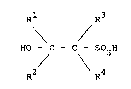Note: Descriptions are shown in the official language in which they were submitted.
2~7~23
T 5404 FF
LUBRICATING COMPOSITIONS CONTAINING ALKAI,INE
EARTH METAL SALTS
The present inventisn relates to lubricating oil
compositions and concentrates containing an alkaline
earth metal salt selected from calcium and magnesium
hydroxyalkyl sulphonates, a procPss for preparing said
compositions, and the use of said sulphonates as
detergent additives in lubricating oil compositions.
The use of alkaline earth metal salts of organic
sulphonic acids as additives for lubricating oil
compositions is well known. These salts have
0 detergent properties so that, when applied in such
luboil compositions, they ensure that the insides of
the engine cylinders remain clean and that deposition
of carbonaceous products on pistons and in piston
grooves is counteracted, thereby preventing
piston-ring sticking.
It is also well-known to prepare basic (or
overbased) alkaline earth metal salts of such acids.
The overbasing provides an alkaline reserve which,
when applied in lubricating oil compositionsr reacts
with and neutralises acidic compounds ~ormed during
the operation of the engine in which the composition
is applied. Hence, sludge which may arise is
maintained dispersed due to the detergent property of
PS25004
2~7~23
the salt, and acids which would enhance sludge and
rust formation are neutralised.
US Patent No. 3,704,105 discloses a process for
preparing an overbased barium olefin derived
r~ sulphonate which comprises reacting, in a hydrocarbon
medium, a sulphonated ole~in derivative obtained by
sulphonating a mixture of acyclic monoolefins having
from about 14 to about 30 carbon atoms; a
stoichiometric excess based on the sulphonated olefin
derivative of an inorganic barium compound, for
example, barium oxide, barium hydroxide and the barium
hydroxide hydrates such as barium hydroxide
monohydrate and the like, mixPd in a C1-C5 alXanol and
a small amount of water; heating said mixture to a
1~ temperature of from about 35C to about 150C, and
introducing a stream of sulphur dioxide or carbon
dioxide into said heated mixture until said mixture
becomes acidic. The product obtained by this process
is useful as a smoke reducing distillate ~uel
additive.
European Patent Application Publication No.
351 928 discloses a process for the preparation of
internal olefin sulphonates ~hich comprises reacting
in a film reactor an internal olefin having from 8 to
26 carbon atoms with a sulphonating agent, in a mol
ratio of sulphonating agent to internal olefin of 1:1
to 1.25:1 while cooling the reactor with a cooling
means having a temperature not exceeding 35C, and
allowing to neutralise and hydrolyse the reaction
3o product of the sulphonating step. The hydrolysis may
be carried out with hydroxides, carbonatPs or
bicarbonates of (earth) alkali metals. EP-A-351 928
does not, however, contain any statement relating to
uses of the products obtained by the process.
PS25004
2~7~2~
US Patent No. 3r896,057 discloses water-soluble
salts or acids of alkene sulphonates and hydroxyalkyl
sulphonates containing from about 10 to about 24
carbon atoms per molecule. The sulphonates have
useful properties as cleaning and laundering active
composltions .
US Patent No. 3,883,583 discloses a high
molecular, oil-soluble anionic surface activ~ agent
consisting essentially of at least one member selected
from the group consisting of a sulphonic acid,
sulphonate (salt), and mixtures thereof, of an
aliphatic monoolefin having a number of carbon atoms
in the range of 32-40 and expressed by the yeneral
formula
R R'
\. /
C = C
R" R"'
wherein R is a saturated alkyl radical having either a
straight chain structure or a branched-chain
structure; R', R" and R"' are respectively either
hydrogen or a saturated alkyl radical having either a
straight chain structure or a branched-chain
structure, providing that said R', X'l and R"' should
not all be hydrogen and the total number of carbon
atoms of said R, R', R", and Rl" is in the range of
30-38. The surface activs agent disclosed is said to
have a good lubricating effect, anti-corrosive
property, emulsifying effect and hydrating effect as
required of a dry-cleaning detergent.
3o
PS25004
~7~823
It has now surprisingly been found that certain
calcium and magnesium hydroxyalkyl sulphonates are
useful as detergent additives in lubricating oils.
In accordance with the present invention there is
provided a lubricating oil composition comprising a
ma;or proportion of a lubricating oil and a minor
proportion of an alkaline earth metal salt selected
from calcium and magnesium salts of a hydroxyalkyl
sulphonic acid of the general formula:
0
Rl R3
HO - C - C - SO H (I)
R2 \ R4
wherein each of Rl, R2, R3 and R4 independently
represents a hydrogen atom, or a linear or branched
alkyl group, subject to the total number of carbon
atoms in Rl, R2, R3 and R4 taken together being in the
range from 8 to 30, preferably from 12 to 22, and
especially from 13 to 17.
The preferred salts are the calcium salts.
It is preferred that at least one of Rl and R2
represents an alXyl group and at least one of R3 and
R4 represents an alkyl group.
The calcium and magnesium salts used in the
present invention may be neutral salts, i.e. those
containing stoichiometrically equivalent amounts of
metal and sulphonate moieties, or they may be
overbased salts as hereinafter defined. Overbased
5alt5 are preferred.
The lubricating base oils present in the
compositions of the invention are preferably
hydrocarbon lubricating oils, which may be mineral or
PS25004
2~7~23
synthetic, but ester-type lubricating base oils and
vegetable oils can also be used. The compositions may
also contain mixtures of lubricating base oils. An
example of such a mixture is a mixture of mineral
lubricating oils, for instance a mixture of a
distillate lubricating oil and a residual lubricating
oil. Another example of such a mixtuxe is a mixture
of a mineral lubricating oil and a synthetic
hydrocarbon lubricating oil. As examples of suitable
synthetic hydrocarbon lubricating oils may be
mentioned polyolefins, e.g. polyisobutylenes.
Preferably the lubricating base oil component of the
compositions according to the present invention is a
mineral lubricating oil or a mixture of mineral
luhricating oils. The viscosity of the lubricating
base oils present in the lubricating oil compositions
may vary within wide ranges, and is generally from 3
to 35 mm2/s at lO0C.
In the preparation of the calcium and magnesium
hydroxyalkyl sulphonates employed in the present
invention, a Clo~C32l preferablY Cl4-C24 and
especially Cl5-Clg, olefin is sulphonated using a
sulphonating agent, for example, as described in
EP-A-351 928. The sulphonation is preferably carried
out with sulphur trioxide in a film reactor.
Preferred is a falling film reactor.
The beta-sultone thus produced is then converted
to a neutral salt by reaction with a magnesium, or
preferably calcium, compound in water according to any
of the known procedures. For example, if the
sulphonation has been carried out in a film reactor,
the reaction mixture containing the beta-sultone is
then conducted from the film reactor to a
hydrolysis/neutralisation unit where it may be mixed
with an aqueous suspension o~ a hydroxide and/or oxide
PS25004
2~7~2~
of calcium or magnesium to yield the neutral calcium
or magnesium salt of the hydroxyalkyl sulphonic acid
of formula I as defined above. The neutral salt is
subsequently purified according to conventional known
techniques and, if desired, converted to an overbased
salt by mixing in an organic solvent with further of
the hydroxide and/or oxide of calcium or magnesium, a
promoter and a small amount of water and passing
carbon dioxide through the resulting mixture. In the
0 present context, an overbased salt denotes a salt in
which the basicity index (BI), defined as the
equivalent ratio of total calcium or magnesium (as
determined by ASTM D874-R2) to calcium or magnesium
salt of the hydroxyalkyl sulphonic acid of formula I
as defined above (as determined by ASTM D2896), is
greater than 1. Detailed descriptions of overbasing
processes are yiven in many patent specifications, for
example UK Patent No. 7861~7.
The organic solvent may conveniently be a
hydrocarbon, such as an aromatic hydrocarbon or a
hydrocarbon fraction rich in aromatics, such as
gasoline, with compounds such as benzene, toluene or,
especially, xylene being preferred.
The promoter may, for example, be a Cl-C3
alcohol, preferably methanol. The amount of water
used is conveniently one mole per equivalent calcium
or magnesium hydroxyalkyl sulphonate.
In the lubricating oil compositions of the
present invention, the total amount of calcium and/or
3o magnesium hydroxyalkyl sulphonate(s) can vary within
wide ranges e.g. from 0.1 to 20%w, preferably from 0.1
to 10%w, and especially ~rom 0.2 to 5%w, based on the
total compositicn~
The present invention further provides a
lubricating oil concentrate comprising a lubricating
PS25004
2~7~2~
-- 7
oil and an alkaline earth metal salt selected from
calcium and magnesium salts of a hydroxyalkyl
sulphonic acid of formula I as defined above, in an
amount of from lO to 80%w based on the total
concentrate. Such a concentrate generally comprises a
lubricating oi1 as solvent/diluent and one or more
additives in a concentrated form.
The present invention further provides a process
for preparing a lubricating oil composition which
0 comprises mixing a lubricating base oil with an
alkaline earth metal salt selected from calcium and
magnesium salts of a hydroxyalkyl sulphonic acid of
formula I as defined above, or with a lubricating oil
concentrate in accordance with the invention.
The lubricating oil compositions o~ the present
invention may further contain a number of other
additives, such as antioxidants, anti-wear agents,
friction modifiers, foam inhibitors, corrosion
inhibitors, viscosity index improvers, and pour point
depressants, as can be established by a person skilled
in the art.
If desired, the lubricating oil compositions may
additionally contain an alkaline earth metal alkyl
salicylate and/or alkylphenate, and/or Cl5 40 alkyl
orthoxylene sulphonate, and/or Cl5 40 alkylbenzene
sulphonate.
The present invention still further provides the
use of at least 0.1%w based on the total composition,
of an alkaline earth metal salt selected from calcium
3o and magnesium salts of a hydroxyalkyl sulphonic acid
of formula I as defined above, as a detergent additive
in a lubricating oil composition comprising a major
proportion of a lubricating oil.
The invention will be further understood from the
following illustrative example.
PS25004
2 ~ 3
Example
(A~ Preparation of Neutral Calcium Beta-Hvdroxyalkyl
Sulphonates
The sulphonation of a mixture of C15-C19 internal
olefins derived from the "SHELL" Higher Olefins
Process, having average molecular weight 230, was
carried out in a continuous falling film reactor
having a diameter of 2.54cm and a length of 6m.
Sulphur trioxide was prepared by reacting sulphur
dioxide with oxygen (dry air) over a vanadium
pentoxide catalyst at about 450C.
The Cl5-C~g internal olefins flowed along the
inner part of the reactor walls as a flowing ~ilm in a
downward direction and they reacted with the sulphur
trioxide (molar ratio sulphur trioxide/olefins was
1.06). The reactor was cooled by flowing water of low
temperature along the outside of the reactor tube so
that the temperature of the reaction mixture did not
exceed 35C. The reaction mixture was subsequently
fed, together with a calcium hydroxide slurry in water
(1.45 equivalents calcium hydroxide with respect to
sulphur trioxide), into a continuous
hydrolysis/neutralisation loop provided with a
combined pump/high shear mixer and two heat
exchan~ers. The resulting mixture was intimately
mixed by means of the pump/high shear mixer at 35-40C
and then conducted into an open ve~sel, equipped with
stirring means, and charged with a mixture of water
and calcium hydroxide. After a residence time of
3o about 40 minutes in the open vessel at a temperature
of 35-40C, the mixture was conducted to a laminar
tubular hydrolysis reactor where it was heated to
170-190C for a period of about 30 minutes.
PS25004
2~32~
Once cooled to ambient temperature (20OC), the
mixture was analysed and then purified using
conventional known techniques. Analysis of the
mixture showed it to contain 24%w neutral calcium
beta-hydroxyalkyl sulphonates (as determined by ISO
2271).
(B) PreParation of Overbased Sulphonates
420g neutral calcium beta-hydroxyalkyl
sulphonates (CaHAS) prepared in (A) above ~nd 1900g
xylene were introduced into a 31 glass reactor and
heated, with stirring, at 50C until the CaHAS had all
dissolved. 129g calcium hydroxide were then added to
the reaction mixture followed by 21g water and 390.7g
methanol (97%). The reaction temperature was
maintained at 50C whilst 23.31 carbon dioxide was
subsequently fed into the ~tirred reaction mixture
over a period of 20 minutes. The reaction mixture
continued to be stirred for a further five minutes and
was then centrifuged for 2 hours at 2300 rpm in order
to remove excess calcium hydroxide. Centri~ugation
yielded two liquid layers~ a thin, upper layer
containing mainly methanol, and a main, lower layer
containing xylene and overbased CaHAS. The lower
layer wa~ isolated and was mixed with 1796g "HVI 60"
base oil (a bright and clear high viscosity index base
oil having ~iscosity at 100C of 4.4 to 4.9 mm2/s
(ASTM D445~ and minimum ~lash point 200UC (AST~ D92)).
Xylene and residual methanol were removed ~rom the oil
mixture by rotary evaporation at 130C and 10Pa
pressure to yield a clear solution of overbased CaHAS
in "HVI 60" base oil having a Total Basicity Number
(TBN) as determined by ASTM D2896 of 56.6mg ~OH/g and
a Basicity Index (BI) of 3~0O
PS25004
2~7~2~
-- 10 --
(C) Preparation of HiqhlY Overbased SulPhonates
23.0g neutral calcium beta-hydroxyalkyl
sulphonates (CaHAS) prepared in (A) above and 105.7g
xylene were introduced into a 500ml glas~ reactor and
heated, with stirring, at 50C until the CaHAS had all
dissolved. 22.16g calcium hydroxide were then added
to the xeaction mixture followed by 1.16g water and
21.9g methanol (97%). The reaction temperature was
maintained at 50C whilst carbon dioxide was
subsequently fed into the stirred reaction mixture at
a rate of 0.064 litres/minute over a period of 84
minutes. The reaction mixture was then centrifuged
for 2 hours at 2300 rpm in order to remove excess
calcium hydroxide. Centrifugation yielded two liquid
layers, a thin, upper layer containing mainly
methanol, and a main, lower layer containing xylene
and overbased CaHAS. The lower layer was isolated and
was mixed with 66.3g "HVI 60" base oil. Xylene and
residual methanol were removed from the oil mixture by
rotary evaporation at 130aC and lOPa pressure to yield
a clear solution of overbased CaHAS in "HVI 60" base
oil having a TBN of 252.9 mg KOH/g and a BI of ~.2.
(D) Enqine Test Performance
The ability of overbased calcium beta-
hydroxyalkyl sulphonates to control the formation ofcarbonaceous and lacquer deposits in a diesel engine
was demonstrated in the 252 hour Caterpillar 1~ piston
cleanliness test. The caterpillar lK is a diesel
engine test which measures the ability of an oil to
control oil consumption and piston deposits an~ is
required for the American Petroleum Inskitute (API)
CF-4 lubricant speci~ication. In this test, a single
cylinder supercharged engine is run for 252 hours
according to ASTM Research Report RR- D.02-1273.
PS25004
2~7~2~
Piston cleanliness is rated in terms of total weighted
demerits, which is based on carbon and lacquer
deposits in all grooves and lands as well as
undercrown and pin bore areas, % heavy carbon on the
crown land and ~ caxbon in the top groove.
To demonstrate diesel detergency of this
component, the overbased sulphonate product according
to (B) above in I'HVI 60" base oil (9.4%w sulphonate
detergent) was blended in an amount giving 2.26%w of
the sulphonate detergent as active matter in a
universal engine oil formulation (SAE 15W40 grade)
comprising VI (viscosity index) improver, zinc-based
anti-wear additives and dispersants, and the
performance of the resl~lting oil was evaluated. The
results obtained are presented in Table I below.
TABLE I
Product according
to (B)
Total weighted
demerits 219
~ Top land
heavy carbon 0
Top groove
fill 3
Brake specific oil
consumption, g/kWh 0.251
PS2~004
2~7~
- 12 -
(E) Enqine Test Performance
The overbased sulphonate product according to (B)
above was ~lended in an amount giving 1.34%w active
matter in a base oil containing an additive package
comprising VI (viscosity index) improver, zinc-based
anti-wear additive and polymethacrylate pour-point
depressant. The resulting oil was evaluated according
to sequence 5E ASTM (as described in "Sequence 5E test
procedure", 7th draft dated l9th May 1988; ASTM
Monitoring Centre, 4400 5th Avenue, Pittsburgh, USA).
The sequence 5E test is a gasoline engine test
required for the API SG lubricant specification. It
tests the ability of an oil to control sludge and
varnish deposits and valve train wear in a 2.3 litre
Ford four cylinder gasoline engine run under a cyclic
operating procedure designed to simulate stop-go
running conditions. The results obtained are shown in
Table II Pollowing:
TABLE II
Engine Test
Product AES RCS AEV PSV
B 9.34 9.32 7.42 7.10
AES = Average Engine Sludge )
RCS = Rocker Cover Sludye ) (scale 0 to lO, where 10
) represents zero sludge
A2V = Average Engine Varnish) or varnish)
PSV = Piston Skirt Varnish
PS25004