Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
D a s c r i 'p t i o n
The invention concerns new nucleotide monomers as well
as oligonucleotides and polynucleotides which contain
such monomers, a process for their production and their
use for regulating gene expression as antisense probes
and as pharmaceutical agents.
Antisense oligonucleotides and polynucleotides are known
to one skilled in the art and are described in summary
for example in "Spektrum der Wissenschaft" (1990), pages
70 to 77. These are understood as the nucleotides which
are complementary to the actual gene and have a sequence
with the opposite orientation. Such antisense molecules
act on gene expression in a regulatory manner and in so
doing play an important role in determining whether a
hereditary sequence coded in a gene is translated into a
protein. In this process the separation of the two DNA
strands is triggered by a short RNA chain, the so-called
primer, which first opens up the DNA double helix and
hybridizes with the origin of replication. It has been
shown that the gene expression does not only depend on
the concentration of these primer molecules but on their
ratio to the antisense RNA. In this manner it is
therefore possible to specifically switch predetermined
genes on and off and thus to regulate the entire cell
function. Thus it has for example already been achieved
to make cells which have been malignantly transformed by
means of a,polyoma virus appear healthy by introducing
expression vectors for an antisense RNA against src into
these polyoma transformed cells. By this means these
cells loose their cancerogenic characteristics. Similar
results with this antisense technique have already been
achieved on the oncogenes fos, ras and sys. Moreover it
208 0231
- 2 -
has already even been possible to inhibit infections by
herpes viruses, influenza viruses and the HIV virus in
tissue cultures using antisense oligonucleotides.
Already with the aid of biotinylated antisense
oligonucleotides it has also been possible to
investigate the formation and action of the splicing
complex in more detail (S. Barabino, B. Sproat et,al.,
The EMBO Journal 8, 4171-4178 (1989)).
However, the antisense oligonucleotides and
polynucleotides known up to now have the disadvantage
that after being introduced into an intact cell they are
attacked and degraded by RNA- and DNA-specific nucleases
which leads to a loss in their activity. Thus it has
already been attempted to inhibit the degradation of
polynucleotides and oligoribonucleotides by nucleases by
means of 2'-0-methyl substitution (B. Sproat et al.,
Nucleic Acids Research 17 (1989), 3373-3386).
This invention seeks to provide new oligonucleotides
and polynucleotides which are resistant to attack by
nucleases and which bind ~:rith improved specificity
to a complementary nucleotide strand.
It has now been surprisingly found that this can be
achieved by substituting the 2' position with an
alkyloxy group having at least two carbon atoms.
n~,.
~~8423~.
- 3 -
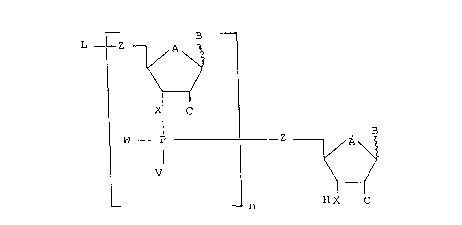
The invention therefore concerns nucleopolymers based on
2'-O-alkylnucleotides having the general formula II
L Z
X
B
W - P Z A
V
H C
in which
B represents an arbitrary derivative known to one
skilled in the art of any nucleoside base and in
particular an adenin-9-yl (A), a cytosin-1-yl (C), a
guanin-9-yl (G), a uracil-1-yl (U), a hypoxanthin-9-yl
(I) or a thymin-1-yl group (T). Of the adenine
derivatives the 2-aminoadenin-9-yl residue is preferred.
It is expedient that one or several of the nucleoside
bases carry a substituent L which facilitates attachment
to particular parts of, the cell or to enzymes or also to
suitable chromatographic material. Such affinity
substituents are known to one skilled in the art.
A denotes an O atom or a CH2 group,
X or Z represent an O atom, a S atom, a NH or CH2 group
and can be, the same or different,
V and W represent an O, S or Se atom or represent an
-OH, -SH, -NH2, alkyl or alkyloxy group.
Preferred alkyl and alkyloxy groups have 1 to 4 carbon
atoms and are in particular -CH3, C2H5 and/or OCH3 or
208 p23,~.
OC2H5. In a monomer unit in the oligonucleotides and
polynucleotide according to the present invention V and
W can be the same or different.
L represents an H atom or a partner of a binding pair.
C denotes a group having the general formula -O-R in
which R is an alkyl group with at least 2 C atom,which
may modified if desired, or an alkenyl or alkinyl group
with at least 2 C atoms which may be modified if
desired, whereby the modification consists of a
substitution by one or several halogen, cyano, carboxy,
hydroxy, nitro or/and mercapto residues. The alkyl group
preferably has 3 to 6 carbon atoms and in particular 3
or 4 carbon atoms. Examples of particularly suitable
alkyl groups are propyl and butyl, however, modified
alkyl groups such as cyanomethyl are also preferred.
Alkenyl chains are particularly preferred and in
particular alk-2-enyl residues of which in turn an allyl
residue is preferred. The propargyl residue may be
mentioned as example of an alkinyl group.
Preferred polymers according to the present invention
have one or several of the monomer units previously
described, if desired in combination with other monomer
units in which -O-R equals -O-allyl, A equals O, X
equals O, Z equals O, W equals O and V equals OH and the
Cl carbon atom of the sugar is in the 13-configuration.
In a further preferred embodiment the oligonucleotides
and polynucleotides according to the present invention
have a 3'-deoxyribonucleoside at their 3' end which
inhibits attack by 3' exonucleases and additionally
impedes degradation by these enzymes.
By incorporating a partner of a binding pair, e.g.
selected from the pairs antibody/antigen or
- 5 -
biotin/avidin or streptavidin, preferably biotin or a
dinitrophenyl residue into the polymer according to the
present invention it is possible to immobilize the
nucleotide polymer according to the present invention
and to carry out an affinity chromatography of proteins,
nucleic acids and/or protein/nucleic acid complexes
which bind to the immobilized nucleotide polymer.
A feature of the oligonucleotides according to the
present invention is their excellent hybridization to
nucleic acids that have a corresponding target sequence
which is complementary to them and that they are
particularly inert towards degradation by nucleases
which is why they have a high biological half-life in
living cells. Moreover, compared to the state of the art
they have a reduced non-specific binding to nucleic acid
binding proteins.
However, the invention also concerns nucleotide monomers
which are suitable for the synthesis of the
oligonucleotides and polynucleotides according to the
present invention and which have in the 2' position of
the sugar moeity an alkyloxy, alkenyloxy or alkinyloxy
residue with at least one carbon atom which, if desired,
is modified by one or several halogen, cyano, carboxy,
hydroxy, nitro or/and mercapto residues.
Particularly preferred residues are O-alk-2-enyl
residues and in particular O-allyl residues. Such a
- 6 -
monomer usually has the general formula I
D B
A
E C
in which A, B and C have the meanings defined above and
D and E are reactive groups capable of forming 3'-5'
internucleotide bonds or denote a -P04H2, -P207H3 or
-P3OlOH4 group. Such groups are known to one skilled in
the art and are for example described in B. Sproat et
al., Nucleic Acids Research 18 (1990), 41-49 as well as
comprehensively in E.L. Winnacker, "Gene and Klone", VCH
Verlagsgesellschaft mbH, Weinheim (Germany) (1985), in
particular pages 44 to 49 and in Froehler/Matteucci,
Tetrahedron Lett. (1986), p. 469-472. The OH group is
particularly preferred as the reactive group. The
-P04H2, -P207H3 and the -P3010H4 group are also
preferred as D and/or E. These mono-, di- or
triphosphates, or salts of the compounds having the
general formula I can be preferably incorporated as
5'-triphosphates into growing nucleic acid chains for
example enzymatically using DNA/RNA polymerases (c. f.
e.g. Random priming, Anal. Biochem. 132 (1983) 6-13,
Nick translation, J. Mol. Biol. 113 (1977) 237-251).
Using the reactive mononucleotides according to the
present invention it is possible to produce in a known
way the oligonucleotides and polynucleotides which are
also according to the present invention in particular on
a solid phase. The production of such polynucleotides
from the corresponding mononucleotides is known to one
skilled in the art and is for example also described in
-
more detail in the above-mentioned literature
references. The invention therefore also concerns a
process for the production of oligonucleotides and
polynucleotides using the nucleotide monomers according
to the present invention.
Finally the invention also concerns the use of the
oligonucleotides and polynucleotides obtained according
to the present invention as antisense probes and as
pharmaceutical agents in particular as pharmaceutical
agents for the treatment of cells infected.with viruses
such as e.g. the herpes, influenza or the AIDS pathogen
as well as for the regulation of gene expression.
The invention is elucidated in more detail by the
following examples.
E x a m p 1 a 1
2'-O-ally-oligoribonucleotides and 2'-O-methyl-
oligoribonucleotides, each having an identical sequence,
were produced by the phosphoramidite method according to
the process described by B. Sproat, B. Beijer and
A. Iribarren in Nucleic Acids Research, Vol. 18 (1990),
41-49. Subsequently both probes labelled with
32p_ phosphate at their 5' end were incubated with a
nuclear extract which was obtained from Hela cells as
described by A. Lamond et al. in Cell, Vol. 58 (1989),
383-390. Both probes were then subjected to gel
chromatography. It turns out that the 2'-O-allyl-
oligonucleotides according to the present invention have
an extraordinarily high specific binding activity and a
negligible non-specific binding activity in comparison
- 8 -
with the 2'-O-Me-oligonucleotides which are part of the
state of the art.
The nucleotide sequence was
5'-AIAACAIAUACUACACUUIA
It binds to human U2 RNA.
E x a m p 1 a 2
Various nucleases were added to the 2'-O-allyl-
oligoribonucleotides produced according to example 1 and
their sensitivity to enzymatic degradation was
determined. In comparison to normal non-modified RNA
with an identical sequence it turned out that on
digestion with pancreatic RNase A, RNase CL-3, RNase T1,
RNase T2 and RNase U2 the 2'-O-allyl-oligonucleotides
according to the present invention are completely
resistant to enzymatic attack by nucleases whereas in
contrast natural RNA is completely degraded in all
cases.
E x a m p 1 a 3
3',5'-O-(tetraisopropyldisiloxan-1,3-diyl)-2-chloro-6-
(2,6-dichlorophenoxy)purine riboside (A) was synthesized
as described by Sproat, B.S., Beijer, B. and
Iribarren, A., Nucleic Acids Research, 1990, 18, 41-49.
The compound A obtained in this way was then allylated
as described below.
Synthesis of 3',5'-O-(tetraisopropyldisiloxan-1,3-diyl)-
2'-O-allyl-2-chloro-6-(2,6-dichlorophenoxy)purine
riboside (B):
- g -
Tris(dibenzylidene-acetone)dipalladium (O) (174 mg,
0.19 mmol) and 1,4-bis (diphenylphosphine)butane
(324 mg, 0.76 mmol) were suspended in dry
tetrahydrofuran (50 ml). A solution of compound A
(13.11 g, 19 mmol) and allylethylcarbonate (4.95 g,
38 mmol) in 50 ml dry tetrahydrofuran was added and the
mixture was heated for 30 minutes under reflux. Silica
gel t.l.c. in petroleum ether/ethyl acetate (2:1 v/v)
showed a complete reaction with a single W-positive
spot of Rf 0.54 (compound A has an Rf 0.41). The solvent
was removed in a vacuum and a red syrup remained which
was dissolved in petroleum ether/ethyl acetate (9:2 v/v)
and the solution was filtered in order to remove the
insoluble Pd-phosphine complex. The product was purified
by preparative liquid chromatography on silica gel and
eluted with petroleum ether/ethyl acetate (9:2 v/v). The
pure compound B was obtained in this manner in the form
of a light yellow foam (12.4 g, 89.4 %}. 13C NMR
spectrum (CDC13)d: 158.12 (C6), 153.13 and 152.54 (C-2
and C-4), 144.66 (phenyl C-1), 142.43 (C-8), 133.75
(-CH = of allyl), 128.77 (phenyl C-2 and C-6), 128.52
(phenyl C-3 and C-5), 127.12 (phenyl C-4), 120.51 (C-5),
117.0 (= CH2 of allyl), 88.26 (C-1'), 81.16 (C-4'), 80.6
(C-2'), 71-4 (O-CH2- of allyl), 69.54 (C-3'), 59.61
(C-5'), 17.19-16.63 (isopropyl CH3s), 13.16, 12.70 and
12.29 p.p.m. (i$opropyl CHs).
Compound B was converted further via various steps into
the corresponding nucleotide monomer, namely 5'-O-
dimethoxytrityl-N2-dimethylaminomethylidene-2'-O-
allylguanosine-3'-O-(2-cyano-ethyl-N,N-
diisopropylphosphoramidite) using the method described
in Nucleic Acids Research, 1990, 18, 41-49.
'~1
- 10 -
Monomers were converted into polymers as described in
Nucleic Acids Research, 1989, 17, 3373-3386.
E x a m p 1 a 4
Synthesis of 3',5'-O-(tetraisopropyldisiloxan-1,3-diyl)-
2'-O-propargyl-4-O-(2,6-dichlorophenyl)uridine (C):
3',5'-O-(tetraisopropyldisiloxan-1,3-diyl)-4-O-(2,6-
dichlorophenyl)uridine (18.95 g, 30 mmol) was dried in a
vacuum by evaporation of acetonitrile. The remaining
foam was dissolved in anhydrous acetonitrile (50 ml), a
80 % by weight solution of propargyl bromide in toluene
(3.56 ml, 33 mmol) followed by 2-tert.-butylimino-2-
diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine
(9.55 ml, 33 mmol) were added while stirring and
excluding humidity. Silica gel t.l.c. in hexane/ethyl
acetate (2:1 v/v) showed a complete reaction after 5
hours with a W-absorbing product spot of Rf 0.38. The
solvent was removed in a vacuum leaving behind a cream-
coloured foam. The product was purified by preparative
liquid chromatography using petroleum
ether/dichloromethane/ethyl acetate (8:2:1 by volume) as
an eluant. The pure product C was obtained as a white
foam (10.7 g, 53.3 %). 13C NMR spectrum (CDC13)d: 169.80
(C-4), 154.60 (C-2), 144.76 (phenyl C-1), 144.41 (C-6),
128.69 (phenyl C-2 and C-6), 128.65 (phenyl C-3 and
C-5), 127.08 (phenyl C-4), 93.80 (C-5), 89.80 (C-1'),
81.70 (C-2'), 80.34 (C-4'), 79.54 (-C_-- of propargyl),
74.63 (-CH of propargyl), 67.63 (C-3'), 59.37 (C-5'),
58.01 (OCH2 propargyl), 17.36, 17.21, 16.90 and 16.73
(isopropyl CH3s), 13.36, 12.92, 12.84 and 12.28 p.p.m.
(isopropyl CHs).
2!~8~~~~.
- 11 -
Compound C could be converted via various steps into
cytidine and uridine monomers for the production of
solid phase polymers.
E x a m p 1 a 5
Synthesis of 3',5'-O-(tetraisopropyldisiloxan-1,3-diyl)-
2'-O-cyanomethyl-4-O-(2,6-dichlorophenyl)uridine (D):
The alkylation was carried out analogous to example 4
using bromoacetonitrile instead of propargylbromide. The
title compound was obtained as a white foam in 55
yield, Rf 0.51 on silica gel t.l.c. in petroleum
ether/ethyl acetate (1:1 v/v). 13C NMR spectrum
(CDC13)s: 169.91 (C-4), 154.57 (C-2), 144.54 (phenyl
C-1), 143.96 (C-6), 128.69 (phenyl C-2 and C-6), 128.60
(phenyl C-3 and C-5), 127.12 (phenyl C-4), 115.72 (CN of
cyanomethyl), 94.10 (C-5), 89.17 (C-1'), 82.34 (C-2'),
81.60 (C-4'), 67.27 (C-3'), 59.06 (C-5'), 55.82 (CH2 of
cyanomethyl), 17.20, 17.08, 16.80 and 16.61 (isopropyl
CH3s), 13.23, 12.70 and 12.24 p.p.m. (isopropyl CHs).
Ex a m p 1 a 6
2'-O-propylation is best carried out by 2'-O-allylation
followed by reduction of the allyl group. 2'-O-
butylation is best carried out by 2'-O-crotylation
(using crotylbromide and the process as described in
example 4) followed by a reduction of the crotyl group.
- 12 -
E x a m p 1 a 7
3',5'-o-(tetraisopropyldisiloxan-1,3-diyl)-4-O-(2,6-
dichlorophenyl)-uridine
14.65 g (60 mmol) dried uridine is dissolved in 150 ml
anhydrous pyridine and the solution is cooled in an ice
bath. A solution of 21 g (67 mmol) 1,3-dichloro-1,1,3,3-
tetraisopropyldisiloxane in 10 ml dichloromethane is
added to this over 15 minutes while stirring and
excluding humidity. After the addition is completed, the
mixture is stirred for a further 3 hours at room
temperature; afterwards the complete conversion into a
product of Rf 0.58 is observed in a thin layer
chromatogram (silica gel; mobile solvent
chloroform/ethanol 9:1). The reaction is stopped by
addition of 5 ml methanol and the mixture is evaporated
in a vacuum. The residue is taken up in 200 ml
dichloromethane and extracted twice with 200 ml 1 mol/1
sodium bicarbonate solution in each case. The organic
phase is dried over Na2S04, filtered and evaporated in a
vacuum. The residue is twice coevaporated in a vacuum
with 25 ml toluene in each case whereafter a white foamy
residue results. This is dissolved in 200 ml anhydrous
1,2-dichloroethane and 42 ml triethylamine (300 mmol)
and 22.5 ml chlorotrimethylsilane (180 mmol) are added
while stirring and excluding humidity. After a reaction
time of 30 minutes a thin layer chromatogram (silica
gel; petroleum ether/ethyl acetate 2:1) shows a complete
conversion~with a spot of Rf 0.39. The reaction mixture
is poured into 500 ml 1 mol/1 sodium bicarbonate
solution while stirring vigorously, the organic phase is
separated and dried over Na2S04. It is evaporated in a
vacuum after filtration and the residue is coevaporated
twice with 25 ml dry toluene in each case. The 2'-O-
- 13 -
trimethylsilyl derivative obtained in this way is
dissolved in 300 ml anhydrous dichloromethane and 42 ml
triethylamine (300 mmol), 19.5 g 2-mesitylenesulphonyl
chloride (90 mmol) and 1.8 g 4-dimethylaminopyridine
(15 mmol) are added while stirring and excluding
humidity. A complete conversion to a product with a Rf
0.63 is observed in TLC (silica gel; petroleum
ether/ethyl acetate 2:1) after a reaction time of 30
minutes. 1.35 g 1,4-diazabicyclo[2.2.2]octane (12 mmol)
and 19.6 g 2,6-dichlorophenol (120 mmol) are added to
the reaction solution and it is stirred for 2 hours at
room temperature. After this time the conversion is
complete as a TLC in petroleum ether/ethyl acetate 2:1
on silica gel shows (Rf 0.56). The reaction mixture is
stirred into 500 ml 1 mol/1 Na bicarbonate solution, the
organic phase is separated, dried over Na2S04, filtered
and evaporated in a vacuum. 2'-O-trimethylsilylether is
obtained as an oily, viscous residue. The syrup is
dissolved in 300 ml dichloromethane and a solution of
28.5 g p-toluene sulfonic acid monohydrate (150 mmol) in
100 ml tetrahydrofuran is added to this while stirring.
After 2.5 minutes 28 ml triethylamine is added in order
to neutralize the acid. Afterwards the reaction solution
is poured into 500 ml 1 mol/1 Na bicarbonate solution
while stirring vigorously. The organic phase is
separated, dried over Na2S04 and the solvent is
distilled off in a vacuum. The TLC (silica gel;
petroleum ether/ethyl acetate 1:1) shows a spot with a
Rf 0.59 of 2,6-dichlorophenyl-2-mesitylenesulfonate and
a further spot with a Rf 0.41 of the desired product.
The crude product is purified in several portions by
preparative chromatography on silica gel using petroleum
ether/ethyl acetate (2:1) as the eluant. After
evaporating the fractions, 25.6 g corresponding to 67.5
of the theoretical yield is obtained as the pure final
product.
- 14 -
Rf value (silica gel; petroleum ether/ethyl acetate 2:1)
0.23
13C NMR spectrum (CDC13) d: 169.82 (C-4), 154.70 (C-2),
144.94 (C-6), 144.77 (phenyl-C-1), 128.92 (phenyl-C-2
and C-6), 128.71 (phenyl C-3 and C-5), 127.11 (phenyl
C-4), 94.05 (C-5), 92.22 (C-1'), 82.01 (C-4'), 74.88
(C-2'), 68.89 (C-3'), 60.35 (C-5'), 17.40 - 16.85
(isopropyl-CH3's), 13.34, 12.91, 12.83 and 12.48 p.p.m.
(isopropyl-CH's).
E x a m p 1 a 8
3',5'-O-(tetraisopropyldisilocan-1,3-diyl)-2'-O-allyl-4-
O-(2,6-dichlorophenyl)uridine
Tris(dibenzylidene-acetone)dipalladium(0) (0.183 g,
0.2 mmol) and 1,4-bis(diphenylphosphino)butane (0.341 g,
0.8 mmol) are suspended in dry tetrahydrofuran (40 ml)
under an argon atmosphere. A solution of the compound
produced according to example 7 (12.63 g, 20 mmol) and
allylethylcarbonate (5.2 g, 40 mmol) in dry
tetrahydrofuran (60 ml) are added and the mixture is
heated for 30 minutes unter reflux. Thin layer
chromatography (silica gel, mobile solvent petroleum
ether/ethyl acetate, 2:1, v/v) is used to check that the
reaction has run to completion. The reaction product is
shown by a new band having a Rf value of 0.48. After
cooling the mixture is filtered and the solvent is
removed in a vacuum. The reaction product is purified by
preparative chromatography on silica gel with 3 % ethyl
acetate in dichloromethane as the mobile solvent.
- 15 - 2oa o231
After evaporating the fractions, 11 g (81.9 % of the
theoretical yield) of the final product is obtained.
Rf value (silica gel thin layer chromatography;
petroleum ether/ethyl acetate 2:1): 0.51
13C NMR specturm (CDC13) d: 169.61 (C-4), 154.49~(C-2),
144.64 (phenyl C-1), 144.37 (C-6), 134.29 (allyl CH),
128.75 (phenyl C-2 and C-6), 128.51 (phenyl C-3 and
C-5), 126.93 (phenyl C-4), 116.85 (allyl = CH2), 93.50.
(C-5), 89.94 (C-1'), 81.64 (C-2'), 80.40 (C-4'), 70.90
(O-CH2 of allyl), 67.49 (C-3'), 59.34 (C-5'), 17.24,
17.10, 16.79 and 16.63 (isopropyl CH3s), 13.21, 12.83,
12.70 and 12.30 p.p.m. (isopropyl CHs).
E x a m p 1 a 9
2'-O-allyl-4-0-(2,6-dichlorophenyl)uridine
5.5 g (8.19 mmol) of the compound produced according to
example 8 is dissolved in 20 ml dry tetrahydrofuran and
1.1 mol/1 tetrabutylammonium fluoride in 18 ml
tetrahydrofuran is added while stirring. The reaction is
completed after 5 minutes as shown by a thin layer
chromatogram onlsilica gel using ethanol/chloroform (5 .
95 v/v) as the mobile solvent (Rf: 0.22).
The reaction is stopped with pyridine/methanol/water
(50 ml, 3:1:1 v/v) and the solution is applied while
stirring to the pyridine form of Dowex 50 Wx4-200
(Trade Mark) resin (30 g suspended in pyridine/methanol/-
water 50 ml 3:1:1 v/v). The mixture is stirred for 20
minutes, the resin is filtered off and washed with
the above-mentioned solvent (3 x 50 ml).
The combined filtrates and washing
- 16 -
solutions are evaporated in a vacuum to dryness, taken
up in toluene and evaporated again. The crude product is
purified in 3 portions by preparative chromatography on
silica gel with 6 % ethanol in chloroform as the eluting
agent. After evaporating the fractions in a vacuum,
ethanol and pyridine residues are removed by addition of
toluene and again evaporating in a vacuum at 45°C. After
evaporation 2.91 g (82.9 % of the theoretical yield) of
the pure final product is obtained.
Rf value (silica gel; ethanol/chloroform 1:4): 0.57
13C ~ spectrum (CDC13)8: 169.74 (C-4); 155.28 (C-2),
146.20 (C-6), 144.42 (phenyl C-1), 133.54 (allyl CH),
128.67 (phenyl C-2 and C-6), 128.57 (phenyl C-3 and
C-5), 127.13 (phenyl C-4), 117.98 (allyl = CH2), 94.41
(C-5), 89.60 (C-1'), 84.54 (C-4'), 81.01 (C-2'), 71.10
(allyl CH20), 67.43 (C-3') and 59.55 p.p.m. (C-5').
E x a m p 1 a 10
2'-O-allyl-uridine
2.91 g (6.79 mmol) of the compound prepared according to
example 9 is dissolved in 20 ml dry acetonitrile. 2.82 g
(16.98 mmol) 2-nitrobenzaldoxime and 1.76 g (15.28 mmol)
1,1,3,3-tetramethylguanidine in 20 ml dry acetonitrile
are added and the mixture is stirred for 18 hours at
room temperature. A thin layer chromatogram on silica
gel using ethanol/chloroform (1:4 v/v) as the mobile
solvent shows that the reaction has run to completion
(Rf 0.37). The solvent is removed by evaporation in a
vacuum and the remaining residue is dissolved in 100 ml .
dichloromethane and the product is extracted with 100 ml
- 17 -
water. The aqueous phase is washed with 100 ml
dichloromethane and subsequently with 100 ml diethyl
ether. The slightly yellow aqueous phase is subsequently
stirred for 5 minutes with the pyridine form of Dowex 50
Wx4-200 resin (25 g). The resin is removed by filtration
and the turbid filtrate is washed twice with 50 ml
dichloromethane and subsequently with 100 ml ether. The
aqueous phase is evaporated in a vacuum. Traces of water
are removed by addition of methanol and tetrahydrofuran
and subsequently evaporation. The desired compound is
crystallized from methanol, filtered off and washed with
ether and dried. 1.83 g (94 % of theory) of 2'-O-
allyluridine is obtained.
Rf value (silica gel; ethanol/chloroform 1:4 v/v): 0.39
13C ~ spectrum (pyridine - d5)s: 164.48 (C-4), 151.72
(C-2), 140.76 (C-6), 135.11 (CH of allyl), 116.96 (allyl
- CH2), 102.17 (C-5), 88.26 (C-1'), 85.72 (C-4'), 82.57
(C-2'), 71.38 (allyl CH20), 69.41 (C-3') and 60.75
p.p.m. (C-5').
E x a m p 1 a 11
2'-O-allyl-uridine-5'-monophosphate
1.42 g 2'-O-allyl-uridine (5.0 mmol) is phosphorylated
according to the method of Yoshikawa et al. (1967)
Tetrahedron Lett. 50, 5065. The yield was 980 mg after
chromatographic purification.
~8p~~~.
- 18 -
E x a m p 1 a 12
2'-O-allyl-uridine-5'-diphosphate and -5'-triphosphate
In order to produce di- and triphosphates, 365 mg
(1 mmol) of each of the monophosphates was reacted in
each case with orthophosphoric acid and pyrophosphoric
acid according to the method of Hoard and Ott (1965) J.
Am. Chem. Soc. 87, 1785. The yields were 220 mg
diphosphate and 140 mg triphosphate.
All 5'-phosphates were characterized by means of
elementary analysis, electrophoresis and 31P-NMR
spectroscopy.