Note: Descriptions are shown in the official language in which they were submitted.
2~78~9
METHOD OF AND APPARATUS FOR TREATING MALODOROUS GASES
BACKGROUND AND SUMMARY OF THE INVENTION
The present invention relates to a method an apparatus for
treating malodorous gases and sulphate soap from a cellulose
pulp mill. The gases and soap (and like fractions thereof)
are treated in a pulp mill in such a way that the recovery
of chemicals entrained therein is improved without any
disturbance to other subprocesses of the mill.
Throughout the world, there is an increasing need to
minimize the sulphur emissions from sulphate pulp mills.
This requires maintaining a substantially constant balance
between sulphur and sodium ("S/Na ratio"), that is
maintaining the sulphidity constant. Maintaining an
appropriate balance can be difficult, however, since
sulphur is added to the process system in the form of
sulphuric acid, and waste acid as a result of the
utilization of chlorine dioxide bleaching chemicals.
A significant source of the sulphuric acid added to a
sulphate mill is that added during the conversion of soap
(formed as a by-product of the manufacture of the pulp) to
tall oil, a malodorous resonance admixture of rosins,
fatty acids, sterols, and other materials. The commercial
significance of tall oil is decreasing, therefore if the
tall oil subprocess can be eliminated there is no
significant adverse commercial impact on the pulp mill,
however the sulphur imbalance contributed by sulphuric
acid normally used in the process of converting soap to
tall oil can be eliminated. However the soap must still
be used or disposed of.
In the past it has been suggested that it is possible to
avoid the tall oil subprocess by combusting soap in a soda
recovery boiler. However such combustion is not
advantageous. Combustion of soap in the soda recovery
,,
- .:: , . .
.': . : : :
.. .. .. . . : ~, ~
2Q78959
boiler carries with it the risk of a melt solution
explosion. Also, combustion of the soap occupies a part
of capacity of the boiler (for example about 3 to 5% of
the capacity). Also combustion of soap might need to be
stopped if the dry substance content of the black liquor
being burned in the soda recovery boiler increases. These
same concerns applicable to a soda recovery boiler are
also typically applicable to combustion of soap in a lime
kiln, and in addition combustion in the lime kiln is
technically complicated.
The method in accordance with the present invention brings
about several advantages compared with previous methods.
The method according to the present invention facilitates
balancing the S/Na ratio of the mill. Further, the
inventive method does not require the addition of
sulphurous compounds that are added to the previous
processes. The sodium in the soap in the present invention
is recovered without disturbing the rest of the mill
process, e.g. the combustion of black liquor in the soda
recovery boiler. Further, it is possible to readily
decrease the environmental loading of a pulp mill because
the sulphur in the malodorous gases may be bound by means
of the sodium in the soap without a significant use of
other chemicals, e.g. sodium hydroxide, that are acquired
elsewhere. The method according to the present invention
is also energy efficient since the burner requires only
minimal energy, e.g. oil or natural gas, acquired elsewhere
during the start-up of the combustion process and/or
during possible breakdowns.
Malodorous gases mainly containing organic sulphur
compounds are generated in the manufacture of sulphate
cellulose pulp. r~hese gases are mainly generated in the
digester and in the black liquor evaporators. Preferably
the odorous organic sulphur compounds in the gases are
removed by combusting them to produce sulphur dioxide in a
..
.
'' . ' ' - .. ~ ' .. , :
- . : . : - : .
.
2~78~5~
separate reactor vessel, or in a lime sludge reburning
kiln. It has been found that the separate combustion
procedure is preferred because the combustion in a lime
kiln disturbs the actual lime sludge reburning process.
Further, during combustion, disturbances in the process
result in the release of malodorous gases to the
environment.
The previous separate combustion systems have the
disadvantage that the sulphur contained therein can only
be recovered by scrubbing the flue gases. This has been
achieved by, for example, scrubbing the flue gases with a
solution containing sodium hydroxide. However, NaOH is
expensive, and disturbs the S/Na balance of the mill.
According to the present invention, a process and apparatus
are provided which eliminate the problem of malodorous
sulphurous gases from a pulp mill, while at the same time
eliminating the use of sulphuric acid for the conversion
of soap to tall oil, thereby helping to maintain the
sulphur sodium ratio in the pulp mill, and minimizing the
adverse environmental impact of a sulphate pulp mill.
This desirable result is accomplished according to the
method of the present invention by destroying sulphurous
malodorous gases while producing recoverable chemicals.
The preferred method according to the present invention
comprises the following steps: (a) Combining sulphurous
malodorous gases with material selected from the group
consisting essentially of soap and black liquor, to produce
a fluid. (b) Combusting the fluid from step (a) under
oxidizing conditions to produce a melt containing sodium
sulphate and sodium carbonate. And, (c) Recovering
chemicals from the melt produced in step (b). Step (c)
may be practiced, for example, by dissolving the melt to
produce a solution, combining the solution with black
liquor, and then combusting the black liquor in a
conventional soda recovery boiler. The gas utilized may
.
. ~, .' ' . ~' .'';'. ' . . ~
- - : .: . . ...
.
- : ~ . ' '' ' :
,
.
2~7~
be any sulphurous off gas from a pulp mill, such as from a
digester or evaporator. The flue gases that are generated
during step (b) may be cooled and then passed to a
scrubber, or they may be fed to a waste heat boiler which
burns bark, sludge, or the like.
Normally soap includes a small amount of sulphur compared
with its sodium content, the average ratio being about 6
kg sodium to about 0.5 kg sulphur. This ratio varies
depending upon the tree species and the site location of
the trees used to produce the cellulose pulp. During the
oxidizing part of the combustion process the sulphur in
the odorous gases reacts with the sodium in the soap
forming Na2SO4 and some Na2CO3. The resulting melt solution
that is formed from this combustion process is then
dissolved in water to an appropriate concentration
(selected in accordance with the precipitation point of
the solution~. The solution is then further mixed with
weak black liquor, and burned in a soda recovery boiler.
This results in the sodium in the soap being recovered
without disturbing the S/Na balance of the mill.
The sulphur-free flue gases that are generated in the
combustion process may be scrubbed with water in a
conventional scrubber. This is done to decrease the
particle emissions from in the mill process. The washing
liquid may be passed to the dissolving section of the melt
solution. Due to the composition of the soap the particle
emissions from the combustion process remain very low.
It has also been discovered according to the invention
that it is possible to combust black liquor with the
odorous gases. The black liquor may be substituted for
the soap or as a mixture along with soap. The treatment
of black liquor comes into question, for example, when
there is not enough soap for the combustion process in
accordance with the present invention. Further, the
treatment of black liquor is useful if the capacity of
2~7~5~
the soda recovery boiler is not sufficient to treat all
black liquor in the system. During the combustion of
black liquor the sulphur in the odorous gases forms SO2.
The resulting SO2 is bound to the melt solution, as it is
in the combustion process of the soap.
The sulphurous odorous gases treated according to the
invention may be any sulphurous gas generated in a pulp
mill and capable of combustion in accordance with the
present invention.
According to another aspect of the present invention, an
apparatus for destroying sulphurous malodorous gases is
provided. The apparatus comprises a reactor vessel
(preferably a cylindrical water-cooled reactor) for
effecting combustion, having a top portion and a bottom
portion. The apparatus further comprises the following
elements: Means for feeding sulphurous malodorous gases,
soap and/or black liquor, and combustion air into the
reactor vessel. Means for discharging melt produced
during the combustion of malodorous gases, and soap and/or
black liquor, in the reactor vessel from the bottom of the
reactor vessel. Means for discharging from the reactor
vessel flue gases produced during the combustion in the
reactor vessel. And, a dissolving vessel communicating
with the melt discharging means, for dissolving the melt.
The dissolving vessel is typically disposed beneath the
reactor vessel, and has a flue discharge for leading flue
gases from the reactor vessel through a cooler and then to
a scrubber, or passing the flue gases to a waste heat
boiler burning bark, sludge, or the like. A melt solution
conduit extends from the dissolving vessel and merges
with a weak black liquor conduit, which is then connected
to a soda recovery boiler.
It is the primary object of the present invention to
destroy malodorous gases from a sulphate pulp mill while
at the same time preserving the S/Na ratio of the pulp
- -
.. . . . . - -- . : . -: . - .... ..
,: - . ~ . : . ' :'- '
:
. .
2~7~
mill. This and other objects of the invention will become
clear from an inspection of the detailed description of
the invention, and from the appended claims.
BRIEF DESCRIPTION OF THE DRAWING
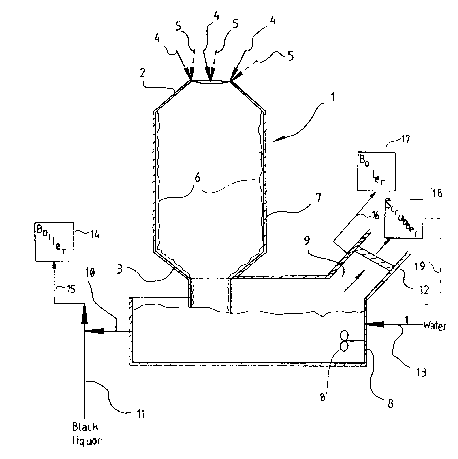
The drawing figure is a longitudinal cross-sectional view
of schematically illustrated exemplary apparatus according
to the invention, and for practicing the method according
to the invention.
DETAILED DESCRIPTION OF THE DRAWING
The drawing illustrates a cylindrical water-cooled
combustion reactor 1 connected to a dissolving vessel 8.
The combustion reactor 1 has a conical upper end 2, a
lower end 3, and vertical wall 7. Soap and/or black
liquor 4, odorous gases 5, combustion air, and possibly
fuel, are introduced homogeneously at several places (e.g.
three to five different places) into the upper end 2 of the
combustion reactor 1. The temperature in the reactor
is preferably 900-1000C during combustion.
When burning soap and/or black liquor and odorous gases
react, a melt layer 6 is continuously generated on the
inside of the reactor wall 7. The continuously generated
melt layer 6 protects the reactor wall 7 from overheating,
and flows downwardly under the force of gravity. The
melt layer 6 is discharged from the lower end 3 of the
reactor 1 into the dissolving vessel 8 that is located
below the reactor 1.
The concentration of melt solution 10 produced in vessel
8 is controlled by an incoming fresh water flow 13 that
enters the dissolving vessel 8, and is mixed with the
melt 6 by mixer 8'. The liquid introduced at 13 may be
wash liquid from a flue gas scrubber.
207~9
Flue gases that are generated in the reactor 1 also
circulate through the dissolving vessel 8. These flue
gases are then transported through a discharge opening 9
and are cooled by a conventional cooler 12. The flue
gases are discharged from cooler 12 to a conventional
scrubber 18 to be treated with water. The scrubber must
also have the capability of treating gases with an
alkaline composition, e.g. NaOH solution, since the
generation of sulphur dioxide may occur in some
circumstances. However, the generation of sulphur dioxide
under most conditions is unlikely. The liquid used for
scrubbing may -- after scrubbing -- be utilized to provide
all or part of the dissolving liquid in line 13, as
indicated by return line 19.
Melt solution 10 containing Na2SO4 and some Na2CO3 is mixed
with weak black liquor in conduit 11. The resultant
mixture of the melt solution 10 and the weak black solution
in line 11 is directed via an evaporation plant (not shown)
to a conventional recovery boiler 14 by the conduit 15 or
other appropriate means. Thus, the chemicals that are
present in the soap and/or black liquor and odorous gases
are recovered for future chemical circulation within the
mill.
The combustion system of the present invention also
operates well at a low dry substance content of soap.
Further, there is no risk of a melt explosion.
The operatir,g capacity efficiency of the combustion system
described above is about 9-25 megawatts (MW). When using
black liquor in the combustion process, higher efficiencies
are possible. Further, it is possible to combine a waste
heat boiler with a system for the recovery of energy from
flue gases in order to obtain high fuel efficiency. That
is, under suitable conditions flue gases from discharge 9
may be passed (e.g. via conduit 16) to a vessel 17 ~e.g.
a boiler) for reaction with bark, sludge or the like, so
': '' : ~.
' -, ~
2~7~
that a separate treatment of flue ~ases in a scrubber is
avoided.
The method in accordance with the present invention is
especially suitable for the type of pulp mill which treats
odorous gases in a separate system.
While the invention has been described in connection with
what is presently considered to be the most practical and
preferred embodiment, it is to be understood that the
invention is not to be limited to the disclosed embodiment,
but on the contrary, is intended to cover various
modifications and equivalent arrangements included within
the spirit and scope of the appended claims.