Note: Descriptions are shown in the official language in which they were submitted.
W091/I9793 2 0 8 5 2 2 ~ PCT/GB9t/00945
, ` - - 1 -
HYBRID PLASMINOGEN ACTIVATORS
The present invention relates to a hybrid fibrinolytic
enzyme, its preparation, pharmaceutical compositions
containing it and its use in the treatment of thromboembolic
~ diseases, in particular acute myocardial infarction.
- European Patent No 0009879 discloses derivatives of ln vivo
fibrinolytic enzymes which are useful therapeutic agents for
10 treating venous thrombosis. The derivatives are
- characterised by the active catalytic site on the enzymes
being blocked by a group which is removable by hydrolysis
such that the pseudo first order rate constant for
.' hydrolysis is in the range 10 6 to 10 3 sec 1.
:.` lS . :.
EP-0155387 discloses a fibrinolytically active hybrid
. . .
protein which comprises one chain of a 2-chain protease
linked to a chain of a different 2-chain protease, or to the
: same chain of the same protease; at least one of the chains
~- 20 in the hybrid protein being derived from a fibrinolytically
active protease, such that the hybrid protein has a
catalytic site essential for fibrinolytic activity which is
optionally blocked by a removable blocking group.
25 EP-A-0297882 discloses a hybrid plasminogen activator which
comprises the five kringle domains of plasminogen linked to
-~; the B-chain of t-PA or u-PA via an amino acid sequence
comprising, respectively, the t-PA cleavage site between
residues 275 and 276 and the cysteine residue 264 of t-PA or
30 the u-PA cleavage site between residues 158 and 159 and the
cysteine residue 148 of u-PA, the catalytic site of the t-PA
or u-PA B-chain being optionally blocked by a removable
. blocking group.
,
35 A large number of possible blocking groups were mentioned in
:
.
.."
. .
' '
WO91/19793 r ~ PCT/G891/00945
-2-
EP-A-0297882 as suitable, particular emphasis being placed
on haloanthraniloyl groups, i.e. the 2-aminobenzoyl group
substituted in the 3- or 9- position with a halogen atom and
optionally further substituted with one or more weakly
~ 5 electron-withdrawing or electron-donating groups.
- Particular blocking groups taught in EP-A-0297882 included
" 2-aminobenzoyl substituted in the 9-position with fluorine,
chlorine or bromine.
o It has now been found, surprisingly, that blocking a hybrid
plasminogen activator (PA) of the type described in
EP-A-0297882 with one particular acyl group (the
4-methoxybenzoyl or p-anisoyl group) affords a derivative
:
with particularly advantageous properties in terms of
15 potency.
.
According to the present invention there is provided
4-methoxybenzoyl plasminogen 1-541/t-PA 262-527 including
one and two chain variants, lys78 and glul variants, and
20 mixtures thereof.
. ~ .
As used herein the terms t-PA and plasminogen have the
meanings and amino acid numbering system given in
EP-A-0297882.
- 2s
In a preferred aspect the acyl hybrid PA of the invention is
the glu1 variant, especially the single chain form thereof.
.' ~ '
The acyl hybrid PA preferably comprises at least 60%, more
30 preferably at least 80% of the preferred glul, single chain
- variant relative to the other chain variants.
.
- In a further aspect the invention provides glu1, single
chain plasminogen 1-541/t-PA 262-527. This variant is
~ 35 preferably present at a level of at least 60%, more
,,
. '~ -
.
- ;,,, ,; ~ ~
.. , ~ . . , .
,
, ~c-, , ., ~ - :.. " , ...... . . .
WO91/19793 2 0 ~ 5 2 2 ~ PCT/GB91/00945
I ~ _3_
~ ~..
~ preferably 80% relative to the other chain variants.
- The acyl hybrid PA of the invention may be prepared as
generally described in EP-A-0297882.
The unacylated hybrid PA iS first prepared by expressing
DNA encoding the hybrid PA in host cell and recovering the
- hybrid PA product.
o The DNA is prepared conventionally by the condensation of
appropriate mono-, di- or oligomeric nucleotide units as
described in EP-A-0297882.
.' .' ` , . :
The host cell is prepared conventionally by transformation ~-
lS with a replicable expression vector capable, in the host
cell, of expressing the coding DNA.
The choice of vector will be determined in part by the host
cell, which may be prokaryotic, such as E. coli, or
20 eukaryotic, such as mouse C127, mouse myeloma, chinese
hamster ovary, fungi e.g. filamentous fungi or unicellular
'yeast' or an insect cell such as Drosophila. The host cell
may also be in a transgenic animal. Suitable vectors
include plasmids, bacteriophages, cosmids and recombinant
25 viruses, derived from, for example, baculoviruses or
vaccinia.
., ~ .
The preferred glu1, single chain form of the hybrid PA may
be obtained by appropriate choice of vector, host cell,
30 expression conditions and purification conditions. Thus,
~- for example, Chinese hamster ovary (DXB11) cells may be
harvested in serum-free medium to prevent proteolysis.
Although this approach usually produces material with a high
content of the glu sc form, it may be necessary, especially
35 in harvests made soon after changing the culture conditions
from one of serum-containing to serum-free, to add protease
: .
~ .
''
.
.: -, , . . - , ~ ' : -
, ' .
:' . : ~ .. : ' , . ' ' ' ' '
~ . ' ~ , ' . ~ . ,
WO91/19793 ~a~ PCT/GB91/00945
_
inhibitor(s) e.g. aprotinin, to prevent nicking. Similarly,
depending on the nature of the purification scheme utilised,
protease inhibitors may be required in all or some of the
purification buffers.
The hybrid PA is reacted with a suitable acylating agent in
order to prepare the 9-methoxybenzoyl derivative.
~' -
- The invention therefore also provides a process for
- lO preparing the acyl hybrid PA of the invention, which process
; comprises reacting the hybrid PA with a blocking agent JK in
which J is a locating group which mediates binding of the
agent to the catalytic site of the enzyme and K is a
p-methoxybenzoyl group.
Examples of group J include 4-amidinophenoxy and
2-chloro-4-amidinophenoxy.
~' .
A preferred acylating agent is 4-amidinophenyl
20 4'-methoxybenzoate, normally used in the form of the HCl
salt. This compound is disclosed in EP-0009879.
The blocking reactions are preferrably carried out in
aqueous media, at a pH in the range 6.75 to 8.5, more
i 25 preferably 6.75 to 7.25 and at temperatures in the range 0C
to 30C, more preferably 15C to 30C.
., .
;~ The preferred concentration range for the blocking agent is
; O.Ol to 2.0 millimolar, and the preferred enzyme
30 concentration range is l to 500 micromolar.
,:
It is desirable to carry out the blocking reaction in the
; presence of enzyme solubilising or stabilising additives
such as l-arginine, l-lysine or 6-aminohexanoic acid.
- 35
.~ .
,
-.~ .
. ~ ' '' ''~ . , . ' .
.
WO91/19793 PCT/GB9t/00945
~5- 208~224
~ The acyl hybrid PA or the glu1, single chain plasminogen 1- -
-` 541/t-PA 262-527 of this invention is preferably ;
administered as a pharmaceutical composition for the
treatment of thrombotic diseases.
Accordingly the present invention also provides a
-- pharmaceutical composition comprising the acyl hybrid PA or
glul, single chain plasminogen 1-541/t-PA 262-527 of the
invention in ~ombination with a pharmaceutically acceptable
o carrier.
., , :
The compositions according to the invention may be
formulated in accordance with routine procedures as
pharmaceutical compositions adapted for intravenous
15 administration to human beings.::
Typically compositions for intravenous administration are
solutions of the sterile hybrid PA in sterile isotonic
aqueous buffer. Where necessary the composition may also
; 20 include a solubilising agent to keep the hybrid PA in
solution and a local anaesthetic such as lignocaine to ease
pain at the site of injection. Generally, the hybrid PA
will be supplied in unit dosage form for example as a dry
~ ! . ,
powder or water free concentrate in a hermetically sealed
;; 25 container such as an ampoule or sachette indicating the
quantity of protein in activity units, as well as, for the
acyl hybrid PA, an indication of the time within which the ``
free protein will be liberated. Where the hybrid PA is to
be administered by infusion, it will be dispensed with an
30 infusion bottle containing sterile pharmaceutical grade
'Water for Injection'. Where the hybrid PA or is to be
administered by injection, it is dispensed with an ampoule
!' of sterile water for injection. The injectable or infusable
- composition will be made up by mixing the ingredients prior
35 to administration.
. .
r:,
. :
W O 91/19793 ~ ~ ~ P ~ /GB91/00945
6-
The quantity of material administered will depend upon the
, ~ .
amount of fibrinolysis required and the speed with which it
is required, the seriousness of the thromboembolic
- condition and position and size of the clot. The precise
5 dose to be employed and mode of administration must Per
force in view of the nature of the complaint be decided
according to the circumstances by the physician supervising
treatment. However, in general, a patient being treated for
a mature, fresh or nascent thrombus will generally receive a
10 daily dose of from 0.01 to 10 mg/kg of body weight either
by injection in up to five doses or by infusion.
Within the above described dosage range, no adverse
toxicological effects are indicated with the hybrid PA of
15 the invention.
'~
Accordingly, in a further aspect of the invention there is
provided a method of treating thrombotic diseases, which
comprises administering to the sufferer an effective
20 non-toxic amount of an acyl hybrid PA or glul, single chain
plasminogen 1-541/t-PA 262-527 of the invention.
, ....
- ~ In a related aspect there is provided the use of an acyl
hybrid PA or glul, single chain plasminogen 1-541/t-PA 262-
25 527 of the invention for the manufacture of a medicament for
the treatment of thrombotic diseases.
The invention also provides an acyl hybrid PA or glul,
~ single chain plasminogen 1-541/t-PA 262-527 of the invention
; 30 for use as an active therapeutic substance and, in
; particular, for use in the treatment of thrombotic diseases.
~ The following Methods and Examples illustrate the invention.
... .
~ ~.
. '. .
.
. .
.
. - ~ - ' - ' .
': ' , ' :' '
WO91/19793 PCTtGB91/00945
~, -7-
A. General Methods used in_Ex~ 2 2 4
ti) DNA Cleavaqe
5 In general the cleavage of about l~g of plasmid DNA or DNA
fragments was effected using about 5 units of a restriction
en~yme (or enzymes) in about 20~1 of an appropriate buffer
solution.
lO (ii) Generation of blunt ends
If blunt ends were required they were produced by treating
the DNA preparation with DNA Polymerase l, Klenow fragment
as described by Maniatis et al, (Molecular Cloning-A
15 Laboratory Manual, Cold Spring Harbor Laboratory, 1982).
Alternatively, sticky ends were removed by digestion using
Mung Bean nuclease (Maniatis et al).
: .
(iii) Generation of 'Sticky_ends' usinq linkers
Short kinased oligonucleotide linkers encoding single or
multiple restriction sites were ligated onto blunt ended
fragments, the linker(s) was digested with the appropriate
restriction endonuclease producing the required 'sticky end'
25 necessary for further manipulation (Maniatis et al).
; ~iv) Liaation of DNA Fraqments
;;~ Ligation reactions were carried out as described in Maniatis
- 30 et al.
. . .
- (~) Transformation
. .
Transformation of plasmid DNA into E. coli HBlOl used
3S competent HBlOl supplied by Gibco BRL (Paisley, Scotland),
- .
" : . .' .' ~: ~' '' . ' '
::
. .
WO91/19793 ~ PCT/GB9t/0094
~ ~ -8-
- according to the manufacturers instructions.
Ivi) Plasmid preParation
.: . .
5 Large scale preparation of plasmid DNA and plasmid
mini-preparations were carried out as described in Maniatis
et al .
(vii) Isolation of DNA fraaments from low-meltina-point
(LMP) aqaroseqels ~
., . , ' ' ' .
DNA fragments were isolated from LMP agarose gels as
` described by Maniatis et al. Alternatively the excised gel
; band was purified using GENECLEAN (Stratech Scientific,
15 London) used according to the manufacturer's instructions.
'' ~ .
~ (viii) Oliqonucleotide linkers
,.':
Oligonucleotides were either purchased or were made on
20 Applied Biosystems 3~1A DNA Synthesizer according to the
manufacturer's instructions and were kinased as described in
.,
~ Maniatis et al. ~
. 6
(ix) Chromoqenic substrate assavs
2s
Hybrid was assayed against the chromogenic substrate S2288
... .
(KabiVitrum, Sweden) at a substrate concentration of lmM in
0.1 M triethanolamine.HCl pH 8.0 at 25C. An SU is defined
as the amount of activity that gives an O.D. increase at
;; 30 405nm of 0.001/min in 0.5 ml substrate in a 1 cm pathlength
cell.
~;, ,
In another form of the assay, specifically designed for the
., , . : - ,. ~ ........................... - .
.
. - '
: : :
W O 91/19793 P ~ /CB91/00945 9 2085224
semi-quantitative assay of chromatography column fractions,
10~1 of each fraction was mixed with 100~1 lmM S-2288 (as
above) in wells of a microtitre plate and the plate
incubated at 37C until such time as yellow colour was
visible. The solutions were read at 410nm using a Dynatech
MR700 Microplate reader.
(x) Fibrinolytic activitv assav
. . .
0 The fibrinolytic activity of hybrid plasminogen activator
solutions was measured on human plasminogen-containing
fibrin plates as described ~Dodd, I., and Carr, K.,
Thrombosis Res. 1989 55 79 - 85). Dose-responses of hybrid
plasminogen activators had slightly different slopes to
. . .
15 those of the tissue-type plasminogen activator standard so
all activities are approximate. Activities are expressed
in IU with reference to the 2nd International standard for
t-PA, Lot 86/670. Based on the activity on fibrin plates
.. . ..
the hybrid protein is estimated to have a specific activity
20 of approximately 150,000 - 250,000 IU/mg protein, equivalent
to a molar specific activity, of approximately 1.5 - 2.5 x
1013IU/mole.
. . .
.
25 (xi) Sodium dodecyl sulphate PolYacrvlamide qel
electroPhoresis (SDS PAGE)
, .
SDS PAGE was carried out to determine the apparent molecular
weight(s) of the hybrid plasminogen activator using
30 essentially the method of Laemmli (Nature 1970 227 680-685).
The activators were identified either by staining for
protein or by a fibrin zymography technique (Dodd, I. et al
Thromb. Haemostasis 1986, 55 94-97). Using these methods
;~ it was possible to determine chain nature (sc v tc) and
35 deduce likely N-termini (Glul v Lys78), but note comments
under 'N-terminus' (Section B.(ii)).
. .
,. .. :
., . , . , . ' ; , ' .
WO91/19793 ~ PCT/GB91/00945
1 0-
(xii) Rate constant determinations
.. :
a) The pseudo first order rate constant was determined
s by hydrolysing the acyl-enzyme under physiological
conditions, i.e. in isotonic aqueous media at pH 7.4 and at
37C. At regular intervals aliquots were withdrawn and
incubated with a chromogenic substrate and the rate of
conversion of the substrate measured as indicated above.
The hydrolysis was followed until such time as the rate of
conversion of substrate reached a maximum. The rate
constant k may then be calculated by plotting:
15 Loge (1-At/AmaX) against t
:~ .
-~ where AmaX is the maximum rate at which an aliquot converts
substrate and At is the rate at which an aliquot converts
substrate at time t.
20 Preferably such rate constants are calculated by
-~ computerised non-linear regression analysis, fitting the At
and time data to the equation:-
At = Ao + (Amax-Ao) tl-e kt)
where Ao is the activity of the acyl-enzyme preparation
before deacylation.
:
b) Acyl-enzyme ~ca. 20 pmol, 10~1) was added to a
- 30 solution of S-2288 (0.5ml of l.OmM in 0.05M sodium
phosphate, O.lM NaCl, 0.01% w/v Tween 80 pH (37C) 7.4) in a
spectrophotometer cuvette thermostatted at 37C.
Absorbance readings at 405nm were recorded at 1.0 min
intervals for 30 min and on-board software tBeckman Inc.)
35 used to calculate the rate of change of absorbance over each
, -
.
, :'
., . . . ~. ~ .
.: . .
. .
. . . : .
. ~
, .
WO g1/19793 2 0 8 ~ 2 2 4 PCT/GB91/00945
successive 1 min interval. AS deacylation proceeded in the
- cuvette, the rate of change of A405nm increased with time.
Rate data obtained when the absorbance exceeded a value of
0.8 were not used because of the effect of substrate
s depletion. The set of rate determinations were fitted to
the following monoexponential function:
f(t) = Ao + (AmaX ~ Ao) x (l-e t)
o where Ao is the initial activity of the acyl-enzyme and AmaX
` is the maximum activity possible after deacylation and was
; determined by deacylation of an aliquot of acyl-enzyme in ~ - O.lM Tris. HCl, 20% w/v glycerol, 0.14M NaC1, 0.01% w/v
Tween 80 pH 7.4 at 37C for lh followed by amidolytic assay
with S-2288 under the above conditions (i.e. phosphate
; buffer pH 7.4, 37C). A and X, the first order deacylation
i~ rate constant, were treated as unknowns in the fitting
process and were derived by non-linear regression analysis
on a VAX 11/750 computer.
~, ~xiii) Determination of plasma deacvlation half-life.
" ~,.
The deacylation half-life for some compounds was determined
~ in human plasma rather than in phosphate buffered saline.
- 2s Measurement of plasma deacylation rate of acyl enzymes
-~ relies on the property of rapid, irreversible inhibition of
hybrid plasminogen activators in plasma following their
deacylation. Acyl enzyme (5nM) was incubated in plasma at
pH7.4 and 37C and 100~1 aliquots removed at various time
30 points.
- - Acyl enzyme not complexed with inhibitors was isolated from
the plasma aliquots by euglobulin precipitation. 18 vol
; acetic acid (0.01% v/v) was added to the aliquot which was
35 then left on ice for 30 minutes before centrifugation at
lOOOg for 15 minutes at 4C. Precipitates were dissolved in
'
. :
:'
' ' " ' ' ' ~' :
'
.. ' , ~ " '
WO9l/19793 ~?p~ PCTtGB91/00945
- ~.J'J~ -12-
- 10 vol PBS-Tween (O.lM NaCl; 0.05M NaH2PO4; 0.01% w/v Tween
80 pH 7.4). Fibrinolytic activity in the stabilised
precipitate was measured on fibrin plates as described in
section (X) by reference to a serially diluted set of
5 standards added to plasma, precipitated immediately (as
above) and processed simultaneously. Incubation time on
fibrin plates was sufficiently long (17-20h) to allow
deacylation of acyl-enzyme and genexation of active
material.
,. 10
The level of fibrinolytic activity measured in the aliquots
was equated with the amount of acylated material remaining
in the plasma at the time of sampling. A semilog plot of
percentage initial theoretical fibrinolytic activity versus
5 time was linear. The deacylation half-life is defined as
the time at which 50 per cent of the initial theoretical
concentration of acyl enzyme had deacylated.
. .
B. Identification of amino acid residues,
N-terminus and chain nature used in the examPles
. ~
(i) Sequences
All t-PA numbering as in Pennica et al (1983) Nature 301,
25 214-221; plasminoqen amino acid numbering based on
Sottrup-Jensen et al (1978) Atlas of Protein Sequence and
~- Structure Vol. 5, Suppl. 3, p91, National Biomedical
Research Foundation, Silver Spring. MD., but updated to
include the extra amino acid residue identified by Forsgren,
30 M. et al (1987) FEBS Letters. 213 254-260. Plasminogen
nucleotide sequences as in Forsgren et al (op. cit.~.
(ii) N-terminus of hybrid Plasminoqen activators
' '
35 Glu1 indicates the protein is believed to comprise the
native (nascent) plasminogen N-terminus i.e. amino acid
'~
''
-
'. ' ' - . : ., ',.; ~ '
' '. ~ :
:
WO91/19793 2 ~ 8 5 2 2 4 PCT/GBgl/0094s
-13-
reisidues 1 onwards.
Lys78 indicates the protein iS believed to comprise the
processed lys78 N-terminus. Alternative processed
N-termini e.g. met69 and val79 are known in nature
s (Miyashita et al 1988, Haemostasis 18 supp. 1, pp 7-13).
References to lys78 N=terminal forms herein are understood I -
to include lys, val and met forms.
-
(iii) Chain nature
- sc. indicates that the protein is in single chain form.
tc. indicates that the protein is in two chain form.
C. Plasmids used in the examPles
pTRH37 ~EP No 0297882)
-expression vector for protein H37 (plasminogen
1-541/t-PA 262-527).
- pTRE24 (EP 0207589) - expression vector for
des(cys5l-aspg7) t-PA
.
D. p-amidinophenyl p'-anisate HCl was prepared as
described in EP 0009879.
. . . .
~; ~
'''''' ' ' ,
':
:
~, .
.
., .
.
-
.,
.`.
,,,,, ~,,, ,, ,. ,. ,j ... . . .
` WO 91tl9793 ~, PCI'/CB91/00945
ExamPle 1'
Expression of Plasminoqen 1-541/t-PA 262-527 (H37) in
Chinese Hamster Ovarv cells
s
The plasmid pSV2dhfr was obtained from the American Type
Culture Collection ~ATCC 37146: Molec. Cell. Biol. 1:
854-864 1981). The plasmid BPV-MT-Xho was obtained from D.
Hamer (National Institutes of Health, Bethesda, Maryland)
10 and contains a version of the mouse metallothionein-l qene
~Hamer and Walling ~1982) J. Mol. Appl. Genet. 1 273-288) in
which the BglII site just 5' to the translation start point
has been converted to an Xhol site by linkering. The dhfr
Chinese hamster ovary cell line ~CHO-DXB11) has been
5 described previously ~Urlaub, G. and L.A. Chasin, Proc.
Natl. Acad. Sci. 77 4216-4220).
:
- 1.1 Construction of an amPlifiable exPreSSiOn vector
20 a. Construction of PTRH69
~, The plasmid pTRH69 was constructed from two fragments A and
i
B isolated from the plasmid PSV2dhfr and the plasmid
` BPV-MT-Xhol respectively.
:
25 Fragment A - The plasmid PSV2dhfr ~Fig. 1) was linearised
- with EcoRI, blunt ended by infilling and linkered with Xhol
~j linkers. The linearised plasmid was then digested with
Xhol and BglII releasing fragment A ~a 3.4kb fragment
; - encoding the dihydrofolate reductase cDNA and SV40 early
30 promoter sequences).
~` Fragment B - The plasmid BPV MT-Xhol was linearised with
i~- Sstl, blunt-ended by nuclease digestion and linkered using
, BglII linkers. The linear plasmid was then digested wi~h
; HindIII, blunt ended by infilling and linkered with Xh~l
ii~1 3s linkers. The plasmid was then digested with BglII and
~ Xhol, releasing fragment B ~0.3kb encoding the 3
:,, .
:.
- ~ . : -, - . .: .: - -
WO91/19793 2 0 8 PCT/G~9t/00945
metallothionein polyadenylation sequences) ~Fig. 2).
Fragments A and B were isolated by LMæ agarose
electrophoresis and ligated together. The ligated DNA was
transformed into E coli HB101 and the plasmid PTRH69 was
s obtained (Fig. 3).
~- b. Construction of pTRH70
.
The plasmid pTRH69 was linearised with Xhol and
` 10 phosphatased. The LMP agarose-purified fragment was
ligated with a 3.3Kb Xhol fragment derived from the plasmid
pTRE24, which encodes a modified t-PA protein. E._coli
HB101 was then transformed with the ligated DNA to obtain
pTRH70 (Fig. 4).
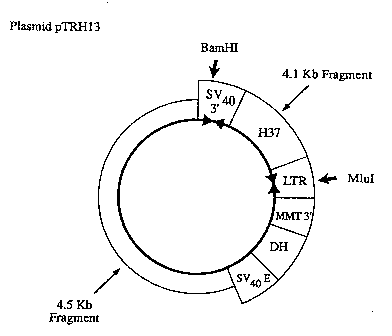
c. Construction of pTRH13
The plasmid pTRH70 was digested with Mlul and BamHl; a ~-
4.5Kb fragment (comprising the vector functions) was ~
20 isolated by LMP agarose gel purification and ligated with a -
4.lKb Mlul/BamHl fragment isolated from pTRH37 (encoding
~ hybrid protein H37). E. coli HB101 was transformed with
i the ligated DN~ and the plasmid pTRH13 was obtained ~Fig.
5).
~, 2s
1.2 ExPression of hvbrid Protein
a. Cell preParation
,
- 30 CHO cells were trypsinised and plated out at 5 x 105 per
60mm dish and left in growth medium (Hams F12 nutrient media
,
~' (041-01765) with 1% stock glutamine (043-05030), 1% stock
penicillin/streptomycin (043-05070) and 10% bovine foetal
- calf serum (011-6290); Gibco, Paisley, Scotland) at 37C in
, 35 a humidified incubator in an atmosphere of 5% C02/95% air.
After 21 hrs the cells were used for DNA transfection.
-
.
-
,:
; , . .. ~ , . : .
',. `, ' ' ~ ' ... ~ , ~ " :
.. . : , - , : . .. . . . .
WO91/19793 ~ PCT/GB91/00945
~ 16-
- b. Transfection procedure
.~ .
The transfection procedure, carried out in growth medium
-~ 5 used calcium coprecipitation and glycerol shock as described
in: DNA Cloning, Ed D.M. Glover (Chap. 15. C. Gorman).
Following transfection with pTRH13 the cells were maintained
in growth medium for 46 hrs under growth conditions (as
above) prior to the selection procedure.
c. Selection and Co-amPlification
The selection and co-amplification procedure was carried out
essentially as described by R. J. Kaufman (1985) Molecular
15 and Cellular Biology 5, 1750-1759. 46hrs post transfection
the cells were medium changed into selective medium MEM
ALPHA (041-02571), 1% stock glutamine (043-05030), 1% stock
penicillin/streptomycin (043-05070) and 10% dialysed bovine
foetal calf serum (220-6300AJ) (Gibco, Paisley, Scotland).
20 The cells were maintained in selective medium for 8-10 days
until colonies of dhfr+ cells appeared. When colonies were
; established the cells were medium changed into selective
medium containing methotrexate, (A6770; Sigma, England).
The methotrexate concentration was initially 0.02~M and was
s increased stepwise to 5~M. Early batches of the hybrid
protein H37 were produced from a mass culture (CHOZ3)
amplified to 3~M methotrexate. Later batches were produced
either from two cell lines (CHOZ5-C4 and CHOZ5-C13) which
were isolated by ring cloning from a mass culture amplified
, .,
0 to 5~M methotrexate (CHOZ5), or CHOZ5-C4E12, which is a
subclone (dilution plated) of CHOZ5-C4.
. .
d. Detection cf hybrid protein H37
35 During the amplification procedure aliquots of growth medium
from growing cells were assayed for plasminogen activator
.; - .
:-"
.;, . ,. . . " ~ . - . ,. ............ . ...... ~ . . .
~. . ~ . , :: ; : . .
WO9lt19793 2 0 8 5 2 2 4 PCT/GBg1/00945
-17-
production by fibrin plate and zymography as described in
Methods. Zymography re~ealed a fibrinolytically active
protein with apparent Mr approximately 100,000, which is
consistent with the expected molecular weight of hybrid
5 protein H37.
. ~,
-~ e. Purification of hvbrid Protein H37
.
CHO cells transfected with pTRH13 (Example l.lc) and
o selected for survival in 3~M methotrexate (Example 1.2c)
were harvested (lOOmls per 175cm2 flask) for 3 or 4 days in -
; Hams F12 nutrient mixture: Dulbecco's modified Eagles medium
(50:50)and 1% pencillin/streptomycin (GIBCO, Paisley,
Scotland) (note, serum free).
~i 5.81 of conditioned medium from a typical 3d harvest was
collected and was purified on zinc chelate Sepharose (vt
240ml) and lysine Sepharose (vt60ml) using essentially the
same method as that described i~ Dodd, I. et al (FEBS ~ett.,
20 1986 209, 13) except that the lysine Sepharose column was
eluted with two arginine-containing buffers rather than just
one. The first was 0.02M Tris/0.5M NaCl/0.25M
L-arginine/0.01% Tween 80 pH 7.4 and the second was 0.02M
Tris/0.5M NaCl/0.5M L-arginine/0.01% Tween 80 pH 7.4. They
25 were applied sequentially in the order given here. The
eluate during these elutions was fractionated. The
hybrid-containing fractions were identified with a rapid
microtitre plate-based assay using S2288. (In this and many
other experiments the hybrid eluted in the 0.5M arginine
-~ 30 wash. However, in other experiments the hybrid elutes in
the 0.25M arginine wash; the reason is not known but appears
to be related to the loading level i.e. mg hybrid bound per
ml of gel of the lysine Sepharose.) They were pooled and
ultrafiltered at ~C using a membrane with a molecular
; 35 weight cut-off of 10,000 (Amicon, 'YM 10'). The
~ ultrafiltered retentate (10.7ml) contained approximately
.. . .
,,, ' ' .
; ' '
', ;` .' ' ' ' .''' . ~'' ` '' ~'' ' ;'' . ' '', ~ :, :;';., : :
,- :, , . : : , . . .
W091/19793 ~ ' PCT/GB91/00945
-18-
220,000 IU/ml and 23,000 SU/ml. These figures generate an
IU:SU ratio of 9.6 (but see comments in Methods:assay)i
other purifications typically yielded good quality sc H37
with lower ratios. Analysis by SDS PAGE under reducing
5 conditions followed by staining for protein using Page Blue
83 (BDH) indicated the retentate contained at least 80 per
cent glu1 sc hybrid protein. The retentate was stored at
-40C.
o Example 2
-
Preparation of two chain plasminoqen 1-541/t-PA 262-527
(tcH37)
15 Single chain H37 ~lOmg ml 1 in 0.02M Tris/0.5M NaCl/0.5M
L-argininetO.01% Tween 80 pH 7.4; 0.5ml) isolated from
CHOZ5-C9 and -C13 cell lines was mixed with human lys
plasminogen (0.5mg ml~l barbitone/saline buffer; 3~1) and
incubated for 2h at 37C and then stored at -40C.
20 Analysis by SDS PAGE followed by protein staining and fibrin
zymography showed that the majority of the incubate was in
, the glu1 two-chain form.
. . .
ExamPle 3
Preparation of two chain plasminoqen 78-541/t-PA 262-527
(tc lys78 H37)
- The preparation of tc lys78 H37 was the same as that
30 described above (Example 2) except that the stock lys
plasminogen was at 5mg ml 1, so that the final plasminogen
concentration was 10-fold higher. The subsequent analyses
confirmed that the material was mainly in the lys78 two
chain form.
- ' ':
,
- ,~
:
. .
. ~ ., .: ~ . ;, ,., , : .
. .
.
, . , . ,, . ~ .
"'.'`''.' ' ' ' ' ` ' ' , , '
W091/19793 PCT/CB91/00945
-19- S22~
~ Example 4
.
Synthesis of p-anisovl sinqle chain plasminoqen l-54l/t-PA
5 262-527
' .
Purified sc H37 isolated from mainly the CHOZ3 mass culture
(80 nmoles in 1.95ml 0.02M Tris/0.2M NaCl/0.2M
L-arginine/0.01% Tween 80pH 7.4) was mixed at 25C with
; lO p-amidinophenyl-p'-anisate. HCl (lOOmM in DMSO, 40
- and incubated at 25C for lh. Assay of the incubate using
the chromogenic substrate S-2288 revealed 99% inhibition of
the activity present at t = O. To remove excess acylating
agent, the 2.0ml was buffer-exchanged into 0.02M Tris/0.2M
5 NaCl/0.2M L-arginine/0.02% Tween 80 pH 7.4 using Sephadex
~ G25 (PDIO) and the product stored at -40C. -
.'"
The solution was thawed and an aliquot diluted in 0.05M
sodium phosphate/O.lM NaCl/O.Ol~ Tween 80 pH 7.4 at 37C.
20 Deacylation was measured using S-2288. Under the above
conditions the deacylation half-life in this buffer was 33+2
min, equivalent to a deacylation rate constant of 3.5 x lO 4
--1
- sec
- .
25 ExamPle 5
-
SYnthesis of p-anisovl two chain plasminoqen 1-54l~t-PA
262-527
. ~
30 Two chain H37 was prepared as described in Example 2. 23
nmoles in l.5ml 0.02M Tris/ 0.5M NaCl/ 0.5 M
L-arginine/0.0l% Tween 80 pH 7.4 was mixed at 25C with
. .,:
p-amidinophenyl p'-anisate. HCl ~lOOmM in DMSO, 30~1) and
incubated at 25C for 30 min. Assay of the incubate using ;
; 3S S-2288 revealed 99% inhibition of the activity present at t
~- = O. The mixture was buffer-exchanged into 0.02M Tris/0.2M
NaCl/0.2M L-arginine/0.01% Tween 80 pH 7.4 using a Sephadex
,. ~ . - :
'.';
;
~, : . ,-.
.. ..
,; , , ' . ' !
. ' ' . ~` . '' . .
WO9l/19793 ~ PCT/GB91/00945
~ 20-
G25 PD10 column. The buffer-exchanged product was stored
at -40C. The deacylation half-life in human plasma at 37C
was 35 min.
,
5 ExamPle 6
Synthesis of p-anisovl two chain plasminoqen 78-541/t-PA
262-527
10 Two chain lys78 H37 was prepared as described in Example 3.
21 nmoles of tc lys78 H37 in 1.5ml 0.02M Tris/ 0.5M
NaCl/ 0.5M L~arginine/0.01% Tween 80 pH 7.4 was mixed at
25C with p-amidinophenyl p'anisate. HCl (lOOmM in DMSO;
30~1) and incubated at 25C for 30 min. Assay of the
15 incubate using S-2288 revealed 99% inhibition of the
acti~ity present at t = Q. The mixture was buffer-exchanged
into 0.02M Tris/0.2M NaC1~0.2M L-arginine/0.01% Tween 80 pH
7.4 using a Sephadex G25 PD10 column. The buffer-exchanged
product was stored at -40C. The deacylation half-life of
20 the product in human plasma at 31C was 34 min.
:
~ Sephadex G25 is a Trademark
. . .
'' :
'
: .
'.
'''
" .. -. : - - ... - . ,. : . , ~
- ., . , ~ , , ...... . :
.. -, : . : ... . ., ;.; . . . . . ..
, . " . ~ :, ~: '. :
WO91/19793 2 0 ~ S 2 2 ~ PCT/GB91/00945
}
-21- .
Leqends to Fiqures :
F i q
5 Plasmid PSV2dhfr ~.
~ ~ :
; P = Simian virus polyadenylation sequence)
. ) SV40 3'
I = Simian virus40 intron sequence
" 10
DH = dihydrofolate reductase cDNA
SV40E = Simian virus40 early promoter
EcoRI = Restriction enzyme s.ite: EcoRI was linkered
: with Xhol linkers after EcoRI digestion
15 BglII = Restriction enzyme site: BglII
.' .
Fiq 2
:
Plasmid BPV-MT-Xhol
; 20
. BPV = Bovine papillomavirus sequences
:~ MT-l = Mouse metallothionein gene sequences - including
3' polyadenylation sequence (0.3Kb fragment B)
Sstl = Restriction enzyme site: Sstl was linkered with
BglII linkers after SstI digestion
.,.~ HindIII= Restriction enzyme site: HindIII was
. linkered with Xhol linkers after HindIII : .
digestion
.
~ .
. , .
,,
: '~
....
;'' .
, i _
... .
.. . .
: ' ' ' ' ,, ; .. ~. : '
' ' ' :''' ' ' .. ' ' . ' ' ' '. ' ' ' '. ~ :'
. . , ~ ' ~ . .. ' .' ' '. '. ' '
. ' . ~' . ' . ' . . ' ~ .
WO9t/19793 ~ PCT/GB9t/00945
F i q 3 9,~ - 2 2 -
Plasmid pTRH69
s MMT 3' = Mouse metallothionein polyadenylation
sequences: Fragment B excised from plasmid
BPV-MT-Xhol
DH = dihydrofolate reductase cDNA ) Fragment A
SV40E = Simian virus40 early promoter) excised from
plasmid PSV2dhfr
Xhol = Restriction enzyme site Xhol
BglII = Restriction enzyme site BglII
~- 15 Fiq 4
Plasmid pTRH70
;;,' .
LTR = Rous sarcoma virus long terminal) 3.3Kb
; 20 repeat ) (XhoI)
SV403' = Simian virus40 intron and ) fragment
- polyadenylation sequences ) from PTRE24
:', MUT = t-PA Mutein cDNA
r~ 2s MMT3' = Mouse metallothionein polyadenylation sequences
DH = dihydrofolate reductase cDNA
SV40E = Simian virus early promoter
Bam HI, MluI, XhoI: indicate restriction sit~s
' ' , .,:
'; ~ : ' '
... .
':' ~;'
.
.
;, , ` ` .
WO 91/19793 2 0 ~ ~ 2 2 ~ Pcr/GB91/00945
Fiq 5
Plasmid PTRH13
s LTR = Rous sarcoma virus long terminal) 4.lKb tBam
repeat ) HI-MluI)
SV403' = Simian virus40 intron and ) fragment
polyadenylation sequences ) from pTRH37
H37 = H37 hybrid protein cDNA
o MMT3' = Mouse metallothionein polyadenylation
sequences DH = Dihydrofolate reductase cDNA
- SV40E = Simian virus40 early promoter
BamHI, MluI: indicate restriction sites
, `'
' .
:'.
! ' ~
,r .
.
i'~
"'
~;
.
; .
.
~' . . : ' . " ' " . . . '
.~ ' ~ . ,: ' '
: . , , : .
;