Note: Descriptions are shown in the official language in which they were submitted.
1- ~0~311
PF/5-l 8887/A
Microbicides
The present invention relates to novel benzimidazolesulfonic acid derivatives of the
formula I below. Furthermore, it relates to the preparadon of these substances and to
agrochemical composidons which comprise, as acdve ingredient, at least one of ~ese
compounds. Equally, the invention relates to the preparation of the compositionsmentioned and to the use of the active ingredients or of the compositions for controlling or
preventing attack of plants by phytopathogenic microorganisms, preferably fungi.
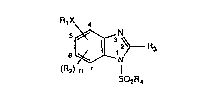
The compounds according to the invention are those of the general formula I
R~X 4
~3~ R3 (I)
(R2) n 7
SO2R4
in which the R4SO2 group occupies the l- or 3-position and, relative to the substituents
RIX- and R2, forms pure or mixed positional isomers, and in which the substituents are
de~med as follows:
Rl is an unsaturated 5-membered heterocycle having not more than two hetero atoms
N and/or S, or an unsaturated 6-membered heterocycle having not more than two
N atoms, it being possible for each of the heterocycles to be unsubstituted or
substituted by at least one of the substituents halogen, Cl-C3alkyl, Cl-C3alkoxy,
Cl-C3haloalkyl, Cl-C3haloalkoxy, COOCl-C3alkyl, C3-C6cycloalkyl, CN and
nitro;
R2 radicals, identical or different, are halogen, Cl-C3alkyl, Cl-C3alkoxy, Cl-C3haloalkyl,
Cl-C3haloalkoxy, nitro;
R3 is cyano, -CS-NH2 or -C(SR')-N~I, where R' is Cl-C4alkyl, Cl-C4haloalkyl, or
ben~yl which is unsubstituted or substituted by halogen and/or CF3;
R4 is Cl-C4alkyl, C3-C6cycloalkyl, -N(R")(R"'), in which R" and R"' are identical or
208~3~
different Cl-C3alkyl radicals;
X is oxygen os sulfur; and
n is an integer 0, 1 or 2.
The term alkyl itself or as part of another substituent such as haloalkyl, alkoxy or
haloaL~oxy, is understood as meaning for example the following straight-chain orbranched groups, depending on the number of the carbon atoms indicated: methyl, ethyl,
propyl, butyl as well as their isomers, isopropyl, isobutyl, sec-butyl, tert-butyl. Halogen
and halo are fluorine, chlorine, bromine or iodine. Haloalkoxy therefore is a mono- to
perhalogenated alkoxy radical, for example, inter alia, OCH2F, OCHF2, OCHFCH3,
OCH2CH2Br or OCF2CHFCl.
The term cycloaL~yl represents cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
~he unsaturated 5-membered heterocycles indicated are understood as meaning pyrrole,
thiophene, pyrazole, imidazole, thiazole and isothiazole, and the corresponding
6-membered heterocycle is understood as meaning pyridine, pyrimidine, pyrazine and
pyridazine.
The compounds of the formula I are oils or solids which are stable at room temperature
and which are distinguished by valuable microbicidal properties. They can be used
preventively and curatively in the agrocultural sector or in related fields for the control of
plant-injurious microorganisms. Applied at low concentrations, the active ingredients of
the formula I according to the invention are distinguished not only by an outstanding
microbicidal, in particular fungicidal, action, but also by the fact that they are particularly
well tolerated by plants.
Important compounds within the scope of the formula I are those in which Rl is an
unsubstituted or substituted pyridine, pyrimidine, pyrazine or pyridazine ring [sub-group
Ib], and, from amongst these, those compounds in which the 6-membered heterocycle is
substituted by one to three substituents selected from amongst halogen, methyl, ethyl,
isopropyl, methoxy, C~-C2haloalkyl where halogen is F and/or Cl, CF30, CHF20 or nitro
[sub-group Ic].
One of the particularly important substance groups within the scope of sub-group Ic is that
in which the 6-membered heterocycle is unsubstituted or mono- to trisubstituted pyridine
and R2 radicals, identical or different, are fluorine, chlorine, bromine, methyl, methoxy,
20~5311
- 3 -
CF3, CF30 or CHF20 where n = O, 1 or 2 [sub-group Id], in particular those where n = O
or I lsub-group Ie3. Pa~icularly important substance gro~ps within the scope of sub-g~oup
Id are those where R2 equal halogen or Cl-C2haloalkyl lsub-group Id'], especially those in
which the pyridine ring is substituted by CF3 [sub-group Id"].
Another important substance group within the scope of sub-group Ic is that in which the
6-membered heterocycle is unsubstituted or halo-substituted or haloalkyl-substituted
pyrimidine and R2 is fluorine, chlorine, bromine, methyl, methoxy or CF3, n being 0, 1 or
2 [sub-group If~. Particularly important within the scope of sub-group If are those
compounds in which R2 is fluorine, chlorine, bromine, methyl, methoxy or CF3, where n is
0~ 1 or 2 ~sub-group If'], and the substance group in which the pyrimidine is linked in the
4-position [sub-group If"].
PrefeIred compounds within the scope of sub-group Id in which Rl is a 6-memberedheterocycle are those in which R4 is methyl, dimethylamino, cyclopropyl, cyclopentyl or
cyclohexyl [sub-group Ig], in particular those in which R4 is methyl [sub-group Ig'] or
those in which R4 is dimethylamino [sub-group Ig"].
Other important compounds within the scope of the formula I are those in which X is
oxygen, those in which X is sulfur and Rl is an unsubstituted or substituted pyrrole,
thiophene, thiazole, isothiazole, imidazole or pyrazole ring ~sub-group Ii].
From amongst these compounds, those in which the 5-membered heterocycle is
substituted by halogen and/or methyl [sub-group Ij] are preferred, in particular those in
which R2, identical or different, are halogen, methyl, methoxy, CF3, CF30 or CHF20, n
being 0, 1 or 2 [sub-group L~c]. Important compounds are, for example, those without
substituents R2 (i.e. n = O).
Important compounds within the scope of the formula I are those in which R3 is cyano or
C(S)NH2 [sub-group Im] or those in which XRI occupies the 5/6-position in the
benzimidazole ring [sub-group In].
Important compounds of the folmula I are those in which X is oxygen, including
sub-groups Ib to In. Preferred individual compounds within the scope of sub-group Id are,
for example,
a) 1(3)-(dimethylaminosulfonyl)-2-cyano-4-bromo-6-(3,5-dichloropyridin-2-yloxy)-
208531~
- 4 -
benzimidazole [Comp. 1.23];
b) 1(3)-methanesulfonyl~2-cyano-6-(5-trilluoromethylpyridin-2-yloxy)-benzimidazole
lComp. 1.2~;
c) 1(3)-methanesulfonyl-2-cyano-6-(3-chloro-5-trifluoromethylpyridin-2-yloxy~-
benzimidazole [Comp. 1.66];
d) 1(3)-cyclopropanesulfonyl-2-cyano-6-(3-chloro-5-trifluoromethylpyridin-2-yloxy)-
benzimidazole [Comp. 1.74].
The compounds of the formula I can be prepared by reacting a compound of the formula II
R1X
~ N>-- 3 (II)
(R2) n
with a compound of the formula IlI
Q-SO2-R4 (III)
in which Rl, R2, R3, R4, X and n are as defined under formula I and M is hyd~ogen or an
alkali metal, preferably Na, K or Li, and Q is a halogen atom, preferably chlorine or
bromine, or the radical O-SO2-R4, in an inert solvent, if appropnate in the presence of a
base, at temperatures from -30 to +180~C, preferably -10 to 80C, under atmospheAc
pressure, reduced or increased pressure, preferably atmospheric pressure.
Suitable solvents are mainly polar reaction media, such as ketones on their own (for
example acetone, methyl ethyl ketone, tert-butyl methyl ketone) or as mixtures with ethers
(such as diethyl ether, dioxane, tetrahydrofuran, 1,2-dimethoxyethane) or, for example,
with dimethylformamide or dimethyl sulfoxide. It is expedient to react the benzimidazole
derivative II in the presence of a strong inorganic base (such as KOH, NaOH).
Due to the reactivity of the thioamide group [R3 = -CSNH21 or of the substitutedisothioamide group [R3 = -C(-SR')=NH], it is expedient to carry out the procedure in such
a way that these groups are introduced in the last reaction step after the sulfonylation.
Thus, in this case, the sulfonylated 2-cyanobenzimidazole of the formula I'
2a~3ll
- s -
R1X
~}C \~ CN (I )
~R2) n S02R4
is reacted aftenvards either with H2S or HS-R', R' being defined as indicated for formula
I. The reaction which leads to the ~ioamide lR3 = -CSNH2] is carried out in po1ar solvents
(for example alcohols, such as ethanol), but preferably in dimethylformamide (DMF) or in
ethers (for exarnple diethyl ether, tetrahydrofuran) or acetonitrile or in pyridine, using a
tert-amine such as trialkylarnine. H2S is passed in at -20 to +80C, in particular -10 to
+40C.
To prepare the substituted isothioamide group, a procedure can expediently be followed in
such a way that the sulfonylated 2-cyanobenzimidazole of the formula I' is treated with
the thiol HS-R' in a polar aprotic solvent (such as acetonitrile) in the presence of weak
bases (such as alkali metal carbonate) at -20 to +100C, preferably -10 to +40C.
The 2-cyanobenzimidazole derivatives of the formula II are prepared by methods known
per se from o-phenylenediamine derivatives or salts thereof:
R,X~NH2 RsO~
~ ~ H "C--CHab -NH4Y
a) (R2) n NH2(-HY) Trihaloimidate -R5
V VI
(Y = anion of an acid; Hal = halogen, preferably Cl)
R1X
b) ~ CHal3 + xNH3 -3NH4Hal [ M=H ]
(R2) n IV H
HY is an acid~ preferably a hydrohalic acid, or sulfi~ric acid. However, the
o-phenylenediamine derivative can also be used in the form of the free base when reaction
208~311
- 6 -
step a) is carried out in glacial acetic acid. Preferred solvents are glacial acetic acid; ethers
such as died~yl ether, dioxane, 1,2-dimethoxyethane; esters such as ethyl acetate or
alcohols such as methanol and ethanol.
The trihaloimidate ~for example methyl trichloro- or chlorodifluoro-methylimidate) is
expediently added to the dissolved or suspended o-phenylenediarnine derivadve at -20C
to +100C. In reaction step b), the 2-trihalomethylbenzimidazole derivative obtained is
expediently added to a concentrated aqueous ammonia solution (USP 3 576 818).
The 2-cyanobenzimidazole derivatives of the formula II can also be prepa~d with the
corresponding o-nitroaniline derivative instead of the o-phenylenediamine derivative by
reaction with formaldehyde and KCN in glacial acetic acid with an addition of zinc
chloride (or another Lewis acid) as catalyst, giving compounds of the fonnula VII
R1X
NH- CH2--CN
~ NO2 (VII)
(R2) n
[K. Dimroth et al., Ber. 98, 3902 (1965)], which can be cyclised with K2CO3 to give
1-hydroxy-2-cyan~benzimidazole derivatives [B. Serafinowa et al. Rocz. Chem. 51, 1783
(1977)] and reacted with PCI3 to give compounds of the forrnula II (M = hydrogen; R3 =
CN).
The intermediates of the formula II in which R3 = CN and M = hydrogen are novel and are
a further object of the present invention. (Compound group II').
R1X
~ \~ CN II'
(R2) n H
Compounds of the formula V can be prepared by reducing the nitro-containing compounds
of the formula VIII. Reducing agents which can be used are the traditional reducing agents
such as iron (Bechamps reduction), tin(II) chloride or else hyclrogen with a catalyst, for
20~531 1
example Raney nickel or palladium/charcoal. The reaction conditions correspond to those
mentioned in the literature (for example Houben Weyl "Methoden der organischen
Chemie" [Methods in Organic Chemistry]).
If Rl is a 6-membered heterocycle, compounds of the formula VIlI can be obtained from
compounds of the formula IX by reacting them with suitably substituted 2-halopyridines,
2-halopyrazines, 3-halopyridazines, 2-halopyrimidines or 4-halopyrimidines (formula
group X). The reacdon takes place in inert organic solvents, preferably in polar solvents
such as DMF, DMSO, DMA or ketones such as acetone or ethyl methyl ketone, alcohols
such as ethanol, propanol, butanol, or ethers such as diethyl ether or tetrahydrofuran, in the
presence of a base, preferably alkali metal (hydrogen) carbonates or hydroxides such as
Na2CO3, K2CO3, NaOH or KOH or amines such as triethylamine or pyridine. The reaction
temperature is bet~,veen 0C and +150C, preferably 80-120C.
Alternatively, compounds of the formula VIII are accessible by reacting compounds of the
formula IX with suitably substituted pyridines, pyrimidines, pyridazines or pyrazines
having a hydroxyl or mercapto group (formula group X1I). The reaction proceeds
preferably under the same conditions as described above.
The compounds of the formulae IX, X, XI and XII are either known from the literature or
can be prepared by known methods.
Compounds of the formula II' can also be synthesised from compounds of the formula
XIII by reacting them with 2-halopyridines, 2-halopyrazines, 3-halopyridazines or 2- or
4-halopyrimidines (formula group X). The reaction is carried out in inert organic solvents,
preferably in polar solvents, for example DMF, DMSO, DMA or ketones such as acetone
or ethyl methyl ketone, alcohols such as ethanol, propanol, butanol, or ethers such as
diethyl ether or tetrahydrofuran, in the presence of a base. Examples of preferred bases are
carbonates such as sodium carbonate or potassium carbonate, but also hydroxides such as
potassium hydroxide or sodium hydroxide or amines such as triethylamine or pyridine.
The reaction temperature is between 0C and 150C, preferably 80C to 120C.
Compounds of the formula XIII can be prepared from compounds of the formula XIV as
described above.
Compounds of the formula XIV can be prepared by reducing the nitro group in
20~:~31~
- 8 -
compounds of the forrnula IX. Examples of reducing agents which can be used are iron
(Bechamps reduction), tin(II) chloride or else hydrogen in the presence of a catalyst, for
example Raney nickel ~r palladium-on-charcoal. The reaction conditions correspond to
those indicated in the literature (for example Houben Weyl).
9 2 0 8 ~ 3 1 1
General reaction scheme 1
(R2~ NH; (R2) N22
XI \ ~} / IX \duction
- ~N2 ~NH2
reduction 1) VI
2) NH3
R(RX2~XNH2 (Rx2)~m' ~CN
1)VI
\~ N X Hal
II~ H
~3 Y = substituted or unsubstituted hydroxypyridine, mercaptopyridine, hydroxy-
pyrimidine, mercaptopyrimidine, hydroxypyrazine, mercaptopyrazine, hydroxypyridazine
or mercaptopyridazine
Z = leaving group (for example halogen) in the o- or p-position relative to
the NO2 group.
-10- 2o~3ll
N~ = substituted or unsubstituted 2-halopyridine, -2-halopyrazine, 2- or
Hal
4-halopyrimidine, 3-halopyridazine
If R2 is fluorine, chlorine or bromine, then the corresponding compounds can also be
obtained by halogenation of compounds in which R2 is hydrogen. This applies to
compounds of the formulae II', V, VII, VIII, IX, XI, XIII and XIV.
Compounds in which Rl is a S-membered heterocycle can be prepared analogously,
depending on eheir reactivity.
If Rl forms a thiazole ring, the following reaction sequence is possible:
General reaction scheme 2
Preparation of the thiazole compounds:
(~2) NH2 acid-binding 'R2' NH2
~ X ~i~ agent R ~ N ~N2
or by construction of the thiazole ring: for example
20~S311
- 11 -
(R2~ + X-CN ~ ~ + H2S ~ C--o~NHz
HO No2 N 3 C--O No2 H2N / O
X=Cl,Br / RJ~
if R=CF3 / ~ ~Y=CL,Br,I
/~S ~ o ~ NH2
CF3 ~R2~ NH thionyl OH (R2) NH
~N ~ chlonde CF3~N ~
If X = S, compounds of the formula IX can also be used in a form in which they are
masked as rhodanide (US Patent 4 076 828).
(R2) 1) NaBH4 ~R2~ N2
NCS ~XNH2 3) X ~ R~S ~NH2
Compounds of the type
R R'
HX ~ R" (IX, xIIr and XIV)
can, if X = S, also exist in the dimeric form as the disulfide. The mercapto compounds can
be obtained herefrom by reduction. Methods for such a reduction are known from the
literature.
20~31.1
- 12 -
Surprisingly, it has now been found that compounds of the formula I have a biocidal
spec~um for the contr~>l of phytopathogenic microorganisms, in particular fungi, which is
very favourable for practical requirements. They have very advantageous curative and
preventive properties and are used for the protection of a large number of crop plants. The
active ingredients of the formula I allow pests which occur on plants or parts of plants
(fIuits, flowers, foliage, stalks, tubers, roots) of various crops of useful plants to be
contained or destroyed, and even newly-forming parts of plants remain unharmed, for
example by phytopathogenic fungi.
The novel active ingredients of the formula I are particularly effective against specific
genera from the fungal class of the Fungi imperfecti (for example Cercospora),
Basidiomycetes (for example Puccinia) as well as Ascomycetes (for exaple Erysiphe and
Venturia) and, in particular, against Oomycetes (for example Plasmopara, Peronospora,
Pythium and Phytophthora). Thus, they complement the compositions for controlling
phytopathogenic fungi in crop protection in a valuable manner. As ~egards their use in
practice, they have the advantage of having curative as well as preventive properties and
can be used for the protection of a large number of crop plants. These active ingredients
allow pests which occur on plants or parts of plants (fruits, flowers, foliage, stalks, tubers,
roots) of various crops of useful plants to be contained or destroyed, and even
newly-forming parts of plants remain unharmed, for example by phytopathogenic fungi.
The compounds of the formula I can furthermore be used as seed-dressing agents for the
treatment of seed (fruits, tubers, grains) and plant cuttings as a protecdon against fungal
infections as well as against soil-borne phytopathogenic fungi.
The invention also relates to the compositions which comprise compounds of the formula
I as active component, in particular crop-protecting compositions, and to their use in the
agricultural sector or in related fields.
In addition, the present invention also embraces the preparation of these compositions,
which comprises intimately mixing the active ingredient with one or more substances or
substance groups described in this publication. It also embraces a method for treating
plants, which is distinguished by application of the novel compounds of the formula I, or
of the novel compositions.
Examples of plant species which are suitable as target crops for the crop-protecting use
disclosed in this publication are, within the scope of this invention, the following: cereals
- 13- 20~5311
(wheat, barley, rye, oats, rice, maize, sorghum and related species); beet (sugar beet and
fodder beet); pomaceous fruit, stone fruit and soft fruit (apples, pears, plums, peaches,
almonds, cherries, strawberries, raspberries and blackberries); pulses (beans, lentils, peas,
soya beans); oil crops (oilseed rape, mustard, poppies, olives, sunflowers, coconut,
castor-oil plants, cacao, groundnuts); curcurbits (pumpkin, cucumbers, melons); fibre
plants (cotton, flax, hemp, jute); citrus fruit (oranges, lemons, grapefruit, tangerines~;
vegetables (spinach, lettuce, asparagus, cabbage species, carrots, onions, tomatoes,
potatoes, bell peppers~; Lauraceae (avocado, cinnamonium, camphor) or plants such as
tobacco, nuts, coffee, sùgar cane, tea, pepper, grape vines, hops, Musaceae and natural
latex plants, as well as ornamentals.
Active ingredients of the forrnula I are customarily used in the form of combinations and
can be applied to the area to be treated or the plant simultaneously or in succession with
other acdve ingredients. These other active ingredients can be fertilizers, trace element
mediators or other preparations which have an effect on the growth of plants. However,
selective herbicides as well as insecticides, fungicides, bactericides, nematicides,
molluscicides or mixtures of a plurality of these preparations, if appropriate together with
other carriers conventionally used in the art of formulation, surfactants or other
application-enhancing additives, can also be used.
Suitable carrier and additives can be solid or liquid and are those substances which are
expedient in the art of formulation, for example natural or regenerated mineral substances,
solvents, dispersants, wetting agents, adhesives, thickeners, binders or fertilizers.
A preferred process for applying an active ingredient of the formula I, or of anagrochemical composition comprising at least one of these active ingredients, is applying
it to the foliage (foliar application). The frequency and rate of application depend on the
risk of infestation with the pathogen in question. Compounds of the formula I can also be
applied to seeds (coating) either by soaking the grains in a liquid composition of the active
ingredient or coating them with a solid composition.
The compounds of the formula I are employed as pure active ingredients or, preferably,
together with auxiliaries customary in the art of formulation. To this end, it is expedient to
process them in a known manner to give, for example, emulsion concentrates, spreadable
pastes, directly sprayable or dilutable solulions, dilute emulsions, wettable powders,
soluble powders, dusts, granules, by encapsulations, for example in polymeric substances.
\
- 14- 208~311
The application methods such as spraying, atomising, dusting, scattering, painting on or
pouring, as well as the nature of the compositions, are selected to suit the intended aims
and the prevailing circumstances.
Favourable application rates are generally 5 g to 2 kg of active ingredient ~a.i.) per hectare
(ha), preferably 10 g to 1 kg of a.i.lha, in particular 20 g to 600 g of a.i./ha.
The forrnulations, i.e. the compositions or combinations comprising the active ingredient
of the formula I and, if desired, a solid or liquid additive, are prepared in a known manner,
for example by indmate1y mixing and/or grinding the active ingredient with extenders
such as solvents, solid carriers and, if appropriate, surface-active compounds (surfactants).
The following are possible as solvents: aromatic hydrocarbons, preferably the fractions C8
to Cl2, such as xylene mixture or substituted naphthalenes, phthalates such as dibutyl
phthalate or dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or paraffins,
alcohols and glycols as well ~s their ethers and esters, such as ethanol, ethylene glycol,
ethylene glycol monomethyl ether or ethylene glycol monoethyl ether, ketones such as
cyclohexanone, strongly polar solvents such as N-methyl-2-pyrrolidone, dimethyl
sulfoxide or dimethylforrnamide, and epoxidised or unepoxidised vegetable oils, such as
epoxidised coconut oil or soya oil, and water.
Solid carriers which are generally used, for example for dusts and dispersible powders, are
ground natural minerals such as calcite, talc, kaolin, montmorillonite or attapulgite. To
improve the physical properlies, it is also possible to add highly-disperse silica or
highly-disperse adsorptive polymers. Possible particulate, adsorptive caIriers for granules
are porous types, such as pumice, brick grit, sepiolite or bentonite, or non-sorptive carrier
materials, for example calcite or sand. Moreover, a large number of pregranulated
materials of inorganic or organic nature, such as dolomite or comminuted plant residues,
can be used.
Suitable surface-active compounds are non-ionic, cationic and/or anionic surfactants
having good emulsifying, dispersing and wetting properties, depending on the nature of
the active ingredient of the formula 1 to be formulated. Surfactants are also to be
understood as meaning mixtures of surfactants.
Suitable anionic surfactants can be either so-called water-soluble soaps or water-soluble
- lS 2~ 3~1
synthetic sur~ace-active c~mpounds~
Examples of non-ionic surfactants which may be mentioned are nonylphenol
polyethoxyethanols, castor oil polyglycol ether, polypropylene/polyethylene oxide
adducts, tributylphenoxypolyethylene-ethanol, polyethylene glycol and
octylphenoxypolyethoxyethanol.
Other suitable substances are fatty acid esters of polyoxyethylene sorbitan, such as
polyoxyethylene sorbitan trioleate.
Cationic surfactants are mainly quaternary ammonium salts which contain, as
N-substituent, at least one alkyl radical having 8 to 22 C atoms, and lower, optionally
halogenated alkyl radicals, benzyl radicals or lower hydroxyalkyl radicals as further
substituents.
Other surfactants convendonally used in the art of formulation are known to those skilled
in the art or can be found in the specialist literature.
As a rule, .he agrochemical preparations comprise 0.1 to 99 per cent by weight, in
particular 0.1 to 95 per cent by weight, of active ingredient of the forrnula I, 99.9 to 1 per
cent by weight, in particular 99.8 to 5 per cent by weight, of a solid or liquid additive and
0 to 25 per cent by weight, in particular 0.1 to 25, pcr cent by weight, of a sur~actant.
While concentrated compositions are often preferred as commercial products, the end
consumer generally uses dilute compositions.
The compositions can also comprise other additives such as stabilisers, defoamers,
viscosity regulators, binders or adhesives, as well as fertili~ers or other active ingredients
for achieving specific effects.
The examples which follow illustrate the abovedescribed invention without imposing any
limitation to the scope thereof. Temperatures are in degree centrigrade.
20~311
- 16-
Preparation Examples
~-1. Preparation of
F3C ~CI
~N ~I\o ~N~02CH3
I (3)-Methanesulfonyl-2-cYano-6-(3-chloro-5-trifluorornethYlpYridin-2-yloxY)
benzimidazole
a) Preparation of the interrnediate 2-cyano-5-(3-chloro-5-trifluoromethylpyridin-
2-yloxy)-benzimidazole.
4.55 g (15 mmol) of 1,2-diarnino-4-(3-chloro-5-trifluoromethylpyridin-2-yloxy)benzene
are dissolvedin 35 ml of glacial acetic acid, and 3.95 g (22 rnmol) of methyl
2,2,2-trichloroacetimidate are added dropwise with stirring at room temperature. Stirring
is continued for 14 hours, the mixture is poured into 150 ml of water and extracted three
times using in each case 40 ml of ethyl ether, the combined extracts are dried over sodium
sulfate, and the solvent is evaporated. The resulting 2-trichloromethyl-5-(3-chloro-5-
trifluoromethylpyridin-2-yloxy)-benzimidazole, in the form of a dark brown oil, is
dissolved without further purification in 30 ml of tetrahydrofuran, 70 ml of a 25 %
aqueous ammonia solution are added in the course of 1 hour at room temperature, with
stirring, and stirring is continued for 1 hour. After the tetrahydrofuran has been distilled
off in vacuo, the mixture is brought to a pH of 5 using concentraled hydrochloric acid, and
the solid which has precipitated is filtered off with suction and chromatographed over a
silica gel column using ethyl acetate/hexane (1:1). After evaporation of the eluent, the
crystalline precipitate is digested with 15 ml of hexane, filtered and dried. The beige
crystals melt >270C.
b) Preparation of the end product
1.02 g (15.5 mmol) of 85 % potassium hydroxide are added in one portion to a solution of
4.14 g (12 mmol) of 2-cyano-5-(3-chloro-S-trifluoromethylpyridin-2-yloxy)-
benzimidazole in 65 ml of acetone, and the mixture is stirred for one hour. 3.2 g
(28 mmol) of methanesulfonyl chloride are added dropwise in the course of one hour at
20~5311
- 17-
room temperature to this mixture, and stirring is continued for 16 hours at 20C. To
complete the seacdon, a further 0.5 g t7.6 mmol) of 85 % potassium hydroxide and, after
half an hour, 1.6 g (14 mmol) of methanesulfonyl chloride are added, and stirring is
continued for four hours at room temperature. The reaction mixture is subsequently poured
into 200 ml of water and extracted three times using in each case 30 ml of ethyl acetate,
the combined organic phases are washed twice using in each case 20 ml of saturated
aqueous sodium carbonate solution and once using 20 ml of water, dried over sodium
sulfate and ~Sltered. The solvent is then evaporated. The bulk of crystals is digested using
20 ml of diethyl ether, filtered and dried. The pale grey crystals melt at 196-206C.
H-2. Preparation of
Br
Cl ~ 5~CN,~ Cl~ mP. 1.304]
SO2N(CH3)2
l-N~N-DirmethvlsulfamoYI-2-cYano-4-bromo-6-(6-chloro-pvridazin-3-Ylthio)-benz-
imidazole
a) Preparation of the intermediate 2-nitro-4-(6-chloro-pyridazin-3-ylthio)aniline.
78.1 g of 2-nitro-4-rhodano-aniline are dissolved in 900 ml of diglyme at 30C and
successively treated with 15.2 g of sodium borohydlide. After the mixture has been stirred
for 1 hour at 60C, it is cooled to room temperature, and 26.4 g of pulverised potassium
hydroxide and 61.4 g of 3,6-dichloropyridazine are added. After the mixture has been
stirred for 1 hour at 60C, it is poured onto ice, and the suspension is filtered.
Recrystallisation from toluene and digestion with diethyl ether give 39.4 g of product a),
m.p.>230C
b) Preparation of the intermediate
2-bromo-4-(6-chloro-pyridazin-3-ylthio)-6-nitro-aniline.
10.0 g of the compound a) and 2.9 g of sodium acetatç are dissolved in 130 ml of glacial
acetic acid.7.5 g of bromine are slowly added dropwise, and the suspension is stirred for
30 minutes at 55C. The mixture is poured onto ice and filtered, and the solid is washed
with water and dried. Chromatography on silica gel using e~hyl acetate:hexane = 1: 1 as the
2 0 ~ ~ 3 1 1
- 18 -
eluent gives 6.4 g of compound b), m.p.>250C.
c) Preparation of the intermediate
1,2-diamino-3-bromo-5-(6-chloro-pyridazin-3-ylthio)benzene.
6.4 g of compound b) are dissolved in 200 ml of tetrahydrofuran, 1.2 g of Raney nickel are
added, and the mixture is stirred for 51 hours at room temperature under a hydrogen
atmosphere. A total of 3.6 g of Raney nickel is added in two portions. The catalyst is
subse~quently filter~d ~ff, and the solvent is evap~rated. 4.3 g of compound c),m.p. 160-161C, are obtained.
d) Preparation of the intermediate
2-cyano-4-bromo-6-(6-chloro-pyridazin-3-ylthio)-benzimidazole.
4.3 g of compound c) are suspended in 30 ml of glacial acetic acid. 3.55 g of methyl
2,2,2-trichloroacetimidate are added dropwise, and the mixture is stiIred for 1 hour at
room temperature. The mixture is poured into ice-water and extracted three times using in
each case 20 ml of diethyl ether. The organic phase is dried over sodium sulfate, filtered
and concentrated. The residue is dissolved in 30 ml of tetrahydrofuran, and the solution is
added dropwise ae room temperature to 60 ml of concentrated ammonia solution. After
1 hour, the reaction mixture is concentrated, diluted with water and acidified with
concentrated HCl solution. The suspension formed is filtered, washed with water and
dried. 4.6 g of compound d), m.p.~250C, are obtained.
e) Preparation of the end product
2.3 g of compound d) are dissolved in 10 ml of N-methyl-2-pyrrolidone, and 0.28 g of
60 % sodium hydride are added. When the evolution of gas has ceased, stirring iscontinued for 10 minutes at 40C, and the mixture is cooled to 15C. 0.876 ml ofN,N-dimethylsulfamic acid chloride are added. The dark brown suspension is stirred for
60 minutes at room temperature and for 30 minutes at 35C. The mixture is poured into
ice-water and extracted three times using in each case 20 ml of ethyl acetate. The organic
phases are washed with water and saline, dried over sodium sulfate, filtered andconcentrated. The residue is chromatographed on silica gel using ethyl acetate:hexane =
1:1. 1.17 g of the title compound are obtained, m.p. 169-170C.
-19- 20~311
H-3. Preparation of
F3C~I /~SO2cH3 [Comp. 3.34]
1(3)-MeehanesulfonYl-2-thioamido-6-(3-chloro-5-trifllloromethvl-pv~idin-2-~loxv~-
benzimidazole
1.12 g of compound No. 1.66 arç dissolved in 12 ml of dioxane and 4 ml of
tetrahydrofuran. 0.43 ml of triethylamine are added, and hydrogen sulfide is passed in for
30 minutes at room temperature. The soludon is concentrated to half its volume and
poured into ice-water. The mixture is extracted using ethyl acetate. The organic phase is
washed with saline, d~ied over sodium sulfate, filtered and concentrated. The crystals
which form are digested with ether. 0.47 g of compound No. 3.34, m.p. 181-1 82C result.
H-4. Preparation of
~N
3 \¢N ~1~ Nl\~ CN [Comp. 1.250]
S S02N(CH3)2
1(3)-N.N-Dimethylsulfamoyl-2-cyano-6-(4-trifluoromethyl-2-oxo-thiazolyl)-
benzimidazole
This product was prepared by the method already described under H-2 from the following
intermediate:
~NH2
3c\¢N /~\N2
-20- 20~3~1
Preparation of the intermediate
50.0 g of 4-amino-3-nitrophenol are dissolved in 40 ml of acetone, and 34.4 g of cyanogen
bromide are subsequently added. 32.9 g of triethylamine are added dropwise at 0-5C in
the cousse of 45 minutes. Towards the end of the addition of the triethylamine, a further
100 ml of acetone a~e added. Stirring is subsequently continued for 15 minutes. The
reaction mixture is filtered through Hyflo, washed with acetone and concentrated. 64.7 g
of an orange-yellow powder are obtained, and this is used directly in the next step.
The product is dissolved in 500 ml of tetrahydrofuran and the solution is refiltered to
remove traces of triethylamine salts. 12.0 g of hydrogen sulflde are subsequently passed in
in the course of 3 hours at 25-30C, and stirring is continued for a further hour. For
work-up, the reaction mixture is poured into ice/water, with stiTring, filtered and washed
with water. The moist solid is dissolved in ethyl acetate, water is removed from the
mixture using magnesium sulfate, and the mixture is filtered and concentrated. 56.0 g of
4-amino-3-nitrophenyl thiocarbamate are obtained as an orange powder.
1.0 g of this aminonitrophenyl thiocarbamate are dissolved in 10 ml of dioxane/toluene
(1:1), and 1.2 g of bromotrifluoroacetone are added dropwise. The reaction mixture is
stirred for i hour at 35C and then filtered, washed with hexane and ether and dried. To
eliminate water, this powder is suspended in dichloroethane and refluxed f~r one hour
together with 2.5 g of thionyl chloride. The reaction mixture is concentrated, the residue is
taken up in ethyl acetate, the mixture is washed with 10 % potassium carbonate solution
and saline, and the organic phase is dried over magnesium sulfate and concentrated. 0.35 g
of an orange oil of l-amino-2-nitro-4-(4-trifluoromethyl-2-oxo-thiazolyl)-benzene, which
is pure according to IH NMR spectrum, are obtained, and this is processed direcdy to give
the end product.
Reaction scheme for the preparation of the intermediate:
-21- 20~3
HO/~(NO2 N----C2--J~(N2 H2N--C--O~(NO2
3 \¢N~ ~I~(NO2 ~ FaC OH ~(NH2
Examples of compounds which can be prepared in this manner or following one of the
methods indicated further above are the following.
-22- 20~J311
Table 1: Compounds of the formula
~R2) n
R1X 2NR4 -SO2R4: Position 1 or Position 3*
Comp. No. RlX (R2)n R4 Physical data
=
~0 H -C~3
F3C ~
1.2~NJI~O H -CH3 m.p. 176-181C
Cl ~CI
1.3~NJI~O H -CH3 m.p. 202-204C
1-4 [~ H -N(CH3)2
N O
1.5 ~ 5-F -CH3
1.6 ~ 5-Cl -CH3
N O--
F3C ~,~
1.7~NJJ~O H -c3H7-n m.p. 168-171C
Cl ~CI
1.8~NJI~O H -C3H7-n m.p. 166-170C
* Isomer mixture AB with regard to -SO2R4 in position 1
or 3; or the pure isomers A or B.
-23- 2~8a311
Comp. No~RIX (R2)n R4 Physicaldata
. . . ~
F3C ~
~N~O - 4-CI -CH3 m.p.203-205C
~O H -N(CH3)2 rn.p.145-146C
1.11 ~ 5-Br -CH3
N O
Cl ~ CI
1.12 ~N~O H -N(CH3)2 m.p.134-135C
F3C ~ ~
1.13 ~N~O S-CI -CH3 m.p.202-20~C
1.14 F3C~ 4-Br -CH3 m.p.213-214C
Cl ~ ~CI
I .15 ~N ~O 4-CI -CH3
1.16 ~ S-Br -N(CH3)2
N O
Cl ~CI
I . 17 ~N~0 4-Br -CH3 m.p.209-210C
Cl ~ CI
1.18 ~N~O 5-CI -CH3 m.p. 185-187C
2 0 ~
- 24 -
Comp. No. RIX (R23n R4 Physical data
1.19 Cl ~CI 4-Br -C3H7-nm.p.139-140.5C
1.20 F3C~ 4-Br -C3H7-nm.p.164-166C
1.21 ~F 4-CF3 -CH3
1.22 Cl ~CI 5-CI -N(CH3)2m.p.170-190C
1.23 Cl ~CI 4-Br -N(CH3)2m.p.156-172C
1.24 Cl ~F H -CH3 m.p.196-199C
1.25 Cl ~ F 4-Br -CH3m.p.219-220.5C
1.26 F3C~ 4-Br -N(CH3)2m.p.160-162C
~ O 4-CF3 -CH3
1.28 F3C ~ 5-Br -CH3
1.29 ~O__ H -CH3m.p.145-146C
-25- 20~:~31 1
Comp. No.RIX (R2)n R4 Physical data
. . _
Cl ~CI
1.30 ~N~O S-Br -CH3
1.31 Cl ~F ~C3H7~n m.p. 143-147C
N O
1.32 Cl ~ CI 5-Br -N(CH3)2
1.33 ~3~ H -N(CH3)2
1.34 ~O_ 5-F -CH3
1.35 Cl ~CI 5-CH3 -CH3
1.36 F3C ~ 5-Br -N(CH3)2
1.37 ~O_ S-CI -Cl~3
1.38 Cl ~ F H -N(CH3)2 m.p. 13~139C
F3C ~ 5-CH3 -CH3
20~5311
- 26 -
Comp. No.RIX (R2)n R4 Physicaldata
1.40Cl ~CI 5-OCH3 -CH3
1.41Cl ~ Cl 4-CF3 -CH3
1.42 ~O 5-Br -CH3
1-43 ~3~O S-OCH3 -CH3
1.44 Cl ~F 4-Cl -CH3
1.45 Cl ~F 4-Br -C3H7-n m.p. 177-178C
1.46 ~o S-Br -N(CH3)2
1.47F3C ~O~ 5-OCH3 -N(CH3)2
N O
1.48 \~O_ S-OCHF2 -CH3
1.49Cl ~[~Cl 5-CF3 -CH3
F3C ~ 4-CF3 -CH3
-27- 20~311
Comp. No.R~X (R2)n R4 Physical data
1.51 Cl ~F 4-CI -N(CH3)2
1.52 ~-- 5-CF3 -CH3
1.53 Cl ~F 5-CI -CH3 m.p. 189-193C
1.54 ~I ~F 4-Br -N(CH3)2 m.p. 174-176C
1 55 ~3~O H -CH3 m.p. 179-180C
1.56 F3C ~ 4-CF3 -N(CH3)2
1.57Cl ~CI S-CF3 -N(CH3~2
1.58CH30~0 H -CH3
1.59 ~F S-CI -N(CH3)2 m.p. 128-132C
1.60 Cl ~F 5-Br -CH3
-28- 20~.~311
Comp. No. RIX (R2)n R4 Physical data
.... _ _
1.61 Br~e3~ H -N(cH3)2 m.p. 121-124C
N 0
F~C~,~,
1.62 ~N JJ~-- S-CF3 -CH3
1.63 Br `~3~ 5-Cl -c~3
N 0
1.64 J~ S-CI -CH3
CH30 N 0--
Cl ~ F
1.65 ~NJ~0 4-CF3 -CH3
F3C ~CI
1.66 ~N~0-- H -CH3 m.p. 196-206C
Br ~
1.67 ~N JJ` O S-Br -CH3
1.68 J~ S-Br -CH3
CH30 N 0
Br ~,~
1.69 ~ Jl~ 4-CF3 -CH3
N 0--
1.70 Cl ~ S-CF3 -CH3
N 0
20~531 1
- 29 -
Comp. No. RIX (R2)n R4 Physicaldata
l .71 F3C ~3~ 5-CF3 -N(CH3)2
1.72 CH30 ~ 5-CF3 -CH3
F3C Cl
~o -C2Hs
F3C ~CI
1.74 ~ Jl~ H cyclopropyl
C1 ~F
~N JJ` -- 5-CF3 -N(CH3)2
Br ~,~
1.76 ~N Jl`o S-CF3 -CH3
F3C ~ Cl
1.77 ~ ~ H -C3H7-n m.p. 130-132C
N 0
F3C ~D~ Cl
1.78 ~ Jl~ H cyclopentyl
F3C ~CI
~N~O 5-OCH3 -CH3 m.p. 201-203C
F3C ~ Cl
1 . 80 ~N Jl~ 4-CF3 -CH3
2 0 ~
- 30-
Comp. No. RIX (R2)n R4 Physical data
F3C ~ Cl
].8] ~NJI~O-- 4-Cl, 5-CI -CH3
F3C ~CI
1.82 ~N~O H ~ -N(CH3)2 m.p. 175-176C
F3C ~,~ Cl
1.83 ~NJJ~O H -N(C2Hs)2
02N ~
1.84 ~N JJ~O-- H -CH3
F3C ~ Cl
1.85 ~NJJ~O S-OCH3 -N(CH3)2
1.86 ~ 5-Br -CH3
N S
F3C ~CI
1.87 ~N J~O H -N(CH3)(C2H5)
F3C ~CI
1.88 ~NJI~O 4-OCHF2 -CH3
1.89 ~S H -CH3 m.p. 157-160C
F3C ~CI
1.9() ~ J¦~ H cyclohexyl
20~53~1
- 31 -
Comp. No.RIX (R2)n R4 Physical data
F3G ~ Cl
1.91 ~NJ~O 5-OCHF2 -CH3
1.92F3C~CI 5-Br -~H3 m.p. 201-205C
02N ~,~
1.93~NJI~O 5-Cl -CH3
F3C~C~
1.94 NJ~O 4-F -CH3
F3C ~CI
1.95~N J~O 4-CF3 -N(CH3)2
1.96 ~3~S H -N(CH3)2 m.p. 137-138C
F3C Cl
1.97 ~ 4-F -CH3
F3G ~CI
1.98 ~N ~O 4-OCF3 -CH3
1.99 ~3~S 5-Cl -CH3 m.p. 170-171C
1.100~3~S-- 5-CF3 -CH3
2 ~ ~ J c~
- 32 -
Comp. No.RIX (R2)n R4 Physicaldata
F3C Cl
1.101 ~ 5-F -CH3
1.102~NJ~S 5-CF3 -N(CH3)2
F3C ~ Cl
1.103~ ,11~ S-F -N(CH3)2
N O
F3C ~CI
1.104¦ Jl~ 4-Cl -CH3 m.p. 206-209C
~N O--
, F3C ~ Cl
1.105~NJJ~O 5-OCF3 -CH3
F3C ~,~CI
1.106 ~N~O 5-CF3 -CH3
F3C ~ Cl
1.107~NJI~O 5-CI -CH3 m.p. 194-225C
N~
1.108~` S-- H -CH3
F3C~CI
1.109~NJJ~O 5-Br -N(CH3)2 m.p. 188-192C
~N
1.110 l ll H -CH3
~N ~S
-33- ~53~1
COMP. N RIX (R2)n R4 PhYSiCaldata
.. . . .
F3C ~CI
1.111~N J~O 5-Cl -C2I~s
1.112 S S H -CH3
F3C ~CI
I.1 13~ ,!1~ 5-Br cyclopentyl
N ~\~
~S-- H -N(CH3)2 m.p~ 113-115C
F3C ~CI
1.115~N~O S-CI -N(CH3)2 m.p. 199-232C
~N
I .1 16~N ~ S H -N(CH3)2
F3C ~CI
1.117~NJJ~O 5-OCF3 -N(CH3)2
F3C ~ Cl
1.1 18~N J~ o _ 5-CI cyclopropyl
02N ~
1.119~N~O-- 5-CF3 H
1.120S ~ S H -N(CH3)~
20~311
- 34 -
Comp. No.RIX (R2)n R4 Physicaldata
1.121o2N~9~o H -CH3
F3C ~CI
1.122 ~N~O 5-CI cyclopentyl
1-123[~ 3~O H -CH3
1.124 S~O H -CH3 m.p. 103-105C
1 125 ~ 3~ S-Cl -CH3
1.126 S~O H -N(CH3)2
1.127F3C~CI S-CI cyclohexyl
1.128O2N~S O -- H -N(CH3)2
F3C ~CI
1.129 ~N~O 5-CF3 -N(CH3)2 m.p. 108-111C
1.130 ~9~O S-CI -CH3
1.131 ¢53~o 5-CF3 -CH3
20~a31~
- 35 -
Comp. No.RIX (R2)n R4 Physicaldata
~N~
1.132~NJI~O-- 5-CF3 -CH3
F3C ~CI
1.133~NJI~O-- 7-Cl -CH3
~N
1.134S~O-- 5-Cl -N(CH3)2
1.135 ~O S-Br -CH3
~ F3C Cl
1.136 ~ 4-Br -CH3 m.p.209-211.5C
1.137 ~o-- 5-OCH3 -CH3
F3C Cl
1.138 ~ S-CF3 cyclopentyl
F3C ~ Cl
1.139~NJI~O 4-Br -C3H7-nm.p. 167-168C
~N
1.140 S~O 5-Br -CH3
Cl ~OCH3
1.141~N Jl`o - H -CH3
1.142 ~O 5-CI -CH3
-36- 20~.~311
Comp. No. RIX (R2)n R4 Physical data
F3C Cl
1.143 ~ 5-Br cyclohexyl
1.144 S~O 5-CF3 -CH3
1.145 CH3 J~ S-CF3 -CH3
F3C ~CI
1.146 ~N~O 4-Br -N(CH3)2m.p. 182-183C
r~N
1.147 ~NJ~O 5-CF3 -CH3
F3C Cl
1.148 ~ S-CF3 cyclohexyl
1.149 CH3 ~lo-- S-CH3 -CH3
F3C ~CI
1.150 ~N Jl~o-- 7-Br -CH3
CH3
1.151 ,,~1 H -CH3 m.p. 197-198C
CH3 N lo -
F3C~CI
1.152 ~NJI~O 4-F, 5-CI -~H3
20~31 1
- 37 -
Comp. No. RIX (R2)n R4 Physical data
F3C ~ Cl
1.153 ~NJJ~O-- 7-Br -N(CH3)2
~N
1~154 ~N~O-- H -~H3
F3C~CI
1.155 bNJJ~o 4-CI, 5-CF3 -CH3
F3C~Cl
1.156 ~N ~O 4-CH3 -CH3
1.157 CH3 J~O 5-Br -CH3
F3C ~ Cl
1.158 ~NJI~O 7-CF3 -CH3
CH3
1.159 ~[~N H -N(CH3)2
CH3 N O
F3C ~CI
1.160 ~NJI~O-- 5-CH3 -CH3 m.p. 197-202C
1.161 ~O 5-CF3 -N(CH3)2
F3C ~CI
1.162 ~NJ~O 7-CF3 -N(CH3~2
3 ~ 1
- 38 -
Comp. No. RIX (R2)n R4 Physical data
Cl ~OCH3
1.163 ~ JJ~ S-CF3 -CH3
N O
Cl ~S~OCH3
1.164 ~ JD~ H -N(CH3)2
N O
~N
1.165 ~N~O H -CH3
1.166 ~ H -N(CH3)2
S O
F3C ~CI
1.167 ~ Jl~ 5-CH3 -N(CH3)2 m.p. 122-123C
N O-
1.168 ~ H -CH3
CH3 N o
Cl ~.,OCH3
1.169 ~NJ~O 5-CI -CH3
F3C ~,~CI
1.170 ~ J~ 4-OCH3 -CH3
N O--
1.171 ~O H -CH3
Cl ~,~OCH3
1.172 l ll 5-Br -CH3
~N ~` O
1.173 J~ H -N(CH3)2
CH3 N O
2o~311
- 39 -
Comp. No. RlX (R2)n R4 Physic~ll ddtd
1.174 ~ S 4-Br -CH3
1.175 ~N~S - H -CH3
1.176 ~N ~ S - 4-CI -N(CH3)2
F3C~CI
1.177 ~N~S - H -CH3 m.p.174-176C
F3C~CI
1.178 ~ ~ 5-CI -CH3 m.p.198-202C
N S--
F3C ~,~CI
1.179 ~ ~ H -N(CH3)2m.p.155-156C
N S--
F3C ~ Cl
1.180 ~N~S - 5-CI -N(CH3)2m.p.142-145C
F3C ~ ~CI
1.181 ~ ~ 4-Br -CH3 m.p.238-240C
N S--
F3C ~D~ Cl
1.182 ~N~S - - 4-CI -N(CH3)2m.p.192-193C
2~8~3l~
- 40 -
Comp. No. RIX (R2)n R4 Physical data
F3C ~
1.183 ~ ~J~ 5-CI -CH3m.p. 217-219C
N S--
F3C~
1.184 ~ ~ 5-Cl -N(CH3)2m.p. 154-156C
N S--
Cl ~CI
1.185 ~N Jl~ S-- S-OCH3 -N(CH3)2
Cl
1.186 N\~S-- H -CH3
Cl
CH3
~N
1.187 ~ S-- 5-Cl -CH3m.p. 195-196.5C
,~= N
CH3
CH30
1.188 N ~ S-- H -CH3m.p. 163-165C
~= N
CH30
CH3
~N
1.189 </ \~ S-- 5-Br -N(CH3)2
,~= N
CH3
208~3~ 1
- 41 ^
Comp. No. RIX(R2)n R4 Physicaldata
CH30
1.190 N~ S-- 4-Br -CH3
)=N
CH30
N
1.191 ~N~ 5-OMe -N(CH3h
CH30
1.192 ~N3~S-- 4-CF3 CH3
~N~CI
1.193 ~NJJ~S-- H -CH3
N CH3
1.194 CH3 ~N3~S-- 5-CI -N(CH3)2
1.195 ~N3~ 5-Cl -N(CH3)2
Cl N S--
Br ~
1.196 ~N~O-- H -N(CH3)2 m.p. 112-115C
1.197 ~S-- -CH3 m.p. 117-119C
-42- 20~311
Comp. No.RIX (R2)n ~4 Physicaldata
F3G ~CI
1.198 ~N~O - S-CI -CH3 m.p.175-179C
CF3 ~CI
1.199 ~N~O - 5-Br -N(CH3)2 m.p.160-162C
CF3 ~CI
1.200 ~N~S - H -N(CH3~ m.p. > 200C
~N~0
1.201 N~CI 4-Br -CH3 m.p.202-205C
~N~O
1.202 N~CI H -N(cH3~2 m.p.142-147C
CH30 ~, N ~ 0
1.203 Cl ~N 5-CI -CH3 m.p.203-205C
f ~N ~0
1.204 N~CI H -CH3 m.p.180-182C
CH30 ~ N ~ 0
1.205 Cl ~N 5-CI N(CH3)2 m.p.132-134C
~43~ 20~a3~1
Comp. No.RIX (R2)n R4 Physical data
CH30 ~ N ~O
1.206Cl ~N 5-CI -NtCH3)2 m.p.163-165C
N O
1.207 CF3~ 5-CI -N(C~H3)2 m.p. l30-131C
1.208 CF3~ S-Cl -N(CH3)2 m.p.141-143C
Cl N O
1.209 ~ ~ 5-Cl -N(CH3)2 m.p.180C
CH3 '~' decomp.
~N~O
1.210 N~CI 5-Cl -N(CH3)2 m.p.204-206C
CH3
1.211 ~CI 5-CI -CH3 m.p.203-205C
CH3
1.212~;~CI 5-CI -CH3 m.p. 206-107C
CH3
Cl N~O
1.213CH3 ~N 5-Cl -CH3 m.p.210-213~C
20~311
- 44 -
Comp. No. RIX (R2)n R4 Physicaldata
Cl N~O
1.214 CH3 ~N 5-CI -CH3 m.p.221-222C
~N O
1.215 N~CI 5-CI -CH3 m.p.180-182C
CH3
1.216 ~ 5~Q -N(CH3)2 m.p.149-152C
CH3
~N~O
1.217 N~3 5-Cl -CH3 m.p.207-208C
~N~I~O
1.218 N~ S-CI -N(CH3)2 m.p. 185-186 C
CF3
1.219 ~CI H -CH3 m.p.209-210C
CH3
1.220 N~CI H -N(CH3)2 m.p. 158-159C
CH3
20~3~
- 45 -
Comp. No.RlX (R2)n R4 Physical data
~N~O
1.221 N~CI CF3 -C~13 m.p~ 183-187C
CH3 N
1.2æ ~¢ \~o H -N(CH3)2 m.p. 146-160C
CH3 N
1.223 ~ \~O H -CH3 m.p. 109-128C
~N ~0
1.224 N~CI Cl -N(CH3)2 m.p. 157-161C
~N~O
1.225 N~CI Cl -CH3 m.p.199-201C
~N~"O
1.226 N~CI CH3 -CH3 m.p.182-186C
Cl ~.N O
1.227 CF~ H -N(CH3)2 m.p.145-149C
Cl ~N O
1.228 CF3 ~ H -CH3 m.p.150-153C
-46- 20~53~ 1
Comp. No.RIX (R2)n R4 Physical dasa
Cl N O
1.229 CF3~ H -CH3 m.p.144-147C
~N ~,O
1.230 CF3 ~ Cl CH3 -N(CH3)2 m.p.137-143C
~N ~0
1.231 N~ H -N(CH3)2 m.p. 125-130C
CF3
1.232 CF~ H CH3 m.p.12~134~C
~N ~,O
1.233 Cl ~CF3 H -N(cH3~2 solid
1.234 ~ H -N(CH3)2 solid
Cl CF3
N O
1.235 Cl ~CF3 H -CH3 m.p.169-170C
Cl N o
1.236 ~CF3 H -CH3 m.p. l34-136C
Cl N O
1.237 ~CF3 H -N(CH3)2 m.p.184-185C
20~311
- 47 -
Comp. No.RIX (R2)n ~4 Physical data
G~N O
1.238 Cl ~CF3 Cl -CH3 m.p.165-166C
CF3 N O
1.239 ~ H -N(C~H3)2 m.p.95-96C
Cl N o
1.240 ~ H -N(CH3)2 m.p.155-160C
CH3 ,~ N ~0
1.241 ~ H -N(CH3)2 m.p.103-105C
CF3
1.242 N j Cl H -CH3 m.p. 165-167C
~N~O
1.243 N~CI H -N(CH3)2 m.p.98-105C
CF3 N O
1.244 ~ H -N(CH3)2 m.p.137-140C
CF3 N O
1.245 ~ H -N(CH3)2 m.p.117-118C
20~5311
- 48 -
Comp. No.RIX (R2)n R4 Physical data
Cl N O
1.246 ~ H -CH3m.p. lSS-157C
CH3 ~N O
1.247 ~ H -CH3 m.p.126-127C
CF3
CF3 N O
1.248 ~ H -CH3 m.p.124-125C
N CF3
1.249 ~ H -CH3 m.p.131-132C
N O
1.250 ~CI H -N(CH3)2m.p.144-145.5C
N O
1.251 ~CI H -CH3m.p. l38-143C
CF3 N
1.252~ \~O H -N(CH3)2m.p.123-125C
CF3 N
1.253~ \~O S-CI -N(CH3)2m.p.182-186C
CF3 N
1.254~ \~O S-CI -CH3m.p.185-188C
2 0 5~ 3 31 1
- 49 -
Comp. No. RIX (R2)n R4Physical cl"ta
.
CF3 N
1.255 ~¢ \~o H -CH3m.p. 168-169C
CH3 N
1.256 ~¢ \~ S-CI -CH3
S
CH3 N
1.257~¢ \~O S-Cl -N(CH3)2
S
CH3 N
1.258~¢ \~O 4-Br -N(CH3)2
CF3 N
1.259~ \~O 4-Br -N(CH3)2
S
CF3 N
1.260~¢ \~O 4-Br -CH3
CF3 N
1.261~¢ \~O S-Br -N(CH3)2
S
CF3 N
1.262~¢ \~O S-Br -CH3
S
CH3 N
1.263~¢ \~O 5-BR -N(CH3)2
20~:~311
- 50-
Comp. No. RIX (R2)n R4 Physical data
CH3 N
1.264 ~ \~O 5-Br -BH3
S
1.265 ~S - S-CI -N(CH3k m.p.169-170C
1.266 ~S - S-CI -N(CH3~2 m.p.142-143C
Cl
1.267 Cl ~S - 4-Br -CH3 m.p.230-232C
N
Cl
1.268 Cl ~S - 4-Br -N(CH3)2 m.p.186-187C
Cl
1.269 Cl ~S - H -CH3 m.p.157 159C
CF3
1.270 Cl - ~O - S-CI -N(CH3)2 m.p.143-145C
N
Cl
1.271 F3C ~ S - 4-Br -CH3 m.p.201-203C
N
Cl
1.272 F3C - ~S- 4-Br -N(CH3)2 m.p.203-204C
N
-sl- 20~33~1
Comp. No. RIX (R2)n R4 Physical data
N~S -
1.273 ~ 5-CI -CH3 m.p.164-167C
N~S -
1.274 ~ 5-CI -N(CH3)2 m.p.166-168C
1.275 CF3 ~S- 4-Br -CH3 m.p.197-198C
1.276 CF3 ~ S - 4-Br -N(CH3)2 m.p.131-133C
N
1.277CF3 ~ ~S- H -CH3 m.p.164-166C
1.278CF3 ~ S - H -N(CH3)2m.p.131-133C
N
1.279 CF3 ~S- 4-CI -CH3 m.p.197-200C
N
1.280 CF3 ~ ~ 4-CI -N(CH3)2 m.p.153-154C
N
1.281 CF3~ 4-Br -CH3 m.p.155-158C
-52- 20~53~1
Comp. No. RIX (R2)n R4 Physical data
1.282 CF3~ 4-Br -N(CH3)2 m.p.190-192C
1.283 CF3~s -CH3 m.p.198-201C
1.284 CF3 ~ S -N(CH3)2 m.p.85-8~C
OCH3
1.285 N~ H -N(CH3)2 m.p.140-142C
CH3O ~N S
N
~\~S-
1.286 ~N 5-Cl -CH3 m.p.14~149C
N
~\~S-
1.287 ~==N 5-Cl -N(C~H3)2 m.p.155-165C
CH3
1.288 ~ ~ H -N(CH3)2 m.p.126-130C
CH3 N
CH3
1.289 ~ ~ 5-CI -N(CH3)2 m.p.18~-187C
CH3 N
CH3 N
1.290 ~ ~S H -CH3 m.p.152-155C
CH ~N
53 203~31~
Comp. N~. RIX ~R2~ R4 Physical data
N
1.291 ~ \~S - H -N(CH3)2m.p.132-142C
N
1.292 CH3 ~ ~S- H -C~H3 m.p.137C
N decomp.
CH3
1.293 ~CI 4-CI -N(CH3)2 m.p.202-204C
N S -
Et
I Cl
1.294 N ~ H -N(CH3)2 m.p.147-149C
~N ~I\S--
CH3
Cl
1.295 ~ 4-Br -N(CH3)2 m.p.201-203C
N S -
CH3
1.296 ~CI 4-Br -CH3 m.p.216-218C
N S -
CH3
Cl
1.297 ~ H -CH3 m.p.197-200C
S--
-54- 20~ ~31~
Comp. No.RIX (R~n R4 Physical data
CH3
Cl
1.298 N~ H -N(CH3)2 m.p.162-164C
~N S--
N Cl
.299 ~ ~ H -N(CH3)2 m.p.88-90C
N S -
N Cl
1.300 ~N~s_ 4-Br -N(CH3)2 m.p.175-177C
N ~CI
1.301 ~N~s_ 4-Br -CH3 m.p.178-181C
1.302 H3C~S- H -CH3 m.p.156-160C
N = N
1.303 CH3 ~S- H -N(CH3)2 m.p.125-130C
N = N
1.304 Cl ~S- H -CH3 m.p. 194-195C
N=N
ss 2085311
Comp. No. RIX (R2)n R4 Physical data
. _
1.305 Cl ~S- H -N(CH3)2m.p. 14S-147C
N=N
1.306 Cl ~S- 4-Br -N(CH3)2m.p. 169-170C
N=N
1.307 ~3 4-CI -N(CH3)2m.p. 158-159C
N
Cl
1.308 ~ 4-CI -N(CH3)2 m.p. 133-149C
N
OCH3
1.309-N~ H -CH3
H30 ~N lo--
OCH3
1.310 ~ 4-Br -CH3
H30 N --
OCH3
1.311N~ H -N(CH3)2
H30~N ~O--
20~53~ 1
- 56 -
Comp. No. RIX (R2)n R4 Physical data
. _
OCH3
1.312 N~ 4-Br -N(CH3)2
H30'bN 0--
1.313 ,~,N3~ H -CH3
Cl N --
1.314 ,~,N3~ H -N(cH3)2
Cl N --
1.315 ,,~N3~ 4-Br -CH3
Cl N --
1.316 ,~N3~ 4-Br -N(CH3)2
Ci N 0--
1.317 ~N3~C2H5 H -CH3
N 0--
N C2H5
1.318 (~ ~ 4-Br -CH3
N 0--
N C2H5
1.319 [~N3~o-- H -N(CH3)2
2O~J311
- 57 -
Comp. No.RIX (R2)n R4 Physical data
N C2Hs
1.320 [~N~0_ 4-Br -N(CH332
1.321 CH3 ~- H -CH3
N -N
1.322 CH3 4~o- H -N(CH3)2
N _N
1.323 CH3 4~o 4-Br -CH3
N=N
1.324 CH3 4~o- 4-Br -N(CH3)2
N=N
1.325 H30 ~ - H -CH3
N_N
1.326 H30 ~ 0 - 4-Br -CH3
N-N
1.327tl30 4~ ~ 4-Br -N(CH3)2
N=N
-58- ~0~3~1
Comp. No. R~X (R2)n R4 Physical data
. _ . .
1.328 H30 4~ 0 - H -N(CH3)2
N=N
1.329 Cl ~4~ ~ H -CH3
N=N
1.330 C~ 4~~ H -N(CH3)2
N=N
1.331 Cl ¢~0- 4-Br -CH3
N-N
1.332 Cl 4~- 4-Br -N(CH3)2
N=N
Cl Cl
1.333 ~ o - ~ -c~3
N -N
Cl Cl
1.334 ~ 0 - 4-Br -N(CH3)2
N -N
Cl Cl
1.335 ;~ 0 _ H -N(CH3)2
N - N
20~53~1
Comp. No.RIX (R2)n R4 Physical dala
Cl Cl
1.336 ~ O _ 4-Br -CH3
N=N
1.337 CF3 ~S- 5-CI -CH3
N
1.338 CF3 ~S- 5-CI -N(CH3)2
N
1.339 N~3 S- 4-Br -CH3
1.340 N3S_ 4-Br -N(CH3)2 m.p. 166-168C
1.341 N~S- 5-Br -CH3 m.p. 141-142C
1.342 N~S- 5-Br -N(cH3)2 m.p. 167-169C
[~0 H -N(CH3)2 m.p. I()3-107C
-60- 208a311
C~mp. N~.RIX (R2)n R4 ~hysical data
EtOOC
1.344 ~ 4-Br -N(CH3~2m.p.162-165C
1.345 ~N S-Cl -N(CH3)2m.p.164-166C
CH3 ~N~O
1.346 ~N 5-CI -CH3 m.p.173-175C
CH3 N lo
1.347 ~ S-CI -N(CH3)2m.p.186-190C
CH3 S
~CN S-Cl -CH3 m.p.199-203C
CH3 ~S
1.349 ~ 5-Cl -N(CH3)2m.p.166-170C
CF3 N
1.350 ~ 5-CI -CH3 m.p.156-160C
CF3 N
N O
1.351 N~ 5-CI -N(CH3)2m.p.149-152C
OCH3
20~31~
- 61 -
Comp. No. R~X (R2)n ~4 Physical d~lt
~N~O
1.352 N~ 5-Cl -CH3 m.p.162-165C
OCH3
CH3
CH3CH2S ~ N
CH ~N~O S-CI -CH3 m.p.160-165C
CH3CH2S ~ N
C H3 ~N ~ O 5-Cl -N(CH3)2 m.p.112- 115C
CH3
1.355 CH3~N~O S-Cl -N(CH3)2 m.p.183-185C
CH3~N O
1.356 N~CI S-CI -N(C~3)2 m.p.190-193C
CH3
CF3 N
1.357 ~ \~ S Cl -N(CH3)2 m.p.136-139C
CH3CH200C
CF3 ~Cl
1.358 ~N~O 7-CI -N(CH3)2 m.p.186-188C
N O
1.359 ~'~ 4-Br -N~CH3)2 m.p. 214-250C
CH3
-62- 20~5311
Comp. No.RIX (R2)n R~ Physical data
.. . _ . . . . . .. .
CH3
1.360 ~ 4-Br -CH3 m.p. 179-184C
N Cl
~zN ~O
1.361 ~ 4-Br -N~CH3)2 m.p. 205-209C
20~5311
- 63 -
Table 2: Compounds of the fonnula
IR1
(R2) n~C ~SO2~4 -SO2R,1: Position lL or 3*
Comp. No. RIX (R2)n R4 Yhysicaldata
~0 H -CH3
2.2 ~ 5-CI -CH3
~ O H -N(CH3)2
F3C ~,
2.4 ~N ~O H -CH3
Br ~.~
2.5 ~N~O H -CH3
2.6 ¢~)~ H -CH3
S O
Cl ~,~CI
2.7 ~N ~ O H -CH3
2.8 [~ 6-F -CH3
* Isomer mixtures AB with regard to -S02R4 in position 1
or 3, or the pure isomers A or B.
208~311
- 64-
Comp. No. RIX (R2)n R4 Physical data
2.9 F3C ~ 6-F -CH3
2.10 S o 6-F -CH3
2.11 ~0 6-F -CH3
2.12 C~ ~ F H -CH3
2.13 F3C ~ 6-F -N(CH3)2
2.14 ~o-- 6-CI -CH3
2.15 ~ 6-F -N(CH3)2
2.16 F3C~ S-CI -CH3
N
0 6-CF3 -CH3
2.18 ~ 6-CI -CH3
N O
2.19 ~ o-- 6-F -N(CH3)2
2085311
- 65 -
C~>mp. No. RIX (R2)n R4 Physical data
Br ~
2.20 ~N J~O 6-CI -CH3
2.21b~o 6-Cl -N(CH3)2
F3C ~,
2.22~NJ~O 6-CI -CH3
C~ ~D~CI
2.23~N ~ O 6-F -~,H3
2.24Cl ~F S-F -CH3
2.25F3C ~ Cl -CH3
N O--
F3C ~ Cl
2.26~N~O 6-CF3 -CH3
2.27 ~O H -CH3
Br ~
2.28~N ~O 6-CF3 -CH3
F3C ~
2.29bNJ~O 6-CI -N~CH3)2
Cl ~,~CI
2.30~NJJ~O-- 6-F -N(CH3)2
20~3~l
- 66 -
Comp. No. R~X (R~)n R4 Physical data
2.31 ~ 6-F -CH3
2.32 Cl ~ F 6-F -CH3
F3C ~,~C!
2.33~N J~O 5-F -CH3
2.34 ~ 6-Cl -CH3
2.35 ~ 6-CF3 -CH3
2 36 ~3~0 6-CF3 -N(CH3)2
F3C ~,~
2.37~N JJ~O-- 6-CF3 -CH3
2.38 ~ 6-CF3 -CH3
2.39 ~0_ 6-CH3 -CH3
2.4()Cl~CI 6-CI -CH3
2.41 ~ 6-CI cyclohexyl
20~311
- 67 -
Comp. No. R~X (R )
2 n R4 Phys~c~l d~t~
Cl ~ F
2.42 ~N Jl~ O _ 6-F -N(CH3)2
F3C ~
2.43 ~N ~O 6-CH3 -CH3
2.44 ~3~ 5-CI, 7-F -CH3
Cl ~
2.45 ~N Jl~ O H -CH3
Cl ~ ~CI
2.46 ~N Jl~ - 6-CF3 -CH3
F3C ~ C I
2.47 ~N ~O-- 6-F -CH3
Cl ~
2.48 `?N J~ 0 5-F -~H3
Cl ~ F
2.49 ~N O 6-CI -CH3
F3C ~ Cl
2.50 ~N ~;) 6-F -N(CH3)2
Cl ~
2.51 ~N J~O 6-F -CH3
Cl ~CI
2.52 ~N ~O 6-CF3 -N(CH3)2
20~531~
- 68 -
Comp. No. RlX (R2)n R4 Physical data
F3C ~CI
2.53 ~N~O-- 5-CI -C~3
F3C~CI
2.54 ~NJ~O 6-CH3 -CH3
Cl ~ F
2.55 ~N Jl~ o 6-CI -NtCH3)2
Cl ~
2.56 ~NJI~O-- 6-F -N(CH3)2
Cl ~ F
2.57 ~NJ~O-- 6-CF3 -CH3
Cl ~
2.58 ~N ~O 6-CH3 -CH3
F3C ~CI
2.59 ~N~O 6-CI -CH3
F3C ~ Cl
2.60 ~N~O--- 5-CI, 6-CI -CH3
Cl ~,~CI
2.61 ~NJI~O 6-CH3 -CH3
Cl ~ F
2.62~N~O 6-CF3 -N(CH3)(C2Hs)
Cl~,
2.63~N ~O 6-CI -CH3
20~3311
- 69 -
Comp. No. RIX (R2)n R4 Physical data
. _ _
F3C~CI
2.64 ~N ~O 6-CI -N(CH3)2
2.65CH3 ~0-- H -CH3
F3C Cl
2.66 ~ 7-Cl -CH3
Cl ~,~ F
2.67~NJI~O-- 6-CF3 -N(CH3)2
2.68CH3 J~o-- 6-F -CH3
F3C ~,~CI
2.69~N J~O-- S-CF3 -CH3
Cl ~,~CI
2 70~NJ~0 6-Cl -N(CH3)2
Cl ~D~ F
2.71bNJI~o 6-CH3 -CH3
F3C ~ Cl
2.72bNJ~o_ 6-CF3 -CH3
2.73 CH3 J~N O 6-CI -CH3
20~31 1
- 70 -
Comp. No. RIX (R2)n R4 Physical data
Cl ~
2.74 ~NJJ~O 6-CF3 -CH3
2.75CH J~ 6-CF3 -CH3
3 N O
Cl~
2.76bN Jl~ S _ H -CH3
Cl ~CI
2.77~N JJ` S-- S-CI -N(CH3)2
2.78b ~ H -N(CH3)2
N S--
F3C ~ Cl
2.79~ ~ 5-Br -CH3
N S--
F3C ~
2.80~ Jl~ H -N(CH3)2
N S--
F3C ~ Cl
2.81~N~S-- H -CH3
CH30
2.82N~ S-- H -N(C~3)2
)= N
CH30
-71 - 2~31 ~
Comp. No. RIX (R2)n R4 Physical data
CH3
2.83 ~ N -CH3
)=N
CH3
N~
2.84 CH30~N J~ S 6-F -N(CH3)2
2.85 ~113~s-- H -CH3
~N ~ Cl
2.86 ~N J~S-- 5-CI -N(CH3)2
N CH3
2.87 CH3 ~N~S-- 6-Cl -CH3
- 72 -2 0 ~
Table 3: Compounds of the formula
(R2) n 3
~ N NH-SO2R4: Position I or 3*
R1X 1 S02R4SR
(if R'=~, then the compound is in the tautomeric form -C(S)-NH2)
Comp. NO. RIX (R2)D R4 R' Physical data
-
,~
bN Jl~o H -CH3 H
Cl ~CI
3.2 ~NJJ~O H -CH3 H
F3C ~CI
3.3 1 ll 5-CI -CH3 H
~N ~O
3.4 ~ H -CH3 -CH3
N O
~o_ -N(CH3)2 H
Cl ~CI
3.6 ~NJ~O H -N(CH3)2 H
F3C ~CI
~NJI~O 5-CI -N(CH3)2 H
* Isomer mixtures AB with regard to -SO2R4 in position 1
or 3, or the pure isomers A or B.
208~3~
- 73 -
Comp. No. RIX (R2)n R4 R' Physical data
3 8 ~3~ 13 -N(CH3~2 -CH3
Cl ~CI
~N Jl~ O H -N(CH3)2 benzyl
F3C~CI
~NJI~O 5-F CH3 H
F3C ~CI
3.11 ~N~O-- 5-CI -N(CH3)2 -CH3
F3C ~ CI
3.12 ~ Jl~ S-Br -N(CH3)2 H
N O
F3C ~ Cl
3.13 ~N Jl`o-- 5-F -CH3 benzyl
C~ ~CI
3.14 ~NJ~O 4-F -N(CH3)2 H
F3C ~ Cl
~N J~O-- 5-Br -N(CH3)2 -CH3
F3C ~CI
3.16 ~N J~O 5-F -N(CH3)2 H
F3C ~ Cl
3.17 ~N J'-- 5-CH3 -CH3 H
Cl ~CI
3.18 ~N~O 4-F -N(CH3)2 -CH3
74 20~531 1
Comp. No. RIX (R2)n R4 R' Physical data
3.19~3~ 5-CI -CH3 H
Cl ~CI
3.20~N~O-- S-Cl -N(CH3)2 H
F3C ~CI
3.21~N~O 5-CH3 -N(CH3)2 H
3.22~NJJ~O 5-CI -N(CH3)2 H
F3C ~ Cl
3.23~NJ~O 5-CH3 -N(CH3)2 -CH3
Cl ~CI
3.24~NJJ~O 5-CI -N(CH3)2 -C2Hs
3.25 ~ 5-Cl -N(cH3)2 -CH3
N O
3-26[~3~ 5-CF3 -CH3 H
F3C ~,~
3.27~N~O-- H -CH3 }I
Cl ~,~CI
3.28~N J~O-- S-Br -N(CH3)2 H
3.29 ~ 5-CF3 -N(CH3)2 H
20~:~311
- 75 -
Comp. No. RIX ~R2~ R4 R' Physical data
- F3C ~ H -N(CH3)2 H
Cl ~CI
3.31~ JJ~ 5-B~ -N(CH3)2 -CH3
N O
F3C ~,~
3.32~ J~ H -N(cH3)2 -CH3
N O
F3C ~
3.33~ J~ 5-F -CH3 H
N O
F3C ~,~ Cl
3.34~ J~ H -CH3 H m.p. 181-182C
N O
F3C ~
3.35~ J¦~ 5-CF3 -N(CH3)2 H
N O
F3C~CI
3.36~ ~1~ H -CH3 -CH3
N O
F3C ~
~N Jl~o 5-F -CH3 -CH3
F3C ~ Cl
3.38~ JJ~ H -N(CH3)2 H
N O
F3C ~CI
3-39~ ~ 5-OCH3 -CH3 H
N O
F3C ~,~
3.40~ ~¦~ 5-F -N(CH3)2 H
N O--
20~.)3~1
- 76-
Comp. No. RlX (R2)n R4 R' Physical data
F3C ~
~NJ~O S-Br -N(CH3)2 H
F3C ~CI
3.42 ~NJJ~O-- H -N(CH3)2 -CH3
F~C ~
~N ~O-- S-F -N(CH3)2 -CH3
F3C ~CI
~NJJ~O S-OCH3 -N(CH3)2 H
F3C ~
~N J~ O --- 5-CI -N(CH3)2 H
F3C ~,~ Cl
3.46 ~N~O S-OCH3 -N(CH3)2 H
F3C ~ Cl
~NJ~O 4-F -N(CH3)2
F3C ~CI
3.48 ~NJI~O S-F -CH3 -C2Hs
F3C ~CI
~N J~ S-CF3 -CH3 H
F3C ~ Cl
~N~O S-CF3 -N(CH3)2 H m.p. 190-192C
F3C ~ Cl
3.51~NJI~O S-F ~N(CH3)2 -CH3
20~3311
Comp. No. RIX (R2)n R4 R' Physical dala
~ = _
F3C ~,~ Cl
3.52 ~N~O-- 5-CF3 -N(CH3)2 -C2Hs
F3C ~CI
~N Jl~o 5-CF3 -N(CH3)2 -CH3
F3C ~,
~N J~ 0 5-CI -N(CH3)2 -CH3
F3C~CI
~N ~O 5-CF3 -N(CH3)2 benzyl
3.56 ~ 5-F -CH3 H
N O
F3C ~,~ Cl
~NJ~O 5-F -N(CH3)2 benzyl
3.58 ~ 5-F -N(CH3)2 H
N O
F3C ~
~N J~---- 5-CF3 -N(CH3)2 -CH3
3.60 ~ 5-F -N~CH3)2 -CH3
N O
~CI ~
3.61 ~NJ~S-- H -CH3 H
20~311
- 78 -
Comp. No. RlX (R2~n R4 R' Physical da~a
. . ~
3.62 CH30~ S-- 4-Br -CH3 H
F3C ~ Cl
3.63 ~NJJ~S-- H -CH3 H
F3C ~ Cl
3.64 ~N ~S-- H -N(CH3)2 H
F3C ~ Cl
3.65 ~NJ~S-- 5-CI -CH3 -CH3
F3C ~CI
3.66 ~NJI~S-- 5-Cl -N(CH3)2 H
F3C ~ Cl
3.67 ~NJI~S-- 5-Br -CH3 H
F3C ~ Cl
3.68 ~N Jl~ S-- 5-Br -N(CH3)2 H
F3C ~
~N J~ S-- S-OCH3 -CH3 H
Cl ~,~CI
3.7() ~ JJ~ H -CH3 -CH3
208~311
- 79 -
Comp. No. RIX (R2)n R4 R' Physical data
OCH3
3.71 N ~ 4-Br -CH3 H
CH30J~N S--
CH3
3.72 <~ ~ 5-Cl -N(CH3)2 H
CH3
CH30
3.73 </ ~ S-- H -CH3 H
)=N
CH30
~N 3~ S _ 4-OCF3 -CH3 -CH3
~N~CI
~N Jl~ S-- S-Cl -N(CH3)2 -CH3
3.76 ~N3~CH3 5-Br -CH3 H
CH3 N S--
3 77 ~ 3~ 4-CF3 -N(CH3)2 H
Cl N S--
~N~O--
3.78 N~CI 5-CI -N(CH3)2 H m.p. 206-208C
20~3~
- 80-
Comp. No. RlX (R2)n R4 R'Physical data
5-CI -N(CH3)2 Hm.p.200-203C
~ CF3 Cl
3.80 Cl ~O- 4-Br -N(CH3)2 Hm.p.225-227C
3.81 CF3 ~S 5-Cl -N(CH3)2 Hm.p.147-148C
3.~2 N~ S 5-CI -N(CH3)2 H
~N~O--
3.83 N~CI 5-Cl -N(CH3)2 llm.p.167-171,5C
CH3
3.84 ~CH3 5-CI -N(CH3)2 H amorph
~N ~ O--
3.85 N~CI H -CH3 H m.p.172-173C
Cl ~~ ,CI
3.86 ~N~O- - 4-Br -N(CI13)2 CH3 m.p.163-164C
20~3~.1
- 81 -
Table 4: Compounds of the formula
XR1
~C ~2cR\4NsH ~ -SO2R4: Position 1 or 3*
Comp. No. R1X (R2)n R4 R' Physical data
41 ~3~ H -CH3 H
4.2 F3C~ 6-CI -CH3 H
F3C~CI H -CH3 H
[~;3~ 0-- -CH3 -CH3
4.5 ~ F 6-C1 -CH3 -CH3
~0 H -N(CH3)2 H
F3C ~ 6-CI -CH3 -CH3
4.8 F3C~CI H -CH3 -CH3
N O
* Isomer mixtures AB with regard to -SO2R4 in position 1
or 3, or the pure isomers A or B.
208~3~1
- 82-
Comp. No. R7X (R2)n R4 R' Physical dat-
F3C ~CI
~N Jl~o H -N(CH3)2 H
F3C ~,~ Cl
~N Jl~ 6-CF3 -N(CH3)2 -CH3
~0 H -N(CH3)2 -CH3
4.12 ~N J~ 0 6-CI -CH3 -CH3
F3C ~
~N JJ~O 6-CI -N(CH3)2 H
4.14 Cl ~ 6-CI -N(CH3)2 H
N O
F3C ~,~CI
4.15 ~N Jl~ O _ H -N(cH3)2 -CH3
F3C ~ Cl
4.16 ~N ~O-- 6-CF3 -N(CH3)2 -C2H5
F3C ~
~N ~0 6-CI -N(CH3)2 -CH3
Cl ~ F
4.18 ~N Jl~ 0 6-CI -N(CH3)2 -CH3
~3`o -N(CH3)2 H
20~)311
- 83 -
C~mp. N~. RIX (R~)n R4 R' Physical data
Cl ~ F
4.20 ~NJI~O 6-CH3 -CH3 H
Cl ~CF3
4.21 ~N Jl~o H -N(CH3)2 -CH3
4.22 ~ 6-Cl -N(CH3)2 -CH3
F3C ~
4.23~ JJ~ 6-Cl -N(CH3~2 -C2Hs
N O
Cl ~ F
4.24~NJJ~O 6-CH3 -CH3 -CH3
F3C ~ Cl
4.25~N ~O-- 6-CH3 -N(CH3)2 H
4.261~3~ 6-CF3 -N(CH3)2 H
Cl ~ F
4.27~NJJ~O H -CH3 -CH3
F3C ~ Cl
4.28~NJI~O-- S-F -N(CH3)2 H
Cl ~D F
4.29~NJ~O-- 6-CH3 -N(CH3)2 H
~3`o -N(CH3)2 -C3~l7-n
-84- 20~531~
Comp. No. RIX (R2~n R4 R' Physical data
.... _ _
4.31 F3C~ 5-F -N(CH3)2 -CH3
4.32 Cl ~F 6-CH3 -N(CH3)2 -CH3
F3C ~ H -CH3 H
4.34 ~ F 6-CF3 -CH3 -CH3
F3C~l~ H -CH3 -CH3
4.36 Cl ~F H -N(CH3)2 H
F3C ~CI 6-CI -CH3 -CH3
N O
4-38~0_-- H -N(CH3)2 H
4.39~ F 6-CF3 -N(CH3)2 benzyl
F3C ~ H -N(CH3)2 -C3H7-n
4.41Cl ~F H -N(CH3)2 -CH3
-85- 20~31~
Comp. No. RIX (R2)n R4 R' Physicaldata
. _ .
Cl ~ F
4.42 ~ J~ 6-CF3 -N(CH3)2 H
N O
F3C ~
~NJJ~O 6-F -N(CH3)2 H
F3C~,CI
~N~O 6-CI -NtCH3)2 H
Cl ~,~CF3
~N Jl~o 6-CF3 -N(CH3)2 -CH3
4.46 F3C ~ 6-F -N(CH3)2 -CH3
Cl ~,~ F
~N~O 6-F -N(CH3)2 H
F3C ~
4,48 ~NJJ~O-- 6-F -N(CH3)2 4-ch]orobcnzyl
F3C ~ Cl
~NJJ~O 6-CI -N(CH3)2 -C2H5
Cl ~ F
~N JJ` O 6-F -N(CH3)2 -CH3
F3C ~ Cl
4.51 ~N~O-- 6-CF3 -N(CH3)2 H
4.5 ~CI 6-CH3 -N(CH3)2 -CH3
N O--
-~6- 20~311
Comp. No. R1X (R2)n R4 R' Physical data
Cl ~ F
~N JJ` 0 6-F -N(CH3)2 -C3H7-
~N ~ S-- H -N(CH3)2 CH3
F3C ~,~
4.55~ JJ~ 6-Br -N(CH3)2 H
N S--
4.56CH30J~ S ~ 5-Cl -CH3 H
F3C ~ Cl
~N~S-- H -CH3 H
F3C~CI
4.58~NJ~S-- 6-CF3 -N(CH3)2 -CH3
Cl ~CI
~N J~ S-- 5-Br -N(CH3)2 -CH3
CH3
4.6()~ ~ H -CH3 -CH3
N
Ct~3
- 87 -
Comp. No. RIX (R2)n R4 R' Physjcalo8a3 311
CH30
~N
4.61 </ ~ S-- 5-OMe -N~CH3)2 H
>=N
CH30
CH30~ S
4.62 N~N H -N(CH3)2 H
CH30
4.63 (~N3~S_ 5-OCF3 -CH3 -CH3
~N CH3
4.64 H3CJ~N3~S-- H -CH3 -CH3
-88- 20~3a3
Formulation eXamQes of active~gredients of the formula ~ (% = ~er cen~ by wei~ht)
2.1. Wettablepowders a) b) c)
Active ingredient from the tables 25 % 50 % 75 %
Sodium iigninsulfonate 5 % 5 %
Sodium lauryl sulfate 3 % - 5 %
Sodium diisobutylnaphthalene sulfonate - 6 % 10 %
Octylphenol polyethylene glycol ether - 2 %
(7-8 mol of ethylene oxide)
Highly-disperse silica 5 % 10 % 10 %
Kaolin 62 % 27 5~ -
The active ingredient is mixed thoroughly with the additives and ground thoroughly in a
suitable mill. This gives wettable powders which can be diluted with water to give
suspensions of any desired concentration.
2.2. Emulsion concentrate
Active ingredient from the tables 10 %
Octylphenol polyethylene glycol ether 3 %
(4-5 mol of ethylene oxide)
Calcium dodecylbenzenesulfonate 3 %
Castor oil polyglycol ether 4 %
(35 mol of ethylene oxide)
Cyclohexanone 34 %
Xylene mixture 50 %
Emulsions of any des*ed dilution can be prepared from this concentrate by dilution with
water.
2.3. Dusts a) b)
Active ingredient from the tables 5 % 8 %
Talc 95 %
Kaolin - 92 %
Ready-for-use dusts are obtained by mixing the ~active ingredient with the carrier and
grinding the mixture in a suitable mill.
-89- 20~5311
2.4. Extruder ~ranules
Active ingredient from the tables 10 %
Sodium ligninsulfonate 2 %
Carboxymethyl cellulose 1 %
Kaolin 87 %
The active ingredient is mixed with the additives, and the mixture is ground and moistened
with water. This mixture is extruded and subsequently dried in a stream of air.
2.5. Coated granules
Active ingredient from the lables 3 %
Polyethylene glycol (MW 200) 3 %
Kaolin 94 %
(MW = molecular weight)
In a mixer, the finely ground active ingredient is applied uniformly to the kaolin which has
been moistened with polyethylene glycol. In this manner, dust-free coated granules are
obtained.
2.6. Suspension concentrate
Active ingredient from the tables 40 %
Ethylene glycol 10 %
Nonylphenol polyethylene glycol ether 6 %
(15 mol of ethylene oxide)
Sodium ligninsulfonate 10 %
Carboxymethylcellulose 1 ~
37 % aqueous formaldehyde solution 0.2%
Silicone oil in the form of a 0.8%
75 % aqueous emulsion
Water 32 %
The finely ground active ingredient is mixed intimately with the additives. In this manner,
a suspension concentrate is obtained from which suspensions of any desired concentration
can be prepared by dilution with water.
-90- 208~3~
3. Biological examples
Example 3.1: Action a~ainst Plasmopara viticola on ~rape vines
a) Residual-protective action
Grapevine seedlings in the 4-5-leaf stage are sprayed with a spray mixture prepared with a
wettable powder of the active ingredient (Q.02 % of active ingredient). After 24 hours, lhe
treated plants are infected with a sporangia suspension of the fungus. The fungus
infestation is assessed after incubation for 6 days at 95-100 % relative atmospheric
humidity and 20C.
b) Residual-curative action
Grapevine seedlings in the 4-5-leaf stage are infected with a sporangia suspension of the
fungus. After incubation for 24 hours in a humid chamber at 95-100 % relative
atmospheric humidity and 20C, the infected plants are dried and sprayed with a spray
mixture prepared with a wettable powder of the active ingredient (0.02 % of active
ingredient). After the spray coating has dried on, the treated plants are returned to the
humid chamber. The fungus infestation is assessed 6 days after the infection.
Compounds *om the tab~les exhibit very good activity against Plasmopara viticola on
grapevines, in particular active ingredients Nos. 1.2,1.24, 1.66, 1.250, 1.276, 1.303, 1.322,
1.346, 3.79 and others cause complete suppression of fungus infestation (residual
infestation 0 to 5 %). In contrast, the Plasmopara infestation of untreated, but infected,
control plants was 100 %.
Example 3.2: Action a~ainst PhYtophthora on tomato plants
Residual-protective action
Tomato plants which have been grown for 3 weeks are sprayed with a spray mixtureprepared with a wettable powder of the active ingredient (0.02 % of active ingredient).
After 24 hours, the treated plants are infected with a sporangia suspension of the fungus.
The fungus infestation is assessed after incubation of the infected plants for S days at
90- 100 % relative atmospheric humidity and 20C.
Compounds from the tables exhibit a sustained activity (fungus infestation less than
20 %). Comps)unds Nos. 1.2, 1.12, 1.23, 1.24, 1.26, 1.54, 1.66, 1.146, 1.177, 1.1~, 1 221,
1.230, 1.275, 1.308, 3.50, 3.79 and others virtually completely prevent infestation (0 to
5 % infestation). In contrast, the Phytophthora infestation of untreated, but infected,
- 91 ~ 3~ ~
control plants is 100 %.
Example 3.3: Action against Phvtophthora on potato plants
Regidual-proeective action
2-3-week-old potato plants (cultivar Bintje) are grown for 3 weeks and then sprayed with a
spray mixture prepared with a wettable powder of the active ingredient (0.02 % of active
ingredient). After ~4 hours, the treated plants are infected with a sporangia suspension of
the fungus. The fungus infestation is assessed after incubation of the infected plants for S
days at 90-100 % relative atmospheric humidity and 20C.
Compounds from the tables exhibit a sustained activity (fungus in~estation below 20 %).
Compounds No.s 1.23, 1.24, 1.66, 1.146 and others prevent infestation virtually
completely (0 to 5 % infestation). In contrast, the Phytophthora infestation of untreated,
but infected, control plants is 100 %.
Example 3.4: Action a~ainst Pythium debarvanum on sugar beet (Beta vul~aris)
a) Action after soi! drench
The fungus is cultured on sterile oat grains and added to a mixture of soil and sand. The
soil which has been infected in this manner is filled into flower pots and sugar beet seeds
are sown in. Immediately after sowing, the test preparations which are formulated as
wettable powders are poured over the soil in the fo~m of an aqueous suspension (20 ppm
of active ingredient based on the soil volume). Hereupon, the pots are placed in the
greenhouse for 2-3 weeks at 20-24C. The soil is constantly kept uniformly moist by
gently spraying it with water. In the evaluation of the test, the emergence of the sugar beet
plants as well as the quantity of healthy and diseased plants are determined.
b) Action after aPplication bv seed-dressin~
The fungus is cultured on sterile oat grains and added to a mixture of soil and sand. The
soil which has been infected in this manner is filled into flower pots and sugar beet seeds
are sown in, which had been treated with the test preparations formulated as powders for
seed-dressing (1000 ppm of active ingredient based on the seed weight). Hereupon, the
pots in which the seeds have been sown are placed in the greenhouse at 20-24C for 2-3
weeks. The soil is constantly kept uniformly moist by gently spraying it with water. In the
evaluation of the test, the emergence of the sugar beet plants as well as the quantity of
healthy and diseased plants are determined.
- 92 - 2085
After treatment with the active ingredients of the formula I of Tables 1 and 3, more than
80 % of the plants emerge and are healthy in appearance. Only a few plants emerge in the
control pots, and their appearance is unhealthy.
Example 3.5: Direct action against PeronosPora tabacina
Formulated active ingredient in a range of concentrations (10, 1, 0.1 ppm) is mixed with
agar prepared with water, and the agar mixture is poured into Pe~ dishes. After cooling,
100 ~Ll of a sporangia suspension (106 spores/ml) are streaked onto the plate. The plates
are incubated for 16 hours at 1 8C.
Compounds of Tables 1 and 3 were not found to inhibit the gerrnination of Peronospora
tabacina.