Note: Descriptions are shown in the official language in which they were submitted.
WO 91/lStOO
,~ PCT/GB91 /OOSl 7
,f ~ :-
r .
Z~ '~3 ~
USE OF TRINITROBENZENES OR CARMINIC ACID
IN THE TREATMENT OF CANCER OR VIRAL DISEASES
The present invention relates generally to compounds and
therapeutic formulat~on~ ba6ed on trinitrobenzene and~or
carminic acid (or their derivatives~ which ln certain low
concentrations exhibit anti-cancer or anti-vlral activity.
Traditional cancer treatments generslly involve surgery,
radiotherapy, chemotherapy, or some combination ~hereof.
w~ e these treatments are often effecti~e in lengthening a
pBtient' 8 life or sometime6 eradicating the cancer, they ha~e
well-known serious side effectq. More recently, alternative
attempt6 to treat cert in cancera have been developed. One
such technique, ~immunotherapy,~ is designed to strengthen the
innate ability of the patlent's immune sy~tem to flght cancer.
While ~iagnostlc and treatment techniques have improved
significantly over the last decades, there has been very
little impro~ement in the overall survi~al rate in patients,
especially those with solid tumours treated by these orthodox
methods. And, although alternative techniques ~uch as
"immunotherapy" show promise, the treatments de~elopsd thus
far can only help a limited number of patients, 6till exhibit
toxic side effects and~or are complex and e~pensive to per~orm.
53U~3~;TITUTE 5~EE~
:~ W O 91/~5200
PC~r/G891/00517
` 2
In the area of anti-viral medicine, many anti-viral drugs
are known, but therapy msy involve hi~h doses or be of limited
effect. In HIv treatment, AZ~ is the only significantly
effective drug foun~ to date, but treatment re6ults are
variable and the drug i~ expen6ive.
The present invention approache6 these problems through
the low dose use of readily avallable trinitrobenzene
compounds ~nd~or carminic acid (either singularly or in
combination). lt has now been found, most surprisingly, that
such compound3 are efficaciou~ a~ anti-cancer or ant~-viral
agents when administered at low concentrationq, reg~rdless of
patient bodyweight. Toxicity and expense problems a~sociated
with the prior art thus do not apply.
Thus, in one aspect the invention proYideq for use in the
therapy or prophylasis of neopl~sm or viral lnfectlon in human
snd non-human animals, a formulation comprising a compound
dis~ol~ed or di6persed in an aqueous medium at a concentration
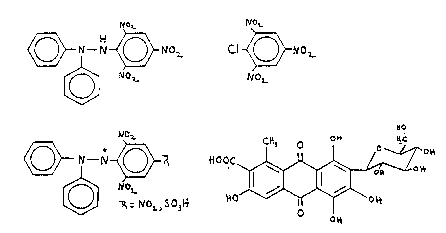
of 10-3 to 1~-15 moles per litre and having the general formula:
~1~
>~ O~L
~J2.
W091/15200 PCT/GB91/00517
f 3 Z~
wherein X is ~elected from OH, NH2 halogen, a 6ulfo group, a
carboxyl group, OCH3, or a ~ubstituted or un6ubstitute~
hydrazyl group of the formuls:
Z - N - N -
Y A
wherein A i5 hydrogen or an unpaired electron of the nitrogen
atom, Y is hydrogen or an organic group and Z i~ an organic
group, or Y and Z together with the ad~acent nitroqen atom
form a nitrogen-contalning heterocycle; provided that when X
is a 6ubstituted or un~ubstituted hydrazyl group as aforesaid
one of the NO2 groups may be replaced by a sulfo group. The
~ulfo group is prefer~bly a sulphonate salt group, optionally,
sodium or potassium sulphonate (SO~Na or SO3R) and the
carboxyl group is ~referably a carbo~ylato salt group,
optionally, sodium or potas~ium carboxylate.
In another aspect the in~ention provide~ for use in the
therapy or prophyla~is of neoplasm or viral infection in human
and non-human anlmals, a formulation comprising:
(a) one or more compounds of the g~neral formula:
X - P
wherein P is a nitrophenyl, and X i~ ~elected from O~,
NH2 halogen, a sulfo group, a carbo~yl ~roup, OCH3 or a
substituted or unsubstituted hydrazyl group of the
formula:
WO 91/15200 PCI'/GB91/00517
2J`7~
Z - N - N -
Y A
wherein A is hydrogen or an unpaired electron of the
nitrogen atom, Y is hydrogen or an organic group and Z is
an organic group, or Y and Z together with the adjacent
nitrog~n atom ~orm a nitrogen-containing heterocycle;
provided that when X is a substituted or unsub~tituted
hydrazyl group QS afore~aid one of the NO2 groups may be
replaced by a sulfo group; and
~b) a quinone, such a~ carminic acid or its derivative3.
A further aspect of the invention i8 a pharmaceutical or
veterinary formulation wherein a compound as defined above i5
di~olved or dispersed in an aqueous medium at a concentration
of from about 10-3 to 10-15 moles per litre.
Signiftcantly, carmlnic acid has been found to axhibit
anti-viral effects when used alon~ at low molar concentration~
accord~ng to the invention. Thus, an important aspect of the
invention ia the use of carminic acid and its derivetives in
the preparation of a medicament for the prophylaxis or therapy
WO91/15200 PCT/GB91/00517
5 2~?7~ 3
of viral disease such as AID5. Such derivatives have the
following general formula:
R~o~l
~ o~
where ~ is COO~ (i.e., carminic acid) or 60me other or~anic or
inorganic functional group such as NH2, SO3LX, H or Na], snd
the C-glycoside is any sugar. The anthraquinone may
optionally be a benzo~uinone (single ring) or napthaquinone
(double ring).
Without being tied to an exact mechani~m, it i3 believed
thst the compounds referred to above function by initiating
and propagating a free radical mechanlsm, thereby producing
active chemical species that ~electively attack abnormal cell
structures. In Ç~SQL ~a~rch 36 (1978), 1745-1750, Bachur
et al describe possible free radical mech~nisms in connection
with the known biological actionc of quinone-containing
anti-cancer drugs. It is postulated that these drugs may
generate oxygen-dependent free radicals ~uch as superoxide or
hydro2yl radicals. In the present invention, it is likely
thst the abo~e mentioned compound~ serve as cataly6ts for a
redo~-recycling mechanism which continuou~ly generates free
radicals such as superoxides. The free radicals, or their
by-p~oducts, selectively attack cancer cell~ or viruses.
WO91/15200 PCT/GB91/00517
2~ J~ 6
Preferred formulations according to the invention
comprise a trinitrobenzene derivative (such as picryl chloride
or diphenyl picrylhydrazine (DPPH)), an anthraquinone having a
glycosidic moiety (such as carmlnic acid or a derivative
thereof), or an admixture of one or more trinitrobenzene
derivatives and an anthraquinone glycoside. Carminlc acid has
been used in the laboratory for staining nucleic acids and,
interestingly, its effect on DNA ls inhibited by, in5~r ~li~,
free radical scavengers (Lown et al., B1ooraanic ~hQm.
(1979), l7-29). Again, such a formulation contains the actiYe
ingred~ents dissolved or su pended in an aqueou~ medlum at a
concentration in the range from 10-3 to lO-1s moles per litre,
and may be admin~stered orally or parenterally. Carminic acid
is especially useful for anti-viral treatment.
For 3 more complete understsnding of the present
invention and the advantage6 thereof, reference ~hould be made
to the following detailed description taken in connection with
the accompanying figures in which:
FIGURE l is a plot of the a~erage survival ti~e in days
of NMRI mice transplanted with MAC16 colon carclnoma cells
after treatment wlth various therapeutically-~ffective
concentrations of picryl chloride according to the teachings
of the pre~ent invention; and
WO91/1520Q PCT/GB91/00517
~ ~ 2~
F~GURE 2 is a plot of the dose response of a GM892 cell
line to diphenyl picrylhydrazyl ~DPPZ) and diphenyl
picrylhydrazine (DPPH) according to the teachings of the
invention.
Turning now to the various specific aspectQ of the
invention, a compound cspable of initiating and propagating a
free radical mechanism ~ay be dissolv~ad or disper6ed in an
aqueous medium at a therapeutically-effective concentratiol~ in
the range of from about 10-3 - 10-15 moles per litre. Such
compounds catalytically trigger free radical chain reactions,
thereby producing active chemical ~pecie6 that selectively
attsck abnormsl cells. One such set of compound~ include
derivati~es of nitrobenzene o~ the following general formula:
X ~~ ~JOL
wherein X is selected ~rom ~H, N~2 halogen, a sulo group, a
csrboxyl group, OCH3 or a ~ubstituted or unsubstituted
hydrazyl group. When X i5 the hydroxyl radicsl, the compound
is picric acid. ~hen X is chloride, picryl chloride is
formed, and so forth. The Sulro group is preferably a
~ulphonate salt group, optionally, sodium or potassium
~ulphsnate (SO3Na or SO3K) and the carboxyl group is
pr~ferably a carboxylate salt group, optionslly, sodium or
WO91/15200 PCT/GB91/00517
2~ ~ ~8`~ 8 ~ ~
potasslum carboxylate. The halogen is Cl, Br or F.
Preferably, the hydrazyl group or derivative ls a radical of
the followinq general formula:
2 - N - N -
Y A
wherein A is hydrogen or the unpaired electron of one nitrogen
atom, Y i~ hydrogen or an organic group and Z i6 an organic
group, or Y znd Z together with the adja~ent nitrogen atom
form a nitrogen-containing heterocycle (e.g., a carbazyl
group); provlded that when X is a sub6tltuted or unsubstituted
hydrazyl group as afore~aid one of the NO2 groups may be
replaced by a sulfo group. For example, where A i9 hy~rogen
and Y and Z are phenyl groups, the overall compound become~
diphenyl picrylhydrazine (DPP~). Where A i5 an unpaired
electron and Y and Z are phenyl groups, th~ diphenyl
picrylhydrazyl (DPPZ) radical ia formed. Carbazyl picrylamine
(CPZ) i~ formed when A i6 hydrogen and Y and Z are paired
phenyl groups, thus forming a nitrogen-containing
heterocycle. One particularly deairable water-soluble
derivative has the following Rtructural formula for the
hydrazyl-derived group:
5O3X - Z - N - N -
Y A
SO3K
WO91/152~ PCT/GB91/~517
9 2~
wherein Y and Z are phenyl groups, and potassium i6 optionally
hydrogen or sodium.
Another particularly ~uitable water soluble derivative is
diphenyl dinitro~ulfonate phenylhydrazyl (DDSH), which
includes a sulfonats potas6ium salt group SO3R (substituted
for the 5-NO2 group) and i9 preferably syntheslzed by the
interactlon of diphenylhydrazine with the pota6sium salt o~
2-chloro-3, 5-dinitro-benzene ~ulfonic acid in dilute alcohol
or dilute dioxane, with subsequent o~idation of the resultin~
hydrazin~ by lead dioxide. Additional detall6 concerninq the
synthesis of the DDS~ radical are set forth in In~estigation
In The Field Of Th~ Chemistry Of F~99L~ 18 Of The
Hy~r~ u~ SeLiQ~ ~ VITI, by M.A. Ikrina et al, Zhurnal
Obschei ~himii, Volume 32, No. 12 at 3952-3957, Decsmber,
1962, incorporated here by reference.
It has been shown that trinitrobenzene deriv~tives as
above, e.g., dip.henyl picrylhydrazine, are effective when
admini~tered to a host in a substantially pure aqueous
solution/.~u~pension ranging at dilutions between about 10-3 -
l0-15 molar concentration (i.e., ~therapeutically-effective
concentrations~). FIGURE l is a pl~t of the average survival
tlme ln days of NMRI mice transplanted with MAC16 colon
carcinoma cells after treatment by subcutaneous lnjection with
various therapeutically-effective concentrations of picryl
W091/152~ PCTtGB9l/~517
2~
chloride according to the teachings of the pre~ent lnvention.
Aq seen in FIGURE 1, the untreated control anlmals lived an
average of les~ than 10 days, whereas animal~ treated with the
variou6 ~pecified concentration~ of picryl chloride had
significant survival rates. The best results were obtained at
10-1Z ~olar concentration when the animals were injected
subcutaneously for five ~5) days (one injection per day). At
this concentration, most of the treated animals were
tumor-free on dsy 60.
FIGURE 2 i~ a plot of the dose response of 3 GM892 cell
line to diphenyl picrylhydrszyl (DPPZ) and diphenyl
picrylhydrazine (DPPH). The figure represent6 an average of
3-6 different experiments, with a Coulter counter u~ed to
determine the cell count. As seen in F~GURE 2, both agents
provide signlficant in-YiSLQ anti-tumor effects on this cell
line when admini6tered in a ~ubstantially pure aqueous
solution/3uspension ranging at dilution6 between about 10-3 -
10-l5 molar concentration.
Sig~ificant therapeutic resultq are obtaine~ u61ng
pharmaceutical or Yeterinary compositions ba~ed on the above
compounds, singularly or in combination, di~salYed or
di6per6ed in an squeous medium in the concentrations
pre~iously described. For example, carminic acid alone (or a
deriYatl~e thareof) is especially us~ful as sn anti-vl~al
WO91/15200 2~ t2~'n ~ PCT/GB91/00517
agent. Carminic acld has a C-glyco~ide (C6H~1O5) side-linked
to a polyhydroxyanthraquinone 88 e~ldenced by the following
formula:
0~
c I~
tn combinatlon form, one preferred compo~itlon i8 an admisture
of one or more trinitrobenzene derl~atives and an
anthraquinone having a glycosidic moiety, optlonally carmlnic
ac~d. For e~ample, one such composition i5 an admi~ture of
picryl chloride (or picryl 6ulfonate) ana car~inic aci~.
Although not meant to be limitin~, the preferred ratio of
picryl chlorlde to carminic acid i9 preferably between l:l and
1:2 but with the concentration of the acti~e ingredients
being in a therapeutically-effecti~e conc~antration of between
about 10-3 - lO-15 molar concentratlon. A
therapeutically-effective amount of the ph~rmaceutically
composition in ~olutton or suspension i~ betw~en 2.0-5.0 mls,
and this amount i8 3pparently substantt~lly tndependent of the
bo~yweight of the ho6t 3nimal.
I~ desired, more than one trinltrobenzene can be
advanta~eously incorporated into the admixture. For e~ample,
when plcric acid and DPPH are uBed, these trinitroben2enes msy
act synergi6tically in gen0rating free radicals and a fre-
W091/15200 PCT/CB91/00517
2~ 2
radical chain reaction mechanism. The qulnone, if used, canalso be a -Qource of OH free radicals. In one embodiment,
predetermined amounts of picric acid, DPPH and carminic acid
are mi~ed in a substantially pure aqueous solution/suspenslon
ranging at dilutions gi~ing between about 10-3 - 10-15 molar
concentration. The DPPH has the highest dilution, followQa by
the carminic acid and then the picric acid. ~hile not meant
to be limiting, a pharmaceutical or veterinary composition may
be formed by firRt dissolving the hydrazine derivative in
double-distilled, deionized water in a clean gla6s container
under ~terile condition~. Therea~ter, the carminic acid is
added and mixed into the solution. The picric acid is then
added and the solutlon throughly mixed. Serial dilution can
then be used to obtain the desired molar concantration.
Alternatively, the three constituents are mi~ed together prior
to dissolution in the carrier.
According to other features of the invention, the
efficacy of a free radical chain reactlon mechanlEm may be
enhanced through administration of iron or any other
transitional metal, especially copper. Although not descrlbed
in detail, it i~ also envisioned that the anti-tu~or agent~
described abo~e can be admini~tered to the host
Qubcutaneously, intravenously or using an acceptable carrier
or e~cipient. Also, while double-distilled, deionized water
i~ the preferred solutlon/suspension liquid, other dilutants,
.
WO91/152~ 2~ ~ 9~ PCT/GB91/00517
f ` 1 3
such as a dimethylsulfoxide/water solution, arachis oil, olive
oil, vegetable oil or corn oil, may be useful as well. Yet
another useful catalyst for the free radical mechanism i9 a
(low concentration) polyunsaturated fatty acid, which i6 a
long chain free carboxylic acid typically found in a lipld.
It should be spprecisted that whlle the preferred
trinitrobenzene derivatives useful in accordance with the
present invention are picric acid, picryl chloride, picryl
sulfonate, diphenyl picrylhydrazine and diphenyl
picrylhydrazyl, other trinitrobenzene compounds are al~o
suitable catalysts for the free radical mechanism. Such
compounds are included within the above general formula.
In the above general formula, when A is hydrogen and Y is
a phenyl group, 2 may be, for e~ample, any aromatic group such
as shown in appendix Table I or an aliphatic group as shown in
appendix Table II. Alternatively, Y and Z may be any of the
aromatic compounds shown in appendi~ Table III. Another ~et
of "phenyl" ~erivatives is derived from the compounds ~hown in
appendis Table IV and a set of "carbazyl~ deri~atives is
defined by the formulae set forth in appendix Table V.
;~ Althou~h not meant to be limiting, the preferred hydrazine
derivatives are diphenyl picrylhydrazine (DPPH) and
d~rivatives thereo~ ~uch a~ diphenyl picrylhydrazyl (DPPZ),
WO91/15200 PCT/CB91/0051/
2~ 3~ ~ 1 4
pnenyl picrylhydrazine ~PPH), carbazyl picrylamine (C~Z) and
2-sulfophenyl, 2-sulfophenyl, picrylhydrazine.
Moreover, while the emphasis in the above discu~6ion has
been on novel anti-cancer and anti-viral therapies, it should
be appreciated that the active chelnical ~pecies generated by
the trinitrobenzene and carminic acid derivatives accordinq to
the invention may al60 evidence ~ignificant anti-fungal and
anti-bacterial effect3. Use of such low concentration
therapies is also being investigated in connection with
treatment of genetic and autoimmune di orders.
In one pilot study of the invention, a sixty four year
old fem~le with advanced cervical cancer wa~ treated with
diphenyl dinitrosulfonate phenylhydrazyl (DDSH). On
~ystematic ex~mination, the patient pre~ented wlth lower
abdominal pain, anaemia, and vaginal bleedin~. On
gynecological examination, a ldrge mass was located protrubing
from the birth canal and extendlng to the lateral pelvic
wall. The patient w3s dlagnosed with stage 3 cervical cancer
(squamou6 cell carcinoma), a finding later confirmed during
exploratory surgery in which extenslve metastatic masses
throughout the abdomen were al-qo discovered. After diagnosed
as terminal, the patient was treated with a ~ingle 2.0 ml.
subcutaneous injection of DDSH di~solved in double-distilled,
deionized water at 10-9 molar concentration. No other
WO91/15200 2~ PCT/GB91/00517
f" ' : 1 5
tre~tm~nt was undertaken. Upon recent clinical examinatlon,
the patient was found to be ree from the cancer, with no
evldence remaining of the m~tastatic masses.
In another pilot study a sixteen year old male, diagnosed
with metastatic pheochromocytoma involving the liver and
jawbone, was al~o treated with DDSH by subcutaneous injection
at 10-9 molar concentration. When fir~t examined, the patient
had extremely high blood pressure, 180/l60, an abnormal heart
rate, and extensive psin ln the jaw area. A first
subcutaneous injection wa~ given in April, 1990, with a
follow-up injection provided in November, 1990. Upon recent
investigation, the patient~s blood pressure is normal, the
extensive jawbone paln has subsided, and general health i~
consldered good.
~ a8ed on ongoing pilot studie~, carminic acid has
evidenced slgnificant anti-~iral effects when dissolved in an
aqueou~ medium at low concentrations. In one pilot study, a
thirty seven year old male was diagnosed as HIV positive by
the 6tandard ELISA test. When fir~t esamined ln November,
199~, the patient had oral thrush, very severe herpes zoost~r
of the lsft facial nerve with invol~ement of the left orbital
region, hard bilateral cervical lymph nodes, and an enlarged
liver and ~pleen. Thereafter, ~he patient was treated with
carminic acid, dissolved in double-distilled, ~eionized water
WO91/15200 PCT/CB91/00517
2 ~ 6
at 10-6 molar concentration, via su~cutaneous injection~. For
five ~ays, the patient received a Ringle 2.0 ml. ln~ection per
day. Ater five days, a similar five day course (one
injection per day for five days) wa~ repeated. After the
ourth cour~e (20 in~ections), the patient wa~ in good general
condition, W8~ no longer anaemlc, and the oral thru5h,
cer~ical lymph nodes and herpes zooster infectton had
cleared. The ~pleen and liv~r were normal and the patient had
gainea weight. Signlfic~ntly, the patient's white blood count
(~3C) had 1ncreased rom 2,000 cells/mm3 to l2,qO0 cell~/mm3,
wtth a correspondlng increase in hemoglobin (Hb) rom ll.3 to
l9.7 grams per deciliter.
TheYe findlngs are significAnt and in~icate that carminic
scid, tn therapeutically-effective concentrations a~
described, appe3rs to stimulate the immune system. It it~
believed that other quinones ha~ing ~ide-chained sugars (and
derivatives thereof) msy al~o e~hiblt anti-viral activity when
admlnistered ac w rding to the teachings herein. Thus, an
important a~pect of the in~ention i8 the use of carminic acl~
and it~ deri~atives in the preparation of ~ medicament for the
prophyla2ls or therapy of viral disease such as AIDS. Such
~Qrivstive~ have the followlng ~ene~al formula:
G~
R 7~~
~
WO91/15200 ~ PCT/GB91/~517
1 7
where R is COO~ (carminic acld) or other organic or lnorganic
functional group 3uch as NH2, SO3[~, ~ or Ns], and the
C-glycoside is any .qugar. The anthraquinone may optionally be
a benzoquinone (single ring) or napthaquinone (double ring).
While the inv~ntion has been described in part by
reference to various preferred embodiments, those skilled in
the art will appreciate that various modificationfi,
substitution6, omi6sions and changes may be made without
departing therefrom.