Note: Descriptions are shown in the official language in which they were submitted.
wo 9m~~mo 1 ;~~:;~~~~~ Pcris~9moo~~3
METHOD AND MEANS TO PERFORM BIOCHEMICAL REACTIONS
The present invention relates to a method to perform biochemical
reactions and a combination of a capillary and a reaction vessel
for use in said method.
The invention is applicable far all small volume biochemical
reactions in which the reagents cannot be mixed beforehand.
Particularly, the invention is intended for the PCR (Polymerise
Chain Reaction)-technique.
The recently developed PCR-technique has led to great advances
in a number of important diagnostic sectors, e.g. the diagnosis
of many different diseases, determinations of paternity, forensic
medicine, etc. When it is desired to detect RNA, a necessary
preliminary stage is the conversion of the RNA into DNA by means
of the enzyme reverse transcriptase. The diagnosis of AzDS is
routinely made by~the detection of antibodies to the HIV-virus
in the blood by means of an ELISA (Enzyme Linked ImmunoSorbent)-
test. A person may, however, be HIV-positive without antibodies
being present if he/she, for instance, is in the early stages of
the disease. In this case the ELISA test gives a negative result
and the person concerned then risks unwittingly transmitting the
infection to others. Therefore the need for a better, i.e. more
sensitive, HIV test is very great. The diagnosis of other
viruses, also, the culture of which previously took a long time,
has been improved with the PCR technique.
As regards the practical procedure, PCR diagnosis comprises three
stages:
l) preparation of.the reaction mixtures, i.e. preparation of the
samples to be tested;
2 ) the actual amplification, r . e. the chain reaction in which the
a DNA.molecules are replicated exponentially; and
3) the detection of positive samples by means of electrophoresis
ar hybridisation.
A disadvantage of the PCR method which the present inventor aims
to eliminate is that stage 1) is time-consuming and demanding
Vd'~ 91/18110 PC'T/SE91/00'~43
e~'v. ~~ i~~3
2
work, primarily because the reagents cannot be mixed in advance,
and thus give rise to many sources of error. It is very important
that stage 1) should be carried out with great care and precision
because the amplification in stage 2) and the detection result
in stage 3) depend absolutely on the reliability of stage 1).
During the various stages of preparing the reagents for a
laiochemical reaction, such as PCR mentioned above, there is a
risk of cross-contamination between the different reaction
vessels or test tubes.
While preparing for a PCR reaction there is also a risk of so-
called '°carry-over contamination" from the person who handles the
sample. This applies especially to routine analysis to detect a
specific DNA if the same person carries out all the stages before
PCR reaction and also handles the PGR product. On skin, hair and
laboratory clothing there may be remnants of PCR products from
amplifications carried out previously which engender '°false'°
positive resluts. The risk of false positive results increases
the more sensitive the test. The test for HIV is very sensitive
and it need scarcely be said that a false positive result causes
needless distress to the individual notified of it.
The object of the invention was to deminish the contamination
risk .as well as the time required to grepare small reagent
volumes for a specific biochemical reaction in which the reagents
canf~ot be mixed in advance and the preparation is time-consuming.
This object is achieved by a method using a combination of a
capillary and a reaction vessel, according to claims 1 and 6,
respectively.
In DD-Al-225 788 a capillary is described, which contains several
reagents separated by intermediate hydrophobic liquid, e.g.
paraffines, oils, alkanes. In this capillary, reagent storage as
well as sample reaction takes place. The sample is added to the
capillary and then the capillary is melted at one end. The mixing
CA 02082933 2001-08-30
69520-30
3
of the sample with the :reagents is done in that a steel pin
is put into the capillary and a magnet is moved in an upward
and downward direction along the outside of the capillary.
After a suitable incubation period the capillary is
centrifugated to obtain the reaction solution and the
hydrophobic liquid in two separate phases. To be able to
analyze the reaction so:.Lution the capillary has to be cut at
the sealed end and also at the boundary between hydrophobic
liquid-reaction solution and thereafter the reaction
1C solution is transferred to a cuvette or the like, for
measurement of, for example, UV absorbance.
This known capillary solves the problem of
preparing reagents which cannot be prepared in advance.
However, because of the above mentioned handling stages,
15 there is no time savings nor reduction of contamination
compared to conventional pipetting techniques.
The invention will now be described in greater_
detail below with reference to the accompanying drawings in
which
20 Fig. 1 is a diagrammatic view if a reaction vessel
including a reagent capillary containing reagent;
Fig. 2 is a diagrammatic view of an alternative
embodiment of a reaction vessel including an alternative
embodiment of a reagent capillary;
25 Fig. 3 is a plan view of the embodiment shown in
Fig. 2;
Fig. 4 shows the reagent capillary depicted in
Fig. 1 on a larger scale; and
CA 02082933 2001-08-30
69520-30
3a
Fig. 5 shows the reagent capillary depicted in
Fig. 2 on a larger scale.
According to one aspect of the present invention,
there is provided a method to perform biochemical reactions
in which the reagents cannot be mixed in advance, wherein a
reagent capillary (3) i;~ used being filled with reagents
separated from each other, characterized in that the reagent
capillary (3) is inserted in a first bore (4a) in the lid
(4) of a reaction vessel (1), in that the reaction vessel
(1) with inserted capil=Lary (3) is centrifugated to bring
the contents of the reagent capillary (3) in the bottom of
the reaction vessel (1), and in that the sample to be
reacted is added to the .reaction vessel (1).
According to another aspect of the present
invention, there is provided a combination of reagent
capillary and reaction vessel, characterized in a reagent
capillary (3) comprising different reagent solutions (6--13)
in predetermined volumes separated from each other by air or
an inert fluid, and a reaction vessel (1) comprising a lid
(4) provided with one or more bores) (4a), the reagent
capillary (3) being intended to be inserted into the bore
(4a) in the lid (4) of the reaction vessel (1) at use.
Fig. 1 shows ~~ ready-prepared reaction vessel 1
according to the present. invention. Inside the reaction
vessel 1, for instance an
WO 91/18110
__ PL I'/S E~ 1 /00343
s~'~~ ~' ~ ~ i.~ a~
4
Eppendorf tube, is placed a reagent capillary 3. The reagent
Capillary 3 is provided with different reagents, which cnn be of
any Sul.table type for a desired reaction. In the bottom of the
reaction vessel 1 there may be water or buffer 2 for subsequent
dilution of the reagents.
Fig. 2 shows an alternative and preferred embodiment of a
reaction vessel 1 and a reagent capillary 3. The reaction vessel
1 is provided with a lid 4 having a bore 4a. The bore 4a is
covered by a permeable membrane 4b. The bore ~4a fitted with a
membrane is located centrally ir. the lid 4 in the shown em-
bodiment but this is not a critical feature. In fact it is
possible to provide the lid with several bores to be able to put
in more than one reagent capillary as desired. The bore 4a forms
a stop collar for the reagent capillary 3 in accordance with Fig.
5, which is described in greater detail below.
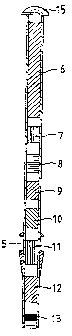
The reagent capillary 3 depicted in Fig. .~ is designed to be
inserted into the reagent vessel 1 shown in Fig. 1. The reagent
Capillary 3 is provided with different reagents 6-13 for a
specific biochemical reaction. The amount of each reagent is
calculated and intended only for this specific reaction. If a PCR
reaction is to be performed the reagent solutions 6-13 comprise
PCR buffer, dCTP, dGTP, dATP, dTTP, two or more oligonucleotides,
all of the reagents being calculated for a specific PCR reaction,
and termostable DNA polymerase. Between the reagents there is air
or do inert fluid. Naturally, the mutual order is optional.
A modified reagent capillary is depicted in Fig. 5. This reagent
capillary is designed to be inserted into a reaction vessel
according to Fig. 2. The reagent capillary differs from the
reagent capillary shown in Fig. 4 in that there is an annular
locking groove 5 on the lower part of the capillary intended to
be snapped into the bore ~la. Moreover, a protective cover 15 is
fitted over the upper end of the capillary. The reaction vessel
according to Fig. 2 and the reagent capillary according to Fig.
are stored separately until use. Upon use, the lower end of the
WO 91/18110 PCT/SE91/003d3
s ''~'~~~~~~
Capillary 3 1S pushed through the permeable membrane 4b in the
lid 4 of the reaction vessel 1, whereupon the locking groove 5
engages with the stop collar formed by the bore 4a. The protec-
tive Cover 15 protects the contents of the reagent capillary 3
from contamination during the process of insertion and pushing
into the reaction vessel 1. When the reagent solutions in the
reagent cagillary 3 have thawed, they are then centrifuged down
and mixed with one another and, where applicable, with the
diluent 2 at the bottom of the vessel 1. After centrifuging, the
lid 4 may be opened without having to remove the capillary 3 from
the lid. The advantage of this is that material can readily be
added to or extracted from the reaction vessel if desired.
After producing the reagent Capillaries, i.e. by aspirating the
different reagents with air or inert fluid in between, either
manually or automatically, they may be packed separately or
placed in a reaction vessel in kits for performing a specific
biochemical reaction. Of course, this packaging takes place under
sterile conditions.
An alternative method of producing the capillaries would be to
aspirate the reagents into capillaries with air or inert fluid
between the reagents, freeze the capillaries, cut the Capillaries
in tha air sections, and to place the desired capillary pieces
in one common outer capillary having an inner diameter eorrespon-
ding to the outer diameter of the capillary pieces. This would
allow combining of the reagents in any desired way.
The reagent capillaries with or without the reaction vessels axe
stored in the frozen state until use. For use the reagent
capillary is thawed and the contents thereof are centrifuged down
in the reaction vessel, being mixed with each other and with
diluent if any. If a PCR reaction is to be performed, all the
reagents, including heat stable DNA polymerise, are now in the
reaction vessel and the only further addition needed before the
amplification is of the sample, e.g. blood.
WO 91/1110
PC'T/SE91 /0343
6
Preferably the sample is added by using a dosing system described
in applicants pending swedish patent application SE 91 0726-0.
Briefly, the sample is drawn up into a capillary, of the same
type as reaction capillary 3, by the capillary effects. Thereaf-
ter the sample capillary is inserted in an unoccupied bore 4a in
the lid 4 of the reaction vessel 1 which thereafter is once again
centrifugated.
For fast detection of the results of the amplification reaction,
the present inventor suggest using material for the reaction
vessels that does not or only slightly absorb W light. If
ethidium bromide is added after the reaction it would then be
possible to detect whether or_not DNA has been amplified by
viewing the vessels under W light with the naked eye. Preferably
the ethidium brom~.de addition is made in the same manner as the
above described sample addition.
It should be appreciated the the shown reagent capillaries 3 have
been prepared for a specific sample volume and a specific
biochemical reaction. Other reactions require different volumes
and number of reagents.
According to the present invention numerous factors are obviated,
e.g. pipetting, changing pipette-tips, changing gloves, repeated
opening and closing of the reaction vessel, whereby the number
of sources of error is substantially reduced and the tests are
more. reliable, quicker and cheaper. The problem of false positive
results with PCR is thus appreciably reduced.
Thus the invention offers the biotechnical industry the chance
to supply a new type of "kits", i.e. complete sets containing
reagents for a specific reaction. Large numbers of such kits are
on the market today; they usually consist of Eppendorf tubes
containing different reagents suitable for about 100 standard
reactions . For each reaction a certain volume is mixed from each
tube. With the aid of reagent capillaries one kit can contain,
e.g. 500 capillaries, each ready to use for the reaction it is
!f0 91/1$110 PCT/SE91/00343
~~L;~~ i~..'~
7
designed for. The advantage of kits based in the reagent
capillaries desribed in the present application is that the user
does not have to pipette the reagent and is able, instead, to
select the appropriate reagent capillary for the relevant
reaction with fingers of tweezers. The simplification of the work
is obvious, above all in regard to the handling of radioactive
reagents, as there is no risk of contaminating pipettes, less
risk of radioactive waste and shorter periods of e.~posure for the
staff. In addition to the economic and operational advantages of
reaction capillaries in PCR technology, there is the saving in
time and the benefits of worker protection in many biotech-
nological sectors.