Note: Descriptions are shown in the official language in which they were submitted.
2079268
The invention relates to new substituted imidazolyl-
propenoic acid derivatives, to a process for their
preparation and to their use in medicaments, in par-
ticular as hypotensive and anti-atherosclerotic agents.
It is known that renin, a proteolytic enzyme, eliminates
the decapeptide angiotensin I in vivo from angioten-
sinogen, which decapeptide is in turn degraded in the
lungs, the kidneys or other tissues to give the hyperten-
sive octapeptide angiotensin II. The various effects of
angiotensin II, such as, for example, vasoconstriction,
Nat retention in the kidneys, aldosterone release in the
adrenal gland and increase in tone of the sympathetic
nervous system, act synergistically in the sense of a
blood pressure increase.
Moreover, angiotensin II has the property of promoting
the growth and the replication of cells such as, for
example, of cardiac muscle cells and smooth muscle cells,
these growing and proliferating in an increased manner in
various disease states (for example hypertension, athero-
sclerosis and cardiac insufficiency).
In addition to the inhibition of renin activity, a
possible starting point for intervention in the renin-
angiotensin syctem (RAS) is the inhibition of the
activity of angiotensin-converting enzyme (ACE) and the
blockade of angioten~in II receptors.
Le A 28 592 - 1 -
.
2~7~2~8
In the publications EP 324,377 A2, ~P 403,158 A2 and
EP 403,159 A2, phenyl(alkyl)imidazole and imidazolyl-
al:kenoic acids are described in which the scope of
meaning of position 2 (see compounds according to the
invention -CH=CH-Co2R4) encompasses propenoic acids and
esters and the terminal phenyl ring is substituted by a
tetrazole ring, but without giving a reference to an
actual substance representative of this substitution
pattern.
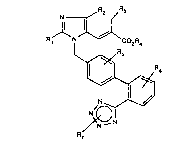
The present invention relates to substituted imidazolyl-
propenoic acid derivatives of the general formula (I)
N ~ R2 ~R3
R J~ N J~ CO2R~
~R6 (I)
N
N_N
in which
R1 represents straight-chain or branched alkyl or
alkenyl each having up to 8 carbon atoms, each of
which is optionally substituted by cycloalkyl
having 3 to 6 carbon atoms, or
represents cycloalkyl having 3 to 8 carbon atoms,
Le A 28 592 - 2 -
2079~68
R2 represents hydrogen, halogen, hydroxyl, nitro,
~ cyano, trifluoromethyl, trifluoromethoxy or penta-
fluoroethyl, or
represents straight-chain or branched alkyl having
up to 6 carbon atoms, or
represents aryl having 6 to 10 carbon atoms,
R3 represents hydrogen or straight-chain or branched
alkyl having up to 10 carbon atoms, or
represents aryl having 6 to 10 carbon atoms or a
5- to 7-membered, saturated or unsaturated hetero-
cycle having up to 4 hetero atoms from the series
comprising S, N and O, each of which is optionally
monosubstituted to trisubstituted by identical or
different halogen, hydroxyl, nitro, cyano, tri-
fluoromethyl or trifluoromethoxy, or by straight-
chain or branched alkyl or alkoxy each having up
to 8 carbon atoms or by a group of the formula
-NR8R~ ~
in which
R8 and R~ are identical or different and denote
hydrogen, straight-chain or branched alkyl
: having up to 6 carbon atoms, or phenyl,
R4 represents hydrogen, straight-chain or branched
alkyl having up to 8 carbon atoms, or phenyl,
R5 and R~ are identical or diferent and
Le A 28 592 - 3 -
,
207926~
represent hydrogen, halogen, cyano, nitro, tri-
fluoromethyl, hydroxyl, trifluoromethoxy or
straight-chain or branched a~kyl or alkoxy each
having up to 6 carbon atoms,
R7 represents hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms
and their salt6.
The substituted imidazolyl-propenoic acid derivatives
according to the invention can also be present in the
form of their salts. In general, salts with organic or
inorganic bases or acids may be mentioned here.
In the context of the present invention, physiologically
acceptable salts are preferred. Physiologically accept-
able salts of the imidazolyl-propenoic acid derivatives
can be salts of the substances according to the invention
with mineral acids, carboxylic acids or sulphonic acids.
Particularly preferred salts are, for example, thos~ with
hydrochloric acid, hydrobromic acid, sulp~uric acid,
phosphoric acid, methanesulphonic acid, ethanesulphonic
acid, toluenesulphonic acid, benzene~ulphonic acid,
naphthalenedisulphonic acid, acetic acid, propionic acid,
lactic acid, tartaric acid, citric acid, fumaric acid,
maleic acid or benzoic acid.
Physiologically acceptable ~alts can also be metal or
ammonium salts of the compounds according to the
Le A 28 592 - 4 -
2Q7~2~8
invention which have a free carboxyl group. Particularly
- preferred salts are, for example, sodium, potassium,
magnesium or calcium salts, and also ammonium salts which
are derived from ammonia or organic amines such as, for
example, ethylamine, di- or triethylamine, di- or tri-
ethanolamine, dicyclohexylamine, dimethylaminoethanol,
arginine, lysine or ethylenediamine.
The compounds according to the invention can exist in
stereoisomeric forms which either behave as image and
mirror image (enantiomers), or which do not behave as
image and mirror image (diastereomers). The invention
relates both to the enantiomers or diastereomers and
their respective mixtures. The racemic forms, like the
diastereomers, can be separated in a known manner into
the stereoisomerically uniform constituents ~cf.
E.L. Eliel, Stereochemistry of Carbon Compounds,
McGraw Hill, 1962].
Heterocycle in general represents a 5- to 7-membered,
preferably 5- to 6-membered, saturated or unsaturated
ring which as hetero atoms can contain up to 2 oxygen,
sulphur and/or nitrogen atoms. 5- and 6-membered rings
containing an oxygen, sulphur and/or up to 2 nitrogen
atoms are preferred. The following may be mentioned as
preferred: thienyl, furyl, pyrrolyl, pyrazolyl, pyridyl,
pyrimidyl, pyrazinyl, pyridazinyl, thiazolyl, oxazolyl,
imidazolyl, pyrrolidinyl, piperidinyl, piperazinyl or
tetrazolyl.
Le A 28 592 - 5 -
". ' ` ~ '
.
2~792~8
Preferred compounds of the general formula (I) are tho~e
in which
R1 represents straight-chain or branched alkyl or
alkenyl each having up to 6 carbon atoms, each of
which is optionally substituted by cyclopropyl,
cyclobutyl, cyclopentyl or cyclohexyl, or
represents cyclopropyl, cyclopentyl or cyclohexyl,
R2 represents hydrogen, fluorine, chlorine, bromine,
iodine, trifluoromethyl, trifluoromethoxy, penta-
fluoroethyl, phenyl or straight-chain or branched
alkyl having up to 6 carbon atoms,
R3 represents hydrogen or straight-chain or branched
alkyl having up to 8 carbon atoms, or
represents phenyl, furyl, thienyl, imidazolyl,
pyrryl or pyridyl, each of which is optionally
substituted up to 2 times by identical or dif-
ferent fluorine, chlorine! bromine, hydroxyl,
nitro, cyano, trifluoromethyl or trifluoromethoxy
or by straight-chain or branched alkyl or alkoxy
each having up to 6 carbon atoms or by a group of
the formula -NR8R9,
: in which
R8 and R9 are identical or different and denote
hydrogen or straight-chain or branched alkyl
Le A 28 592 - 6 -
2~792~8
having up to 4 carbon atoms,
R4 represents hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms,
Rs and R5 are identical or different and
represent hydrogen, fluorine, chlorine, bromine,
trifluoromethyl, trifluoromethoxy or straight-
chain or branched alkyl having up to 4 carbon
atoms,
R7 represents hydrogen or straight-chain or branched
alkyl having up to 4 carbon atoms
and their salts.
Particularly preferred compounds of the general formula
(I) are those
in which
15 R1 represents qtraight-chain or branched alkyl or
alkenyl each having up to 4 carbon atoms, or
cyclopropyl,
R2 represents hydrogen, fluorine, chlorine, bromine,
iodine, trifluoromethyl, pentafluoroethyl, phenyl
or straight-chain or branched alkyl having up to .
4 carbon atoms,
Le A 28 592 - 7 -
~. , . . ' '' ` .. : ~ ' .
.. ': . .,
207926~
R3 represent~ hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms, or
represents phenyl, furyl or thienyl, each of which
is optionally substituted by fluorine, chlorine,
cyano, hydroxyl, trifluoromethyl or trifluoro-
methoxy or by straight-chain or branched alkyl or
alkoxy each having up to 4 carbon atoms or by a
group of the formula -NR9R9,
in which
R8 and R9 are identical or different and denote
hydrogen, methyl or ethyl,
R4 represents hydrogen or straight-chain or branched
alkyl having up to 4 carbon atoms,
R5 and R~ are identical or different and
represent hydrogen, fluorine, chlorine or bromine,
R7 represents hydrogen, methyl, ethyl, propyl or
isopropyl
and their salts.
Very particularly preferred compounds of the general
formula (I) are those
in which
Le A 28 592 - 8 -
207~68
Rl represents straight-chain or branched alkyl or
alkenyl each having up to 4 carbon atoms,
R2 represents hydrogen, fluorine, chlorine, iodine,
trifluoromethyl or pentafluoroethyl,
R3 represents hydrogen or straight-chain or branched
alkyl having up to 4 carbon atoms, or
represents phenyl, furyl or thienyl, each of which
is optionally substituted by fluorine, chlorine,
cyano or methoxy,
R4 represents hydrogen, methyl or ethyl
and
R5, R6 and R7 represent hydrogen
and their salts.
In addition, a process for the preparation of the com-
pounds of the general formula (I~ according to the
invention and their salts has been found, characterised
in that
aldehydes of the general formula (II)
Le A 28 592 - 9 -
~: .- ' `
.
.
2~7926~
N--
RJ~N
~ R6 (II)
6Hd3C ~,N
N~N
in which
R1, R2, R5 and R~ have the abovementioned meaning,
are first converted by reaction with compounds of the
general formula (III)
R3-(CH2)2-Co2Rl (III)
in which
: ; R~ has the abovementioned meaning
and
Rl has the abovementioned meaning of R~, but does not
represent hydrogen,
Le A 28 592 - 10 -
;.
, . - ,
:' ,
207926~
in inert solvents, in the presence of a base, to give the
compounds of the general formula (IV)
R2 R3
R 1¦~(CO2R10
OH Rs
~ R6 (IV)
(C6HS)3C ~N ~J
N~)
N_N
in which
R1, R2, R3, R5, R6 and R10 have the abovementioned meaning,
then the free hydroxyl function i6 blocked by introduc-
tion of a protective group and in a last step an elimina-
tion in inert solvents in the presence of a base is
carried out,
and in the case of the acids (R4 = H) the esters are
hydrolysed
and in the case in which R7 does not represent hydroqen,
the -NH function is alkylated.
~he proce~ according to the invention can be illu~trated
by way of ex~mple by the following reaction scheme~
,:
Le A 28 592 - 11 -
.
.
,
. . ~ , . . -
2~79268
N ~CI
H3C-(CH2)3 ~ N ~
~ ~ (CH2)2-C02-CH3
(C6Hs)3C ~ ~
Cl C6Hs
H9C-(CH2)3 1~3~CC~O CH3 H3C-(CHZ)3 J~N~f (CO2CH3
OH AC20
~q CH2CI2 ~
DMAP ~q
(c6Hs)3c ~ (C6Hs)3C~
DBU H,C ICH,~, l~CC,HCH,
~ .
toluene l
;: ~ ~
(C~Hs)3C~ N
N~
.
';~
Le A 28 592 - 12 -
.~
2079~68
N ~ Cl ~ C6Hs
l.)NaOH ~C-(CH2)3 ~ N ~ CO2H
2.)HCI
N
\NGN
Hydroxyl protective gro~p in the context of the abovemen-
tioned definition in general represents a protective
group from the series comprising: benzyloxycarbonyl,
methanesulphonyl, toluenesulphonyl, 2-nitrobenzyl, 4-
nitrobenzyl, 2-nitrobenzyloxycarbonyl, 4-nitro-
benzyloxycarbonyl,tert-butoxycarbonyl,allyloxycarbonyl,
4-methoxycarbonyl, acetyl, trichloroacetyl, 2,2,2-tri-
chloroethoxycarbonyl, 2,4-dimethoxybenzyloxycarbonyl, 2-
(methylthiomethoxy)ethoxycarbonyl, benzoyl, 4-methylben-
: 10 : zoyl, 4-nitrobenzoyl, 4-fluorobenzoyl, 4-chlorobenzoyl or
4-methoxydbenzoyl. Acetyl, methanesulphonyl and toluene-
::sulphonyl are preferred.
Suitable solvents for the procass are the customaryorganic ~olvents which do not change under the reaction
conditions. These prsferably include ether~ such a~
:~ :
` ~:
~ Le A 28 592 - 13 -
,
j
2~79268
diethyl ether, dioxane, tetrahydrofuran, glycol dimethyl
- ether, and hydrocarbons such as benzene, toluene, xylene,
hexane, cyclohexane or mineral oil fractions, and
halogenohydrocarbons such as dichloromethane, trichloro-
methane, tetrachloromethane, dichloroethylene, trichloro-
ethylene or chlorobenzene, and ethyl acetate, tri-
ethylamine, pyridine, dimethyl sulphoxide, dimethylfor-
mamide, hexamethylphosphoric triamide, acetonitrile,
acetone and nitromethane. It is al~o possible to use
mixtures of the solvents mentioned. Tetrahydrofuran,
methylene chloride and toluene are preferred for the
various steps.
Bases which can be employed for the process according to
the in~ention are in general inorganic or organic bases.
These preferably include alkali metal hydroxides such as,
for example, sodium hydroxide or potassium hydroxide,
alkaline earth metal hydroxides such as, for example,
barium hydroxide, alkali metal carbonates such as sodium
carbonate or potassium carbonate, alkaline earth metal
carbonates such as calcium carbonate, and alkali metal or
alkaline earth metal alkoxides or amides such as sodium
methoxide or potassium methoxide, sodium ethoxide or
potassium ethoxide or potassium tert-butoxide or lithium
diisopropylamide (LDA), and organic amines (tri-
alkyl(Cl-C6)amine~) such as triethylamine, and hetero-
cycles uch as 1,4-diazabicyclo~2.2.2~octane (DA~C0),
1,8-diazabicyclot5.4.0]undec-7-ene (DB~), pyrid~ne, di-
aminopyridine, methylpiperidine or morpholine. It ~8 al~o
possible to employ as bases alkali metals, such as
Le A 28 592 - 14 -
2~79268
sodium, or their hydrides such as sodium hydride. Lithium
diisopropylamide (LDA) and DBU are preferred.
In general, the base is employed in an amount from
0.05 mol to 10 mol, preferably from 1 mol to 2 mol,
relative to 1 mol of the compound of the formula (III).
The process according to the invention is in general
carried out in a temperature range from -lOO~C to +100C,
preferably at -78C.
The process according to the invention is in general
carried out at normal pressure. ~owever, it is also
possible to carry out the process at elevated pressure or
at reduced pres~ure (for example in a range from 0.S to
5 bar).
The introduction of the protective group is in general
carried out in one of the abovementioned solvents and a
base, preferably in methylene chloride using dimethyl-
aminopyridine.
The blocking i~ in general carried out in a temperature
range from 0C to +60C, preferably at room temperature
and at normal pressure.
The elimination is in general carried out in one of the
abovementioned solvents, preferably in toluene and in the
pre~ence of one of the bases mentioned, preferably DBU.
Le A 28 592 - lS -
.
2~7926~
The elimination is in general carried out in a tempera-
ture range from +30C to +130C, preferably at +50C to
+100C and at normal pressure.
Suitable bases for the hydrolysis are the customary
inorganic bases. These preferably include alkali metal
hydroxides and alkaline earth metal hydroxides such as,
for example, sodium hydroxide, potassium hydroxide or
barium hydroxide, and alkali metal carbonates such as
sodium carbonate or potassium carbonate or sodium
hydrogen carbonate and alkali metal alkoxides such as
sodium methoxide, sodium ethoxide, potassium methoxide,
potassium ethoxide or potassium tert-butoxide. Sodium
hydroxide or potassium hydroxide is particularly prefer-
ably employed.
Suitable solvents for the hydrolysis are wster or the
organic solvents customary for hydrolysis. These prefer-
ably include alcohols such as methanol, ethanol,
propanol, isopropanol or butanol, and ethers such as
tetrahydrofuran or dioxane, and dimethylformamide, and
dimethyl sulphoxide. Particularly preferably, alcohols
such as methanol, ethanol, propanol or isopropanol are
used. It is also possible to employ mixtures of the
sol~ents mentioned.
~ he hydrolysis can optionally also be carried out using
acids such as, for example, trifluoroacetic acid, acetic
acid, hydrochloric acid, hydrobromic acid, methane-
sulphonic acid, sulphuric acid or perchloric acid,
Le A 28 592 - 16 -
- .~ , . -
..:
.
. . . . . . .
: ~ ' ' -.
:' ': ~ .
' . .
.
207926~
preferably using trifluoroacetic acid.
The hydrolysis is in general carried out in a temperature
range from 0C to +100C, preferably from +20C to +80~C.
In general, the hydrolysis is carried out at normal
pressure. However, it is also possible to work at reduced
pressure or at elevated pressure (for example from O.S
to 5 bar).
When carrying out the hydrolysis, the base is in general
employed in an amount from 1 to 3 mol, preferably from 1
to l.S mol, relative to 1 mol of the ester. Particularly
preferably, molar amounts of the reactants are used.
When carrying out the reaction, in the first step the
carboxylates of the compounds according to the invention
are formed as intermediates which can be isolated. The
acids according to the invention are obtained by treating
the carboxylates with customary inorganic acids. These
preferably include mineral acids such as, for example,
hydrochloric acid, hydrobromic acid, sulphuric acid or
phosphoric acid. It has proved advantageous in the
preparation of the carboxylic acids in this connection to
acidify the basic reaction mix~ure from the hydrolysis in
a second step without isolation of the carboxylates. The
acids can then be isolated in a customary manner. In the
case of the basic heterocycles, by treatment of the
solutions of the carboxylates with the abovementioned
acids the salts of the heterocycle~ with the inorganic
Le A 28 592 - 17 -
2~79~6~
acids can also be obtained.
The alkylation is in general carried out using alkylating
agents such as, for example, (Cl-C~)-alkyl halides,
sulphonic acid esters or substituted or unsubstituted
(Cl-C6)-dialkyl or (Cl-C~)-diaryl sulphonates, preferably
methyl iodide or dimethyl sulphate.
The alkylation i8 in general carried out in one of the
abovementioned solvents, preferably in dimethylformamide,
in a temperature range from 0C to +70C, preferably from
0C to +30C and at normal pressure.
The compounds of the general formula (II) are known per
se or can be prepared by a customary method
[cf. PCT WO 91/00277~.
The compounds of the general formula (III) are known or
can be prepared by a customary method, [cf., for example,
Beilstein 9, 511].
The compounds of the general formula (IV) are, as actual
substance r0presentatives, new and can be prepared, for
example, by the abovementioned process.
The abovementioned preparation processes are only given
for clarification. The preparation of the compounds of
the general formula (I) according to tha invention is not
restricted to these processe~, and any modification of
these processes can be used in the same manner for the
Le A 28 592 - 18 -
2~7~2~8
preparation.
The substituted imidazolyl-propenoic acid derivatives
according to the invention exhibit an unforeseeable,
useful pharmacological spectrum of action.
The compounds according to the invention have a specific
A II antagonistic action, since they competitively
inhibit the binding of angiotensin II to the receptors.
They suppress the vasoconstrictory and aldosterone
æecretion-stimulating effects of angiotensin II. More-
over, they inhibit the proliferation of smooth muscle
cells.
They can therefore be employed in medicaments for the
treatment of arterial hypertension and atherosclerosis.
Moreover, they can be used for the treatment of coronary
heart diseases, cardiac insufficiency, disorders of
cerebral function, ischaemic brain disorders, peripheral
circulatory disorders, functional disorders of the
kidneys and adrenal gland, bronchospastic and vascularly
conditioned disorders of the airways, sodium retention
and oedemas.
Investi~ation of the inhibition of the contraction
induced with a~onists
Rabbits of both sexes are anaesthetised by a blow to the
necX and bled, or alternativaly anaesthetised with
Nembutal (about 60-80 mg/kg i.v.) and sacrificed by
Le A 28 592 - 19 -
2~7~26~
opening the thorax. The thorax aorta is taken out, freed
from adhering connective tissue, divided into 1.5 mm-wide
ring segments and these are individually transferred
under an initial loading of about 3.5 g to 10-ml organ
baths containing 95% 2/5~ CO2-aerated Rrebs-Henseleit
nutrient solution, thermostated at 37C, of the following
composition: 119 mmol/l NaCl; 2.5 mmol/l CaCl2 x 2H20;
1.2 mmol/l RH2PO4; 10 mmol/l glucose; 4.8 mmol/l KCl;
1.4 mmol/l MgSO4 x 7 H2O and 25 mmol/l NaHCO3.
The contraction~ are detected isometrically by Statham
UC2 cells by means of a bridge amplifier (ifd M~lheim or
DSM Aalen) and digitised and evaluated by means of an A/D
converter (System 570, Keithley, Munich). The determina-
tion of agonist dose-response curves (DRC) is carried out
hourly. With each DRC, 3 or 4 individual concentrations
are applied to the baths at 4-min intervals. After the
end of the DRC and subsequent washing-out cycles (16
times, in each case about 5 sec/min with the above-
mentioned nutrient solution), a 28-minute rest or
incubation phase follows, in the course of which the
contractions as a rule reach the starting value again.
The height of, in the normal case, the 3rd DRC is used as
a reference quantity for the assessment of the test
sub~tance to be investigated in further passages, which
test substance in the following DRCs is applied at the
stsrt of the incubation time to the bath~, in each case
in an increasing dose. Each aorta rlng is in this case
stimulsted for the whole day, always with the same
Le A 28 592 - 20 -
2~79~6~
agonist.
Aqonists and their standard concentrations (administr-
ation volume ~er individual dose = 100 ~
XCl 22.7;32.7;42.7;52.7mmol/l
l-Noradrenaline 3x10-9;3x108;3x10-7;3x10-6 gtml
Serotonin lOa;10';106;10 g/ml
B-HT 920 10-7 lo~6 10-5 g/ml
Methoxamine 10-' 10-6 10-5 g/ml
Angiotensin II 3x109;108;3xlOa;107g/ml
For the calculation of the IC50 (concentration at which
the substance to be investigated causes a 50% inhibi-
tion), the effect in each case at the 3rd = submaximal
agonist concentration i9 used as a basis.
The compounds according to the invention inhibit the
contraction of the isolated rabbit aorta induced by
angiotensin II in a dose-dependent manner. The con-
traction induced by potassium depolarisation or other
agonists was not inhibited, or only weakly inhibited at
high con~entrations.
Blood ~ressure measurements on the an~iotensin II-infused
rat
Male ~istar rats ~Moellegaard, Copenhagen, Denmark)
having a body weight of 300-350 g are anaesthetised with
thiopental (100 mg/kg i.p.). After tracheotomy, one
Le A 28 592 - 21 -
2~79268
catheter is inserted in the femoral artery for blood
pressure measurement and one catheter is inserted for
angiotensin II infusion and one catheter is inserted for
substance administration, both in the femoral veins.
After administration of the ganglionic blocker pento-
linium (5 mg/kg i.v.), the angiotensin II infusion
~0.3 ~g/kg/min) is started. As soon as the blood pressure
values have reached a stable plateau, the test substances
are administered either intravenously or orally as a
suspension or solution in 0.5% Tylose~ The blood pressure
changes under the influence of substance are indicated in
the table as average values + SEM.
Determination of the antihypertensive activity in
conscious hypertensive rats
Th~ oral antihypertensive activity of the compounds
according to the invention was tested on conscious rats
having surgically induced unilateral renal artery sten-
osis. For this, the right renal artery was constricted
with a silver clip of 0.18-mm internal width. In this
form of hypertension, the plasma renin activity is
increased in the first six weeks after intervention.
The arterial blood pressure of these animals was measured
by bloodless means at defined time intervals af~er
substance administration using the ~tail cuff". The
substances to be tested were administered intragastrally
(llorallyl') by ~tomach tube in different doses, suspended
in a ~ylose su~pension. ~he compound~ according to the
invention lower the arterial blood pressure of the
Le A 28 592 - 22 -
~' ''.`'~
,
2~79268
hypertensive rats at a clinically relevant dosage.
In addition, the compounds according to the invention
inhibit the specific binding of radioactive angiotensin
I I in a concentration-dependent manner.
Interaction of the compounds accordina to the invention
-
with the angiotensin II receptor on membrane fractions of
the adrenal aland cortex of cattle
Adrenal gland cortice~ of cattle (AGC), which have been
freshly removed and carefully freed from gland medulla,
are comminuted in sucro~e solution (0.32 M) with the aid
of an ~ltra-Turrax (Janke & Kunkel, Staufen i.B.) to give
a coarse membrane homogenate and are partially purified
in two centrifugation steps to give membrane fractions.
The receptor binding investigations are carried out on
partially purified membrane fractions of bovine AGC using
radioactive angiotensin II in an assay volume of 0.25 ml,
which in detail contains the partially purified membranes
(50-80 ~g)~ 3H-angiotensin II (3-5 nM), test buffer
solution (50 mM tris, pH 7.2, 5 mM MgCl2, 0.25% BSA) and
the substances to be investigated. After an incubation
time of 60 min at room temperature, the unbound radio-
activity of the samples is separated by means of
moistened glass fibre filters (Whatman GF/C) and the
bound radioactivity is measured spectrophotometrically in
a scintillation cocktail after washing the protein with
ice-cold buffer solution (50 mN tris/HCl, pH 7.4,
5% PEG 6000). ~he analysis of the raw data was carried
Le A 28 592 - 23 -
207~2~8
out using computer programs to give K~ or IC50 values
(Kl: IC50 values corrected for the radioactivity used; IC50
values: concentration at which the substance to be
investigated causes a sO% inhibition of the total binding
of the radioligand).
Investiaation of inhibition of the proliferation of
smooth muscle cells by the comPounds accordina to the
invention
To determine the antiproliferative action of the com-
pounds, smooth muscle cells are used which have been
obtained from the aortas of rats by the media explant
technique ~R. Ross, J. Cell. Biol. 50, 172, 1971]. The
cells are inoculated in suitable culture dishes, as a
rule 24-hole plates, and cultured at 37C in 5% C02 for
2-3 days in medium 199 containing 7.5% FCS and 7.5~ NCS,
2 mM L-glutamine and 15 mM HEPES, pH 7.4. After this, the
cells are synchronised by withdrawal of serum for
2-3 days and then stimulated into growth with AII, serum
or other factors. At the same time, test compounds are
added. After 16-20 hours, 1 ~Ci of 3H-thymidine is added
and the incorporation of this substance into the TCA-pre-
cipitable DNA of the cells is determined after a further
4 hours.
The new active substance can be converted in a known
manner into the customary formulations, such as tablets,
coated tablets, pills, granules, aerosols, syrups, emul-
sions, suspensions and solu~ions, using inert, non-toxic,
Le A 28 592 - 24 -
' ' ' ' ' ' ~ , : , .
. -: , . ~
2~7~2~
23189-7405
pharmaceutically suitable excipients or solvents. The therapeuti-
cally active compound should in each case be present ir. a concen-
tration of about 0.5 to 90% by weight of the total mixture, i.e.
in amounts which are sufficient in order to achieve the dosage
range indicated.
The formulations are prepared, for example, by extend-
ing the active substances with solvents and/or excipients, if
appropriate using emulsifiers and/or dispersants, where, for
example, in the case of the use of water as a diluent, organic
solvents can be used as auxiliary solvents if appropriate.
Administration is carried out in a customary manner,
preferably orally or parenterally, in particular perlingually or
intravenously.
In the case of parenteral administration, solutions
of the active substance using suitable liquid excipients can be
employed.
The invention also extends to a commercial package
containing, as active pharmaceutical ingredient, a compound of the
invention together with instructions for its use for treating
arterial hypertension and atherosclerosis.
In general, it has proved advantageous on intravenous
administration to administer amounts of about 0.001 to 1 mg/kg,
preferably about 0.01 to 0.5 mg/kg of body weight to achieve
effective results, and on oral administration the dosage is about
0.01 to 20 mgtkg, preferably 0.1 to 10 mg/kg of body weight.
In spite of this, it may be necessary to depart from
the amounts mentioned, in particular depending on the body
- 25 -
, . ` '~
' , '
2079268
weight or the type of administration route, on individual
behaviour towards the medicament, the manner of its
fo~mulation and the time or interval at which admini-
stration takes place. Thus, in some cases, it may be
sufficient to manage with less than the abovementioned
minimum amount, while in other cases the upper limit
mentioned must be exceeded. In the case of the admini-
stration of larger amounts, it may be advisable to divide
these into several individual doses over the course of
the day.
Startin~ Com~ounds
ExamPle I
Methyl 2-benzyl-3-t2-n-butyl-4-chloro-1-{(2'-(N-tri-
phenylmethyl-tetrazol-5-yl)biphenyl-4-yl)methyl}-lH-
imidazol-5-yl]-3-hydroxy-propionate
c~
~ ~\CO2CH~
l~
(C6H5)3C y~ ~
Le A 28 592 - 26 -
. . :.:'' :
2~7~268
180.4 mg (l.1 mmol) of methyl 3-phenylpropionate are
dissolved in 2 ml of THF, then 0.8 ml of a l.S M s~lution
of lithium diisopropylamide in cyclohexane (1.2 mmol) is
added at -78C. The mixture is stirred at -78C for
30 min, 662.5 mg tl.0 mmol) of 2-n-butyl-4-chloro-1-t(2~-
(N-triphenylmethyl-tetrazol-5-yl)biphenyl-4-yl)methyl]-
lH-imidazol-5-carboxaldehyde in 5 ml of THF are added,
the mixture is stirred at -78C for 30 min, 5 ml of
saturated ammonium chloride solution are added at 0C and
the mixture is extracted three times with 20 ml of ether.
The organic phase is dried over sodium sulphate and the
residue is chromatographed on silica gel 60 using ethyl
acetate/petroleum ether (l:1).
Yield: 300 mg (36~ of theory)
~L = 0.53 and 0.48 diastereomer mixture (ethyl acetate:
petroleum ether l:1)
Example II
Methyl (E)-2-benzyl-3-[2-n-butyl-4-chloro-1-{t2'-(N-
triphenylmethyl-tetrazol-5-yl)biphenyl-4-yl)methyl}-lH-
imidazol-5-yl]-2-propenoate
Le A 28 592 - 27 -
207926~
~ N CO2CH3
(C6Hd3C N ~)
N~3 1
N_N
300 mg (O.36 mmol) of the compound from ExEmple I are
dissolved in 3 ml of dichloromethane, then 16 mg
tO.13 mmol) of N,N-dimethylaminopyridine ~DMAP) and 41 mg
(O.4 mmol) of acetic anhydride are added successively,
the mixture is stirred at 2SC for 1 h, and 2 ml of water
and 10 ml of ether are added. The organic phase is
extracted successively with 3 ml each of saturated sodium
hydro~en carbonate solution and saturated sodium chloride
solution, and the extracts are dried over sodium sulphate
and concentrated. The crude product thus obtained is
dissolved in 3 ml of toluene, then 183 mg (1.2 mmol) of
~;~ 1,8-diazabicyclo[5.4.03undec-7-ene (DBU) are added and
the mixture is stirred at 90C for 20 h. After cooling,
3 ml of ether are added, the mixture i9 extracted with
5 ml of ~aturnted ~odium chloride ~olution, the organic
pha~e i~ concentrated and the residue is chromatographed
:
` .
Le A 28 592 - 28 -
,
2~79268
on sil~ca gel 60 using ethyl acetate/petroleum ether
(1:2).
Yield: 120 mg (41~ of theory)
Rf = 0.63 (ethyl acetate: petroleum ether 1:1)
ExamPle III
Ethyl (E)-3-[2-n-butyl-4-chloro-1-{(2'-(N-triphenyl-
methyltetrazol-5-yl)-biphenyl-4-yl)-methyl)-lH-imidazol-
S-yl]-2-(2-methylpropyl)-2-propenoate
J\CO2C2HS
~C6Hd3C
In analogy to the procedure of Example II, the title
compound was prepared from 1.72 g (2.13 mmol) of ethyl
3-[2-n-butyl-4-chloro-1-{(2'-(N-triphenylmethyltetrazol-
5-yl)bi-phenyl-4-yl)methyl}-lH-imidazol-5-yl]-3-hydroxy-
2-(2-methylpropyl)-2-propenoate.
Yield: 440 mg ~27~ of theory)
Rs ~ 0.71 (ethyl acetate~petroleum ether ~ ls2)
Le A 28 592. - 29 -
,, :
.
2~7~268
xample IV
2-n-Butyl-1-[(2'-(N-triphenylmethyl-tetrazol-5-yl)-
b:iphenyl-4-yl)methyl]-lH-imidazole-5-carboxaldehyde
N
H
~JN
Ph ~C
A solution of 12.0 g (18.1 mmol) of 2-n-butyl-4-chloro-
1-[(2'-(N-triphenylmethyl-tetrazol-5-yl)biphenyl-4-
yl)methyl]-lH-imidazole-5-carboxaldehyde in 150 ml of
methanol is hydrogenated at about 3 bar for 1.5 h at 25C
in the presence of 1.2 q of palladium on carbon (5~
strength) and 2.46 g ~18.1 mmol) of sodium acetate
trihydrate. The solution is then filtered off from the
catalyst and concentrated and the residue i6 chromato-
graphed on silica gel using ethyl acetate/petroleum ether
( 1 : 1 ) .
Yield~ 3.85 g (34~ of theory)
R~ ~ O.41 (ethyl a¢etate/petroleum ether - 1s1)
Le A 28 S92 - 30 -
:
.
:
207926~
Example V
Ethyl 3-[2-n-butyl-1-{(2~-(N-triphenylmethyltetrazol-5-
yl)biphenyl-4-yl)methyl}-lH-imidazol-5-yl]-2-(2-methyl-
propyl)-3-hydroxy-propionate
N
N ~ CO2CH2CH3
OH
j~)N
Ph3~
5.15 ml (8.25 mmol) of a 1.6 N solution of n-butyllithium
in n-hexane are injected under protective gas at -78C
into a solution of 0.89 g (8.75 mmol) of N,N-diisopropyl-
amine in 10 ml of THF. The reaction solution is then
briefly warmed to 0C, cooled again to -789C and 1.24 ml
(7.5 mmol) of ethyl isocaproate in 5 ml of THF are added.
~he mixture is stirred at -78C for 30 min, 3.14 g
(5 mmol) of the compound from Example IV in 20 ml of THF
are added and the mixture is additionally stirred at
-78C for 1 h. It i9 then 810wly warmed to 25C, 20 ml of
satd. ammon~um chloride solution are added and it i9
extrscted three time~ with 50 ml of ethyl acetste in each
Le A 28 592 - 31 -
2Q79268
case. The organic phase is dried over sodium sulphate and
concentrated and the residue is purified on silica gel
using ethyl acetate/petroleum ether (3:1).
Yield: 1.82 g (47~ of theory)
R~ = 0.27 (ethyl acetate/petroleum ether = 2:1,
diastereomer mixture)
Example VI
Ethyl 3-acetoxy-3-[2-n-butyl-1-{(2'-(N-triphenylmethyl-
tetrazol-S-yl)biphenyl-4-yl)-methyl}-lH-imidazol-S-yl]-
2-(2-propylmethyl)-propionate
`N CO2CH2CH3
OAc
N
j~N
Ph~C
11.1 g (14.4 mmol) of the compound from Example V are
dissolved in 100 ml of dichloromethane, treated with
Le A 28 592 - 32 -
2~79,~68
626 mg (5.13 mmol) of N,N~-dimethylaminopyridine (DMAP)
and 2.04 ml (21.6 mmol) of acetic anhyride and the
mixture is stirred at 25C for 16 h. It is diluted with
ether, washed with water (l x 50 ml), satd. sodium
hydrogen carbonate solution (l x 50 ml) and satd. sodium
chloride solution (1 x 50 ml), and the organic phase is
dried over sodium sulphate and concentrated. The crude
product thus obtained is chromatographed on silica gel
using ethyl acetate/petroleum ether (2:1).
Yield: 11.7 g (lO0~ of theory)
Rf = 0.52 (ethyl acetate/petroleum ether = 2:1,
diastereomer mixture)
Example VII
Ethyl 3-[2-n-butyl-1-{(2'-(N-triphenylmethyltetrazol-5-
yl)biphenyl-4-yl)methyl}-lH imidazol-5-yl]-2-(2-methyl-
propyl)-2-propenoate
~CO2CH2CH3
\~1
j~) N
PhJC
Le A 28 592 - 33 -
207926~
11.7 g (14.3 mmol) of the compound from Example VI are
dissolved in 150 ml of toluene, 5.3 ml (35.8 mmol) of
ll8-diazabicyclo[5.4.o]undec-7-ene (DBU) are added and
the mixture is boiled under reflux for S h. A further
2 ml (13.5 mmol) of DBU are then added and the mixture is
boiled under reflux for an additional 2.5 h. After
cooling, it is washed with satd. sodium chloride solution
(1 x 70 ml), and the organic phase is dried over sodium
sulphate, filtered and concentrated. The residue is
chromatographed on silica gel using ethyl
acetate/petroleum ether (1:1).
Yield: 3 g (28~ of theory)
R~ = 0.68 (ethyl acetate/petroleum ether 1:1)
Le A 28 592 - 34 -
2~79268
reparation Examples
ExamPle 1
(E)-2-Benzyl-3-~2-n-butyl-4-chloro-1-{(2'-(tetrazol-5-
yl)biphenyl-4-yl)methyl}-lH-imidazol-S-yl~-2-propenoic
S acid
~3
/~ N CO2H
~1
N
\\N_NH
81 mg (0.1 mmol) of the compound from Example II are
dissolved in 3 ml of 2 N methanolic sodium hydroxide
solution, and the mixture is heated to boiling for
30 min, and acified, after cooling to pH 1 with conc.
hydrochloric acid and extracted with 20 ml of dichloro-
methane. After concentration, the residue is chromato-
graphed on silica gel 6Q using dichloromethane/methanol/
glacial acetic acid (lO:lsO.05).
Yield: 37 mg (67% of theory)
Rr - 0.40 (dichloromethane/methanol/glacial acetic acid -
10:1:0.05)
Le A 28 592 - 35 -
' ' ' , .
207926~
Exam~le 2
(E)-3-~2-n-Butyl-4-chloro-1-{(2'-(tetrazol-S-yl)-bi-
phenyl-4-yl)-methyl}-lH-imidazol-5-yl]-2-(4-methoxy-
benzyl)-2-propenoic acid
~OCH3
N--
~ N J~CO2H
~ .,
N ' _ /~
N
\\N_.NH
2 ml of water and 2 ml of trifluoroacetic acid are added
to a solution of 356 mg (0.42 mmol) of methyl 3-[2-n-
butyl-4-chloro-1-~(2'-(N-tripheny}methyltetrazol-5-yl)bi-
phenyl-4-yl)methyl}-lH-imidazol-5-yl]-2-(4-methoxy-
benzyl)-2-propenoate in 6 ml of THF. The mixture is
stirred at 25DC for 6 h, concentrated, taken up with
dioxane/water (1:1) and rendered alkaline with conc.
lithium hydroxide solution. The reaction mixture is
stirred at 25C for 3 h, extracted by shaking with ethyl
acetate, and the aqueous phase i9 acidified with dil.
hydroahloric acid and extracted once again with ethyl
Le A 28 592 - 36 -
207~26~
acetate. The organic phase is dried over sodium sulphate
and concentrated and the residue is chromatographed on
silica gel 60 using dichloromethane/methanol (8:1).
Yield: 230 mg (94% of theory)
S Rf = 0.63 (dichloromethane/methanol = 8:1)
Le A 28 592 - 37 -
~, . : . . ., ~ . .
.
2~7~8
Example 3
Ethyl (E)-3-[2-n-butyl-4-chloro-1-{(2'-(tetrazol-5-yl)-
biphenyl-4-yl)methyl} lH-imidazol-5-yl]-2-(2-methyl-
propyl)-2-propenoate
c~
~\CO2Et
\\N--NH
~: 5 1.5 ml of H2O and 1.5 ml of trifluoroacetic acid are added
successiv21y to a solution of 440 mg (0.56 mmol) of the
compound ~rom Example III in 10 ml of ~HF. The mixture is
stirred at 25C for 24 h, rendered basic with conc.
sodium hyd.roxide solution and washed with 20 ml of ether,
~:~ 10 and tha~aqueous phase iB :acidified with half-concentrated
hydrochloric acid and extracted three:times with 30 ml of
; ethyl acetate each time. The organic phases are dried
over sodium sulphate and concentrated and tha residue is
, chromatographed on sllica gel 60 using
~ dichloromethane/methanol (10:1).
Yield:~130 m~ (43% of theory~
: R~ - 0.31 (dichloromethane/methanol = 10:1)
: Le A 28 592 - 38 -
: ~
,~: ~ . , ;. : '. ,.,: - ~ :
'
: .
. . - .
2~7~26~
ExamPle 4
( E ) - 3-t2-n-sutyl-4-chloro-l-~t2~-(tetrazol-5-yl)biphenyl-
4 yl)methyl}-lH-imidazol-S-yl]-2-(2-thienylmethyl)-2-
pxopenoic acid
Cl ~3
--~\COOH
N
\\N_NH
In analogy to the procedure of Example 1, the title
compound was prepared from 410 mg (0.72 mmol) of methyl
(E)-3-[2-n-butyl-4-chloro-1-{(2'-(tetrazol-5-yl)biphenyl-
4-yl)methyl}-lH-imidazol-5-yl]-2-(2-thienylmethyl)-2-
propenoate.
Yield: 110 mg (28% of theory)
Rf = 0.27 (dichloromethane/methanol = 10:1)
Le A 28 592 - 39 -
2~79~6~
xample 5
Et.hyl 3-[2-n-butyl-1-{(2'-ttetrazol-5-yl)biphenyl~4-yl)-
me!thyl}-lH-imidazol-5-yl]-2-(2-methylpropyl)-2-propènoate
N ,
H3C ~ 1~,
CO2CH2CH3
N
\~N_NH
A solution of 3.0 g (3.9 mmol) of the compound from
S Example VII in 30 ml of methanol is 810wly treated with
2 ml of conc. hydrochloric acid. After 15 min, the
reaction solution is poured into 300 ml of water and
extracted with dichloromethane (4 x 70 ml), and the
organic phase is dried over sodium sulphate, filtered and
concentrated. The residue is chromatographed on silica
gel using toluene/methanol~glacial acetic acid
: (3555:0.5).
Yield: 1.88 g (94% of theory)
R~ - 0.21 (toluene/methanol/glacial acetic acid -
35s5~0.2)
Le A 28 592 - 40 -
207926~
Example 6
3-~2-n-Butyl-1-{(2'-(tetrazol-S-yl)biphenyl-4-yl)methyl~-
lH-imidazol-5-yl]-2-(2-methylpropyl)-2-propenoic acid
/~
H3C N ~3~J~co2
N
~\N_NH
A solution of 1 g of sodium hydroxide in 10 ml of
methanol is added to a solution of 1.44 g (2.8 mmol) of
the compound from Example 5 in 50 ml of methanol and the
mixture is stirred at 50C for 16 h. After cooling, it is
rendered acidic with dil. hydrochloric acid, extracted
with dichloromethane (3 x 75 ml) and ethyl acetate (3 x
75 ml), and the combined organic phases are dried over
sodium sulphate, filtered and concentrated. The residue
is chromatographed on silica gel using
toluene/methanol/glacial acetic acid (35:5:0.2).
Yield: 0.41 g (30% of theory)
R~ = 0.12 (toluene/methanol~glacial acetic acid
35:5:0.2)
The compounds shown in Table 1 are prepared in analoqy
to the abovementioned procedures.
General rocedure for the re aration of the salts:
P P P
A solution of the corresponding imidazolyl-propenic acid in dioxane/water
is neutralized with equimolar amounts of 1 N NaOH, freezed and lyophilized ~~
overnight.
Le A 28 592 - 41 -
:,
2~7~26~
Table1:
R2 R3
N----~' ,~
H3C'`~ N ~ Co2R4
~,
~fq
,N
N
\`N_NH
Ex.No. R2 R3 R4 Isomer Rf
7 CI ~ H (Z) 0,32b)
8 H ~ -CH3 0~l8c)
9 H ~ H 0,17d)
s
H ~ H 0,19a)
11 H ~ OCH3 H O,lSd)
12 H ~ H 0,16d)
13 H ~F H 0~l6d)
~: 14 H ~ -CH3 0,24d)
:
a) dichloromethane/methanol ~ 10:1
~; b) dichloromethane/methanol ~ 5:1
c) toluenetmethanol/glacial acetic acid = 35:5:0.2
d) toluene/methanol/glacial acetic acid = 35:5:1
~: e) dichloromethane/methanol/glacial acetic acid
10:1:0.05
LeA2859_ -42-
207926~
The compounds of Table 2 are prepared according t the above prepartion procedure.
Table 2:
N
H3C-(CH2)3--~';~ Co2R4
,~
N ~J
N~ ,~M~3
N
Ex.No. R3 R4 M
CH(CH3)2 C2H5 Na
16 CH(CH3)2 Na Na
17 ~ CH3 Na
18 J~3 Na Na
19 J3' CH3 Na
~¢~ F Na Na
:
:
Le A 28 592 - 43 -
, :
~; ' . . ~ .. . .
. : '`
'' , , ~,
. ~