 CH3 -~ CH2- 123
Le A 28 679 - 66 --
~D'~8~~.
Table 3 - continuation
Melting
Ex. R1 R2 H3
point ('C)
No.
OCHF2
/
95 CH3 CH3 _\ CH2- 96
COOCH3
/
96 C2H5 C2H5 ~\ 151
CF3
/
97 C2H5 C2H5 ~\ 134
OCH3
/
98 C2H5 C2H5 \ 120
H3C0
"' isolated in the form of the Na-salt.
Le A 28 679 - 67 -
Startincr substances of the formula ~ II) a
example (TI-11
O
H-N~-QH
-CH3
A mixture of 42 g (0.46 mol) of 4-hydroxysemicarbazide
[R. Ohme, H. Preuschhof, J. Prakt. Chem. ,~13, (1971),
p. 626], 73.3 g (0.54 mol) of tetramethyl orthocarbonate
and 300 ml of methanol is refluxed for 18 hours. When the
reaction mixture is cool, 250 ml of the solvent employed
are distilled off under a water pump vacuum. The colour-
less product, which is obtained in the residue in crys-
talline form, is isolated by filtration with suction.
22 g (36 ~ of theory) of 4-hydroxy-5-methoxy-2,4-dihydro-.
3H-1,2,4-triazol-3-one of melting point 211°C (exothermal
decomposition) are obtained; [cf. Arch. Pharm. Vol. 301,
p. 829 (1968)].
- 68 -
Le A 28 679
20'~88~~
Exam~~le III-21
H-N~ld-OCZH~
~CH3
3.7 g (28 mmol) of 4-hydroxy-5-methoxy-2,4-dihydro-3H-
1,2,4-triazol-3-one are suspended in 30 ml of ethanol,
and the suspension is treated with 1.12 g (28 mmol) of
sodium hydroxide, dissolved in 5 ml of water. After
stirring has been continued for 2 hours at room tempera-
ture (20°C), 4.3 g (28 mmol) of diethyl sulphate are
added, and the mixture is stirred for a further 18 hours
at room temperature. The reaction mixture is acidified
with dilute hydrochloric acid (pH = 3-4), filtered and
concentrated under a water pump vacuum. The residue is
stirred with 50 ml of methanol/diethyl ether (1:4), and
the insoluble constituents are removed by filtration. The
crude product, which is abtained by concentrating the
filtrate, is purified by recrystallisation from
isopropanol.
1.8 g (40 % of theory) of 4-ethoxy-5-methoxy-2,4-dihydro
3H-1,2,4-triazol-3-one are obtained as a colourless solid
c~f melting point 57°C.
Other compounds of the formula (II) which can be prepared
analogously to Examples (II-1) and (II-2) are, for
example, those listed in Table 4 below.
- 69 -
Le A 28 679
2~'~881~.
0
x-N~rr-o-R1 c a a ~
~_R2
Table 4s Examples of the startingsubstances of
the
formula (II)
Ex. R' Ra Melting
No.point (C)
I I-3 CH3 CH3 14 7
I I-4 H CZHS 145
I I-5 CH3 CZHS 118
- 70 -
Le A 28 679
2Q'~88~~.
Use Examoples:
In the use Examples, the following compound (A) is used
as comparison substance:
CZ O
C. ,r-50x-NH-CO-N~N°CFi~~ (~)
~.J ~H~
2-(2-Chloro-phenylsulphonylaminocarbonyl)-4,5-dimethyl-
2,4-dihydro-3H-1,2,4-triazol-3-one (disclosed in
EP-A 341,489).
Example A
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active compound, 1
part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the
desired concentration.
Test plants which have a height of 5 - 15 cm are sprayed
with the preparation of the active compound in such a way
-- 71 -
Le A 28 679
~0~~83.
as to apply the particular amounts of active compound
desired per unit area. The concentration of the spray
liquor is so chosen that the particular amounts of active
compound desired are applied in 1,000 1 of water/ha.
After three weeks, the degree of damage to the plants is
rated in ~ damage in comparison to the development of the
untreated control. The figures denote:
0$ = no action (like untreated control)
100 = total destruction
A clearly superior activity compared with the prior art
is shown, in this test, for example by the compounds of
the following Preparation Examples: 5, 12 and 13.
Example B
Pre-emergence test
i5 Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active compound, 1
part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the
desired concentration.
Seeds of the test plants are sown in normal soil and,
- 72 -
Le A 28 679
~o~~s~~
after 24 hours, watered with the preparation of the
active compound. It is expedient to keep constant the
amount of water per unit area. The concentration of the
active compound in the preparation is of no importance,
only the amount of active compound applied per unit area
being decisive. After three weeks, the degree of damage
to the plants is rated in ~ damage in comparison to the
development of the untreated control. The figures denote:
0$ = no action (like untreated control)
100$ = total destruction
A clearly superior activity compared with the prior art
is shown, in this test, for example by the compounds of
the following Preparation Examples: 2, 4 and 20.
- 73 -
Le A 28 679