Note: Descriptions are shown in the official language in which they were submitted.
WO 91/17~3 ~ 8 ~ PCT/US9~/02837
.
~;~
A METHOD OF DETERMINING TYP~ SPECIFIC ~NTIBODIES TO
~ERPES SIMæLEX VIRUS TYPES 1 AND 2
FIELD OF TH~ E~IQ~
This invention concerns a method of serotyping
antibodies to herpes simplex virus types 1 and 2, and,
in particular, to a method of serotyping which employs
monoclonal antibodies to HSV l gG and HSV-2 gG which are
not susceptible to heterologous blocking.
B~CK~RO~ OF ~ YEN~
T~.e most striking characteri~tic of herpes simplex
virus (HSV) is its propensity ~or persisting in a
quiescent or latent state in man, with recurrence of
activity at irregular intervals. ~here are two main
immunologic variants, types 1 and 2, which crossreact.
One of the major problems in seroepidemiologic studies
of HSV infection is the differentiatiôn of antibodies in
human serum directed against HSV l and HSV-2.:
~0 Researchers have attempted to overcome these probl~ms
using purified HSV glycoproteins and absorption to
remove cross reacting antibodies in an ef ort to develop
a rapid method of distinguishing ~SY-l and HSV-2
~ --antibodies. Such methods have important clinical and
; 25 epidemiological significance because it permits
diagnosis of persons with asymptomatic HSV infection,
identificat~on of candidates for antiviral therapy, as
well as identification of pregnant women who may
transmit a potentially ~a~al H5V infect~on to the
newborn.
U.~. Patent 4,764,459, lssued to Hampar et al. on
- August 16, 1988, and Hampar et al., J. Clin. Microbiol.,
21: 496--500 ~l985), describe an ELISA to determine
antibodies against HSV ~ypes 1 a~d 2 in hum~n sera. The
method utalizes heterologous virus-infected cell
.
~, :
W0 91~17443 2 ~ s
PCr/US91 /02837
extracts to absorb human sera to remove intertypic
cross-reactive antibodies. Specifically, the sera were
absorbed with heterologous virus-infected cell extracts-
to remove cross-reacting antibodies and then were
applied to E~ISA plates containing t~e target antigens,
immunoaffinity-purified HSV-1 glycoproteins gC and gD
and HSV-2 glycoproteins gD and gF. T~e absor~ance
index, defined as the ratio of the absorbance generated
by a serum sample absorbed with a heterologo~s virus-
infected cell extract ~ersus the absorbance generated bya serum sample absorbed with an u~infected cell extract,
was used to determine the pres~nce or absence of
antib~diPs to HSV-1 and HSV-2.
U.S. Patent 4,855,224, issued to Berman et al. on
August 8, 1989, describes the use of a molecularly
cloned polypeptide with antigenic determinan~s capable
of specifically binding complementa-y antibody. Using
an HSV-1 glycoprotein D as the polypeptide, HSV
antib~di~s could be determined. ~SV-2 antibodies co~ld
be detected using HSV-2 glycoprotein C fragmen~.
Arvin et al. J Infection and Immunity, 40: 189-189
~1983), describe an immunoaffinity purification of HSV-l
glycoprotein C with a monoclonal antibody and the use of
this purified glycoprotein in a solid phase RIA for
type-specific antibodies to HSV-l.
Svenner~olm et al., ~. Clin. Microb~ol., l9:
235-239 (February 1984), describe the use Of h~
~Qm~S~a lectin puri~ied ~SV-1 and HSV-2 antigens to
determine type specific a~tibodies in huma~ sera.
Identi~cation cf ~he h~ Qm~i~ lectin purified HSV
antigens ~HSV-l gC and HSV-? gG) was described by
Olofsson et al., J. gen. Virol., 67: 737-744 ~1986).
Lee et al., J. Clin. Mirrobtol., 22: 641-644
(1985), describe the ev~ ~tion or an HSV-2 typo-
.
... .
WO91/17~3 2 ~ ~ ~ g ~ 8
PCl/VS9l/02837
specific en~yme-linked immunodot serological assay based
on af~ini~y-purified gG-2 as antigen.
Lee et al., J. Virol. Methods, 14: 111-118 ~1986),
describe immunoaffinity purification of HSV-1
glycoprotein G using a monoclonal antibody. The
purified antigen was coated on a solid phase ~n an
lmmunodot enzymatic assay to detect HSV-1 antibodies.
Ross et al., J. Virology, 54: 851-855 ~1985),
describe a competitive ELISA to test for human
antibodies to antigenic site~ on HSV glycoproteins C and
D, which are recognized by mouse monoclonal antibodies.
It was shown that there were antibodies i~ human sera
that were capable of blocking the binding of monoclonal
antibodies. Thus, even though the monoclonal antibodles
used were specific for HSV-l or ~SV-2 the assay çould
not be used to distinguish HSV-1 and HSV-2 antibodies
because blockinq by heterologous human antibodies
occurred~ i.e.,-human antibodies to HSV-l blocXed
monoclonal antibodies spec~fic for HSV-2 and human
anti~odies to HSV-2 bloeked monoclonal antibodies
specific for HSV-1.
SU~ARY_Q~ ~HE I~VENTION
This invention concerns a method of serotyping
- antibodies to HSV 2 or 1 or in a sample suspected to
contain HSV antibodies wh~ch comprises:
a) reacting the sample and at least one
monoclonal antibody sp cific for HSV-~ or HSV-l
glycopro~ein G with an anti~en wherein said monoclonal
antibody ls not susceptible to heter~logous blocking;
and
b) determining whether antibody binding has
` occurred.
The serotyping method of the invention can be done
in a variety o~ formats such as ~equential or
simultaneous.
~ . .
. .
. . . .. . . ...... . . ... . .
- , . . . . . . : - . .:. .~ .
WO 9]/17443
~ ~ ~ 8 ~ ~ PCT/US91/0~83,
4 .
In another embodiment, this invention concerns kits
for serotyping antibodies to ~SV 1 and HSV-2.
BRIEF 12ESCI~IP~I!;?~ OF THE DRA~
- Figure 1 is a yraph depicting the absorbance values
for an assay used to determine anti-HSV-l antibodies.
Figure 2 is a graph depicting the absorbance values
for an assay to determine HSV-2 anti}:~odies.
DETAILED DEs~IpTI~2L~l~l~3~
The term "antigen" refers to an antigenic substance
which can be natural, recombinant, or synt~etic, and
which is capable of binding to the monoclonal antibodies
and to the antibodies in the sample. The antigen need
not be substantially pure as long as the contaminants do
not interfere with the assay method.
~5 The term BSV-2 ylycoprotein G ~HSV-2 gG) refers to
the HSV-2 glycoprotein- as described by Roizman et al.j
Virology 133 242-24~, 1984; Marsden et al., J. Virology
~iQ, 547-554, l9B4; Lee et al., J. Clin. Microbiol. ~,
641-644, 1985; Balachandran et al., J. Virology ~,
825-B32, 1985; and Su et al., J. Yirology .fi2, 3668-3679,
19B8.
The term HSV-1 glycoprotein G (HSV-1 gG) refers to
the BSV-l glycoprotein as described by Ackermann et al.,
Vir;:>logy-~Q, 207-220, 1986; Richmarl et al., J. Virolo~y
~ Z, 647-655, 1986; and Sullivan et al., J. Gen. Virol.
~, 25~7-2S98, 1987.
The assay of tbe present invention is an
extsaordinarily simple and speclfic methsd to serotype
antibodies to HSV-2 or HSY~l which overcomes the cross-
reactivity problems which have plagued researchers in
differentiatirlg these antibodies. HSV-1 antibodles are
sero~cypecl by using at le~st one monoclonal antibody
specific for ~SV-1 glycc~protein G which is not
suscep~le to heterologous blocking. ~SV-~ an~ibodies
are serot:yped by using at least one monoclonal ant~body
~,
'... '. ... ..
.. ~ , . .. .. . . . .
WO91/17~3
2 ~ P~T/US91/02837
S
specific for HSV-2 glyooproteln G which is not
susceptible to heterolo~ous blocking. These monoclonal
antibodies can be unlabeled or labeled as is discussed
below.
The following is one illustration of the method of
the invention: HSV antigen, types 1 and 2, is
immobilized on a suita~le support. It is then reacted
with a sample, usually serum or plasma, which is
suspected to contain HSV antibodies whioh, ~f present,
will bind to the antigen, and, thus, block binding of
the monoclonal antibody. After excess reagents are
removed by washing, a labeled monoclonal antibody is
incubated with the mixture. Antibodies to HSV-1 are
detected by using a monoclonal antibody specific for
HSV-l glycoprotein G . Antibodies to HSV-2 are detected
by usiny a monoclonal antibody specific ~or HSV-2
glycoprotein G. The mixture is washed again and
antibody binding is determined by measuring the amount
of reporter bound to the solid support.
The ~ample need not be limited to serum or plasma.
Any biological fluid suspected to con~ain HSV antibodies
can be used as the sample.
Techniques for preparing monoclonal antibodies
- which can~~e used to practice the invention are well
known. The preparation o~ monoclonal antibodies which
recognize either RSV-l or HSV-2 glycoprotein ~ have been
described in a variety of publications such as Roizman
et al., Virology 1~, 242-247, 1984; ~arsden ~t al., J.
Yirology ~Q, 547-554, ~984; Balachandran et al., J.
- 30 ~irology ~, 825-832, l9B5; Lee et al., J. Clin.
Microbio~. 2~, 641-644, 1985; Ackermann e~ alO, Virology
l~Q, 20---220, 198Ç; and R~chman et al. r J. Virology ~,
6~7-~55, 19~.
~; T~e antibody can be labeled with a reporter sr wit~
a m~mber of an immune or ^on-immune speeific binding
'~'
W~1/17~3 2 0 '3 ~ PCT/US91/02837
pair. When the latter is used, detection is effected by
reacting the complex with the other member of the
binding pair whic~ is labeled.
Members of specific binding pairs suitable for
practicing the invention can be of the immune or non-
immune type. Examples of immune specific binding pairs
include antigen-antibody sy~tems o~ hapten-anti-hapten
systems. The antibody member of the specific binding
pair can be produced by customary methods familiar to
those skilled in the art. Such methods involve
immunizing an animal with the antigen member of the
specific binding pair. If the antigen member of the
specific binding pair is not immunogenic, i.e., a
hapten, it can be covalently coupled to a carrier
protein to render it immunogenic. The antibody member
whether polyclonal, monoclonal, or an immunoreactive
fragment thereof can be produced by conventional
technlques well known to those skiiled in ~he art. The
terms immunoreactive antibody fragment or immunoreactive
fragment mean fra~ments which contain the binding region
of the antibody. Such fragments can be Fab-type
fragments which are defined as fragments devoid o~ the
Fc portion, e.g., Fab, Fab' and F(ab'~2 fragments, or
may be so-called "half-molecule~' fragments obtained by
reductive cleavage of the disulfide bonds connecting t~e
heavy chain components of the intact antibody.
Non-immune binding pairs include systems wherein
the two components share a natural affinity for each
other but are not antibod~es. Exemplary non-lmmune
binding pairs are biotin-avidin or b~otin-streptavidin,
folic acid-fola~e binding protei~, complementary probe
nucleic acids, etc.
A variety of methods are available ~o 20valently
~abel monoclonal an~ibodies with members of spec~f~c
blndin~ pairs. ~ethods are selected based upon the
: .
., .
WO91/17443 2~ PCr/us9l/0~837
nature o~ the member of the specific binding pair, the
type of linkage desired, and the tolerance of the
antibody to ~arlous conjugation chemistries. Biotin can
be covalently coupled to monoclonal antibodies by
utilizing commercially available active derivatives.
Some of these are biotin-N-hydroxysuccinimide which
- binds to amine groups on proteins; biotin hydrazide
which binds to carbohydrate moieties, aldehydes and
carboxyl groups via a carbodiimide coupling; and biotin
maleimide and iodoacetyl biotin which bind to sulfhydryl
groups. Fluorescein can be coupled ~o pro~ei~ amine
groups using fluorescein isothiocyanate. Dinitrophenyl
groups can be coupled ~o protein amine groups using
2,4-dinitrobenzene sulfonate or 2,4-dinitrofluoro-
benzene. Other standard methods of conjugate may beemployed ~o couple monoclonal antibodies to a member of
a specific binding pair lncluding dialdehyde,
carbodiimide coupling, homofunctional crosslinking, and
heterobifunctional crosslinking. Carbodiimide coupling
is an effective method of coupling carboxyl groups on
one substance to amine groups on another. Carbodiimide
coupling is facilitated by using the commercially
available reagent, l-ethyl-3-(dimethylaminopropyl)-
carbodiimide ~EDAC).
- 25 Homobifunctional crossli~kers, including the
bifunctional imidoesters and b~functional N-hydroxy-
- succinimide esters, are oommercially available and are
employed for coupling amine groups on one suhstance to
ami~e groups on another. Heterobifunctional
crosslinkers are reagents which possess diffPrent
functional groups. The most common commercially
available heterobifunctionaI crosslinker~ have an amine
~ reacti~e N-hydroxysuccinimide ester as one functlonal
- group, a~d a sulfhydryl reactive group as the ~econd
~ 35 func~ional group. The most common sulfhy~ryl reac~ive
, .
., .
Wo91/17~3 2a~
,~ PCT/U591/02837
groups are maleimides, pyridyl disulfides and active
halogens. One of the funct.ional groups may be a
photoactiv~ aryl nitrene, wh~h upon irradiation reacts
with a variety of groups.
A reporter can be linked directly or indirectly,
covalently or non-covalent:Ly to the monoclonal
antibodies or to a member of a specific binding pair.
Reporters can be radioactive isotopes7 enzymes,
fluorogenic, chemiluminescent or electrochemlcal
materials. Two commonly used radioisotopes are 125I and
3H. Standard radioactive iso~op~c l~beling procedures
include the chloramine T, lactoperoxidase and Bolton-
Hunter methods for 125I and reductive methylation for 3H~
Enzymes are also used as reporters fo~
immunoassays. These include, but are-~ot limited to,
horseradish peroxidase, alkaline phosphatase,
~-galactosidase, glucose oxidase, luciferas~,
~-lactamase, urease and lysozyme. Labeling with enzymes
is facilitated by using dialdehyde, carbodi1mide
coupling, homobifunctional crosslin~ers and
heterobifunctional crosslinkers as described above for
labeling monoclonal antibodies with members of specific
` binding pairs. The labeling method chosen depends on
.. . . . . .
the functional groups available on the enzyme and the
material to be labeled, and the tolerance of both ~o the
conjugation conditions. The labeling method used in the
presen~ invention may be one ofl bu~ no~ limited to, any
conventional methods currently employed including those
described by Engvall and Pearlmann, Immunochemistry 8,
871 (1971), Avramea and Ternynck, Immunochemistry ~,
-: 1175 (1971~, Ishikawa e~ al., J. Immunoa~say 4(3):209-
327 ~1983) and Jablonski, Anal. ~iochem. 1~8:199 (1985).
Labellng may be aceomplished by indirec~ methods such as
using spacers or other members of specific binding
: 35 pairs. An example of this is the detection of
:, .
WO91/17~3
~ J PCT/US~1/02837
biotinylated antibodies wi~h unlabeled streptavidin and
biotinylated enzyme, wit~ the u~labeled streptavidin and
biotinylated en~yme being adcled either sequentially or
simultaneously. Detection of enzyme acti~lty may be
facilita~ed by measuring chromogenic, fluorogenic,
chemiluminescent or electrochemical changes by commonly
known methods.
Reporters can be fluorogenic ox chemiluminesce~t in
nature. In addition, rep~rtl~rs can be detectable by
electrochemical means. Some methods of labeling with
these reporters are de cribed above. In a preferred
embodiment, the enzyme, horseradish peroxidase is used
as the reporter.
In another variation, the monoclonal antibody need
not be labeled. If it is unlabeled, it can be detected
using a labeled anti-species immunoglobulin reagent.
If more than one monoclonal antibody is used, the
combination should be specific for ei~her HSV-l gG or
HSV-2 gG.
Another embodimen~ involves the s~multaneous,
rather than sequential, addition of sample and
monoclonal antibody to a support containing immobilized
antigen. This is illustrated ln ~he examples described
below.
AlternatiYely, at leas~ one monoclonal antibody can
be immobilized on a support. It can then be reacted
with labeled an~igen and sample or the labeled antigen
and sample can be preincubated and then reacted with the
- support con~aining immobilized monoclonal antibody.
Suitable supports include synthetic polymer
supports, such as polys~yrene, polypropylene,
subs~ituted polystyrene, e.g., amina~ed or carboxylated
poIystyre~P; polyacrylamides; polyamides; polyvinyl
chlorlde, etc.; glass beads; agarose; nitrocellulose,
~ 35 etc.
:` :
't
WO~1/17~3 ~ ~ g ~ PCT/U~91/02837
This invention also concerns a kit for serotyplng
antibodies to HSV l or BSV-.2 or bo~h HSV-1 and HSV-2.
kit according to the present invention comprises ~a) a
suppor~ con~aining immobilized ~SV antigen, ~b) a
quantity of at least one monoclonal antibody speci~ic
for HSV-1, ~SV-2 or a combination thereof wherein sa~d
antibody is not susceptible to heterolog~us blocking and
-. further wherein said antibo~y is either (i) unlabeled,
lii) labeled with a reporter, or ~iii) labeled with a
first member of a specific binding pair, and (c) a
quantity of labeled anti-antibody when the monoclonal
antibody is unlabeled or a quantity of a labeled second
member of the binding pair when the mcnoclonal an~ibody
is labeled with the first mem~er of ~he specific binding
pair.
The following examples are intended to illustrate
the invention.
~
:`. The monoclonal antibodies were prepared according
to the methods o~ L. Pereira et al., a~ described in
25 Infection and Immunity, Vol. 29, No. 2, pp. 724~732
~Aug. 1980) and Oi, et al., as described in
Immuno~lobulin Prod~cing ~ybrid Cell Lines, pp. 351~371,
~n B. ~ishell and S. Schitgi ~ed.), Selected Methods in
Cellular Immunology, W. H. Freeman Co., San Francisco.
Monoclonal antibodies were purified from ascites
fluid by salt fractionation with 40% saturated ammonlum
sulfa e and gel filtratio~ on Sephacryl S~300
~P~armacia, Inc., Piscatzway, NJ). Purified monoclonal
antibodies were labeled with horseradish perox$dase
~HRP) accordin~ to the SMCC method described by Ishikawa
- . ,. ::, , -
WO 91/17443 2 ~ 8 ~
PCT/US91 /02837
11
et al., Journal of Immunoassay, Vol. 4, No. 3, pp.
209-327, 1~83.
~SS~
Polystyrene microplate wells were coated with HSV-2
antigen ~Scripps Laboratories, San Diego, CA) dlluted in
O.lM carbonate buffer pH 9.6, and blocked with ~% bovine
serum albumin (BSA) in 10 mM phosphate buffered saline
(PBS), pH 7.4.
For the sequential (Seq.~ format, a serum specimen
containing antibodies to HSV-2 ~Pos.) or a specimen
without antibodies to HSV (Neg.) were incubated for 1 hr
at 37C. Following washing with PBS containing O.OS~
Tween 20 (PBST), HRP labeled anti-HSV-2 gG monoclonal
antibodies 55311, 55312, or 55311 and 55312 combined at
1:1, diluted in 1% BSA-PBST were incubated for 1 hr a~
37C. After washing with PBST, a tetramethylbenzidine
(TMB) substrate (SOMA Laboratories, Romeo, MI~ was added
for 30 min., the reaction stopped with H2SO4, and the
absorbance at 450 nm read on a microplate reader (Vmax,
Molecular Devices Corp., Menlo Park, CA).
For the simultaneous (Sim.~ format, the monoclonal
- antibodies were diluted into the serum specimens, added
to the wells, and incubated for 1 hr at 37C. Following
washing with PBST, TMB was added and absorbance
determined as described for the sequential ~ormat.
The results in Ta~le I below indicate that ~he
~ anti-~SV-2 containing serum-blocked th~ binding of both
- anti-HSV-2 gG monoclonal antibodies when used alone, or
in combination, whether in a sequential or simultaneous
asaay cormat.
..
. .
.
WO91/17~3 ~ ~ ~
2 ~6 ~ PCT/US91/02837
12
. ~ ~e~
Blocking of Anti-HSV 2 gG Monoclonal Antibodies
by Serum Containi~g Anti-HSV 2 Antibodies
rmat ~im~ 0.1:)~
~5311 Seq. Neg. 1.441
: P~s. O.lg3
55312 Seq. Neg. 0.999
Pos . O . 010
55311 + 55312 Seq. Neg. 1.510
Pos. 0.098
55311 Sim. Neg. 0.836
Pos . O . 000
55312 Sim. Meg. 1.033
Pos. O.000
55311 + 55312 Sim. Neg. 1.195
P~s . O . 000
- 5
Polystyrene microplate wells were coated with a
combination of HSV-1 and HSV-2 antigens ~Scripps
; . La~oratories, San Diego, CA) as described in Example 1
above.
For each of the two specificity determinations,
twenty serum samples containing antibodies to HSV-1 and
twenty serum samples contain~ng~antibodies to HSV-2,
diluted 1:1 with 1% BSA-PBS 0.5% triton X-100, were
added to the wells, incubated for 1 hr at 37C, ~nd
washed with PBST. To determine specificity to
anti-HSV-2,-HRP labeled monoclonal antibody 55312
(anti-HSV-2 gG) dilu~ed $n 1% BSA~PBST, was added for 1
hr at 3~C. To determine specificlty to anti-~SV~ RP
labeled monoclonal antibody 5531~ ~anti-HSV-1 gG)
dilut~d i.n 1% BS~-P~Sl~, was added for 1 hr ~t 3~C.
Follo~ing wash~ny with PBS~, TMB was added, and the
absorbance determined as described in Example 1.
',, ` .
-
WO9~/]7~3 2 ~ 3 ~'
~(s~ ~ PCT/US9l/02837
13
~ ~u Lt ~
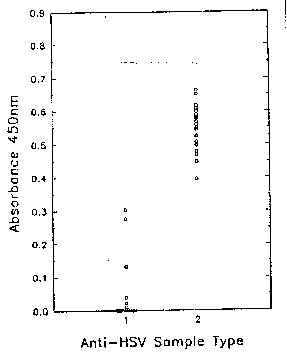
Figure 1 is ~ graph sho~ing the absorbance valuesfor the twenty anti-HSV-1 and twenty anti-HSV-2 antibody
containing sera used in an assay to determlne anti HSV-1
antibodies. The sera containlng anti-HSV 1 an~ibodies
blocked the binding of t~e anti-HSV-l gG monoelonal
antibody. No such blocking was o~served with the sera
containing anti-~SV-2 antibodies. Figure 2 is a graph
showing the absorbance values obtained for an assay to
determine an~i-HSV-2 antibodies. ~he sera containing
anti-HSV-~ antibodies blocked the binding of the
anti-HSV-~ gG monoclonal antibody. No such blocking was
observed with the sera containing anti-~SV-1 antibodies.
~EI~l
~
Polystyrene microplate wells were coa~ed with a
combination of ~SV-l and HSV-2 antigens as described in ~-
Example l above.
Twenty serum samples containing antibod~es to ~V
and one negative serum sample were added to the wells in
duplicate for 1 hr at 37C, and washed with PBST. To
one o~ the duplicate wells, HRP labeled 55312
` (anti-HSV-2 gG) was added, and to the oth~r, HRP label~d
`- 55315 (anti-HSV-l gG) was added.--Labeled antibodies
were diluted in 1% BSA-PBST, and incubated for 1 hr at
37C. Following washing wi~h PBST, TMB was added, a~d
the absorbance determined as described in Fxample l.
The absorbances are shown in Table II. ~ positive
r~sponse was chosen as at le~st a 50% reduction in the
- absorbance of the negative control specimen.
Determining an~i-HSV types by this method does not
require ~edious antlgen purif~cation procedures,
heterol~gsus absorption me~ods, or rat~o d~termi~ati~ns
as all the previo~sly described methods reyuire.
:`
' .
WO 9]/17443
2 0 ~3 1 ~ ~ ~ PCr/VS91/02837
14
~;
Typing Anti-HSV Antibodies in Serum Specimens
Absorbar~ce 450 nm
De~ectors
~pecimen No. . ~n~i~Z=;~ ~i~!~
Neg . Control 0 . 621 0 . 5S2 Neg .
0 . 001 0 . 006 1 & 2
2 0 . 62 6 0 . 000
3 0 . 000 0 . 001 1 & 2
4 0 . 000 0 . 001 1 & 2
0 . 569 0 . 003
6 0 . 000 0 . 001 1 & 2
7 0.579 0.003
8 0.489 0.000
9 0 . 513 0 . 001
0 . 000 0 . 003 1 & 2
11 0 . 01 6 0 . 002 l ~ 2
12 0 . 002 0 . 002 1 & 2
13 0 . 53~ 0 . 002
14 0 . 000 0 . 001 l & 2
0 . 003 ~ . 000 1 & 2
16 0.003 9.001 1 Ji 2
17 0 . 617 0 . 002
18 0 . ~1 0 . 001 1 &
~9 0.002 9.0a2 1 ~ 2
0.023 0.330 2
'`'
. ,