Note: Descriptions are shown in the official language in which they were submitted.
W092/0~99 PCT/~091/000~3
~ 8~3~2
Method of introducing a peptide into the cytosol
Field of the Invention
The present invention is direc~ed to a method of in-
S troducing a peptide into the cytosol, and more specifically toa novel principle in vaccine production against viruses,
intracellular parasites and bacteria and against malignant
cells.
o Backqround of the Invention
In the protection against pathogenic organisms and in
their elimination antigen presentation by major histocompati-
bility antigens (MHC) of class I plays an important role.
Cytotoxic T-lymphocytes recognize cells that express foreign
15 or unusual antigens on their surface and destroy the cells,
which is important to eliminate an infection. The same mechan-
ism is operating in the elimination of malignant cells. Anti-
gen presentation by Class I MHC requires that the antigen to
be presented is found in the cytosol or in the endoplasmic
20 reticulum (Germain, R.N. Nature 322, 687-689 (1986)). Extern-
ally added polypeptides therefore do normally not elicit a
class I response. However, if the antigen is artificially
introduced into the cytosol, presentation by MHC Class I may
occur (Moore, M.W., Carbone, F.R. & Bevan, M.J. Cell 54, 777-
zS 785 (1988)). The rommon way today to immunize against suchstructures is to use attenuated live viruses that are able to
enter cells and replicate such that the peptides in question
are formed in the cells and can be presented at the cell
surface. In this way the population of the relevant cytotoxic
30 CD8~ cells is expanded and upon later exposure to the corres-
ponding virulant virus strain, the organism has an immune
protection. The problems with this approach are partly due to
the fact that the attenuated viruses may sometimes revert to
virulence and partly to the problems of making attenuated
35 viruses in many cases. Convenient and non-damaging methods to
introduce into the cytosol foreign peptides, such as viral
antigens, could th~reforP be useful for vaccine purposes to
expand the relevant population of CD8~ MHC Class I restricted
cytotoxic T-lymphocytes.
.,.,, , . . .
... . :
:
,, : '' ' '
W092/0~99 PCT/NO91/00~93
208~3'1~ 2
The only established examples of external proteins that
enter the cytosol are certain bacterial and plant toxins, such
as diphtheria toxin, Pseudomonas aeruqinosa exotoxin A, ricin,
abrin, viscumin, modeccin, Shigella toxin, cholera toxin,
5 pertussis toxin (Olsnes, S. & Sandvig, K. In: "I~munotoxins"
(A.E. Frankel, ed.), Kluwer Academic Publishers, Boston 19~8,
pp. 39-73; Olsnes, S. & Sandvig, K. In "Receptor-mediated
endocytosis" (I. Pas-tan & M.C. Willingham, eds.), Plenum Publ.
Corp., 1985, pp. 195-234). Toxins of this group enter the
10 cytosol where they carry out enzymatic reactions that are
deleterious to the cell or to the organism. By gene manipu-
lations it is possible to form toxin molecules that are of
very low toxicity (Barbieri, J.T. & Collier, R.J. Infect.
Immun. 55, 1647-1651 (1987)). If the toxins were able to carry
15 into cells additional peptide material, such non-toxic mutants
could be useful for vaccine purposes to carry into the cytosol
anti~enic peptides (Cerundolo et al. Nature 345, 449 (1990))
that can be presented by Class I MHC antigens. Such antigenic
sequences can be obtained from a number of viruses, bacteria
20 and parasites, and it is also possible to derive such struc-
tures from certain malignant cells.
It is an object of the present invention to provide a
mechanism of translocating antigenic peptide se~uences to the
25 cytosol in a safe way to expand the population of cytotoxic T-
lymphocytes that are able to react with the corresponding
antigen and eliminate those cells that are presenting the
antigenic peptides. Although the entry mechanism for the
different toxins mentioned above is in principle the same, it
~o has been worked out in most detail in the case of diphtheria
toxin. This is the toxin we have used in most of our studies
in connection with this application.
Summary of the Invention
We here demonstrate that an essentially non-toxic
mutant of diphtheria ~oxin is able to translocate to the cyto-
sol oligopeptides linked to its N-terminal end. The peptides
we have studied are sufficiently different in sequence to
allow the conclusion that a wide variety of peptides can be
. .
,, : :,-
..
.
W092/0~99 PCTtNO91/00093
~ 3 2~8~3~
carried into the cells in the same way.
Thus, the present invention relates to a method of
introducing a peptide into -the cytosol by linking the peptide
to a bacterial or plant toxin, or a mutant ~hereof. Further,
5 the present invention relates to a method of preparing a
vaccine by linking a peptide to a bacterial or plant toxin, or
a mutant thereof to translocate the peptide into the cytosol
for subsequent presentation at the cell surfac:e by Class I MHC
antigens to elicit a Class I restricted immune response and to
10 expand the relevant population o* CD8~ T-lymphocytes. Also, the
present invention relates to vaccines which have been produced
by the above-mentioned method, as well as the use of such
vaccines against viruses, intracellular bacteria and para-
sites, and against molecules associated with malignancies.
Fiaure Leaends
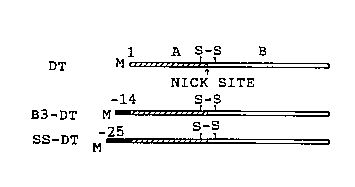
FIG. 1. N-terminal extensions of diphtheria toxin.
A. The coding region of the diphtheria toxin gene
carrying a triple mutation changing Glu14'3 to Ser, and where
20 Gly~ was replaced by initiator Met placed behind a T3 promotor
to give pBD-lS (McGill, S., Stenmark, H., Sandvig, K. &
Olsnes, S.: EMBO J. 8, 2843-2848 (1989)). To obtain pB-B3-D1,
pBD-1 was cleaved with NcoI, and an oligonucleotide encoding
the oligopeptide MGVDEYNEMPMPVN (referred to as B3) was
25 inserted. pGD-2 encodes diphtheria toxin with its natural
signal sequence, MSRKLFASILIGALLGIGAPPSAHA (referred to as
ss), after an SP6 promotor. The plasmid was obtained by
digesting pGD-l (McGill, S., Stenmark, H., Sandvig, K. ~
Olsnes, S.: EMBO J. 8, 2843-2848 (1989)) with HindIII and
30 PstI, removing the overhangs with S1-nuclease and religating to
form pGD-2.
B. The genes were transcribed in vitro and the mRNAs
obtained were translated in rahbit reticulocyte lysate systems
in the presence of [35S]methionine (McGill, S., Stenmark, H.,
3S Sandvig, K. & Olsnes, S.: EMBO J. 8, 2843-2848 (1989)). To
remove reducing agents and to allow disulfide bridges to be
formed, the translation mixture was dialyzed over night
against PBS ( O. 14 M NaCl, 10 mM Na-phosphate, pH 7.4), and
then for 4 h against Hepes medium (Dulbecco-modified Eagles
.~
W092/0~99 PCT/NO91/00093 ~ '
~ 3 1 2 4 ~
medium wherein the bicarbonate had been replaced by ~O mM
Hepes, pH 7.4). An ali~uot of each sample was analyzed by
polyacrylamide gel electrophoresis in the presence of sodium
dodecyl sulfate ~SDS-PAGE) under reducing cond:Ltions (Olsnes,
5 S. & Eiklid, K. J. Biol. Chem. 255, 284-289 (1980)). In some
cases the translation product was treated with protein A-
Sepharose (Pharmacia, Sweden), which had previously been
incubated with rabbit anti-B3 antiserum (lanes 3 and 4) or
anti-ricin (lane 5). The adsorbed material was analyzed by
lO S~S-PAGE. DT, translation product from pBD-l; B3-DT, trans-
lation product from pB-B3-D1; ss-DT, translation product from
pGD-2.
FIG. 2. Translocation to the cytosol of A-fragment with
N-terminally added B3 oligopeptide. p~D-1 and pB-B3-Dl were
15 transcribed and translated in vitro. ~he corresponding trans-
lation products (DT and B3-DT) were added to Vero cells grow-
ing as monolayers in 24-well microtiter plates and kept at
~4C for 20 min in the presence of 10 ,uM monensin ~McGill, S.,
Stenmark, H., Sandvig, ~. & Olsnes, S.: EMBO J. 8, 2843-2848
20 ( 1989)). The cells were washed twice with Hepes medium and
subsequently treated with 0.4 lug/ml TPCK (N-tosyl-L-phenyl-
alanine chloromethyl ketone)-treated trypsin in Hepes medium
containing lO ~M monensin for 5 min at 20C. The cells were
washed and exposed to Hepes medium, pH 4.8, containing 10 mM
zS Na-gluconate to increase the buffering capacity at the low pH.
After 2 min at 37C, the cells were washed with Hepes medium,
pH 7.4, and then treatPd with 3 mg/ml pronase in Hepes medium,
pH 7O4~ containing 10 ~M monensin for 5 min at 37C. The
cells, which were detached from the plastic by the treatment,
30 were recovered by centrifugation and washed once with Hepes
medium containing l mM NEM (N-ethyl maleimide) and 1 mM PMSF
(phenylmethylsulfonyl fluoride). In some cases, (lanes 1-3 and
8-lO) the cells were lysed with Triton X-lOO in phosphate
buffered saline containing l mM PMSF and 1 mM NEM, nuclei were
35 removed by centrifugation and the protein in the supernatant
fraction was precipitated with lO~ (w/v~ trichloroacetic acid
or immunoprecipitated with anti-B3 antibodies adsorbed to
protein A-SepharosP. In other cases (lanes 4-7) the cells were
treated with 50 ,ug/ml saponin in PBS containing l mM PMSF and
: :: .
-
. :
. .
W092/0~99 PCT/NO91/OOOg3
3 ~ 2
1 mM NEM to release translocated A fragment, and then theproteins both in the pellet and in ~he supernatant fractions
were precipitated with trichloroacetic acid. ]:n all cases the
precipitated material was analyzed by SDS-PAGE (13.5~ gel)
5 under non-reducinq conditions.
FIG.3. Translocation to the cytosol of diphtheria toxin
with signal sequence. Lanes 1-4: l25I-labelled natural toxin
(wt-DT, lane 1) and in vitro translated pGD-2 ([35S]methionine
labelled toxin with signal sequence, ss-DT) were bound to Vero
o cells and nicked on the cells (lanes 1 and 2). In lane 3 the
cells were treated as in lane 2, except that 6 times more
translation product was used and the cells were then exposed
to pH 4.8 and pronase as in Fig. 2. The cells were lysed with
Tri-ton X-100 and the nuclei were removed. The supernatants
were incubated with protein A-sepharose that had been pre-
incubated with rabbit anti-diphtheria toxin serum. The adsorb-
ed material was analyzed by reducing (lanes 1 and 2) or non-
reducinq (lanes 3 and 4) SDS-PAGE (10% gel). In lane 4 the
pronase-treated cells were treated with 50 ~g/ml saponin and
20 the material released to the medium was analyzed directly.
Lanes 5-12: Translation products from pBD-1 (DT) and pGD-2
(ss-DT) were bound to Vero cells, nicked, exposed to pH 4.8
and then treated with pronase. The lysed cells were either
analyzed with non-reducina SDS-PAGE (15~ gel) directly (lanes
25 5-8) or they were treated with saponin and the membrane
pellets (l~nes 9 and 10) and the supernatant fractions (lanes
11 and 12) were analyzed separately.
Detailed Description
. Diphtheria toxin is synthesized by pathogenic strains
of Corvnebacterium diphtheriae as a single chain polypeptide.
The protein is easily split ("nicked") at a trypsin-sensitive
site to yield two disulfide-linked fragments, A and B (Pappen-
haimer, A.M., Jr. Annu. Rev. Biochem. 46, 69-94 (1977)).
The B-fragment ~37 kD) binds to cell surface receptors,
whereas the A-fragment (21 kD) is an enzyme that is trans-
located to the cytosol where it inactivates elongation factor
2 by ADP-ribosylation and thus blocks protein synthesis (Van
Ness, B.G., Hovard, J.B. & Bodley, J.W. J. Biol. Chem. 255,
. . .
.. ~, .. . ' . '- - '
W092/0~99 PCT/NO91/00093
208~3~2 6 ~`
10710-10716 (1980)). The translocation, which normally occurs
across the limi-ting membrane of endosomes, is triggered by the
low pH in the acidic vesicles (Draper, R.K. & Simon, M.I. J.
Cell Biol. 87, 849-854 (1980); Sandvig, K. & Olsnes, S. J.
S Cell Biol. 87, 828-832 (1980)). When cells with surface-bound
toxin are exposed to acidic medium, translocation occurs from
the cell surface (Sandvig, K. & Olsnes, S. J. Biol. Chem. 256,
9068-9076 (1981)). We have in the presented examples used this
artificial system, because it enables us to distinguish
10 between translocated and non-translocated material (Moskaug,
JØ, Sandvig, K. & Olsnes, S. JO Biol. Chem. 262, 10339-10345
(1987); Moskaug, JØ, Sandvig, K. & Olsnes, S. J. Biol. Chem.
2~3, 2518-2525 (1988)).
To avoid toxic effect on the cells by the diphtheria
15 toxin vec-tor, a mutant toxin was used which contains a triple
mutation changing Glu14a, which is located in the enzymatica:Lly
active site of the toxin, to Ser (Barbieri, J. T. & Collier,
R.J. Infect. Immun. 55, 1647-1651 (1987)). The modified toxin
has strongly reduced toxicity.
ExamPles
We used two variants of the mutated toxin gene, one
without (pBD-1) (McGill, S., Stenmark, H., Sandvig, K. &
Olsnes, S.: EMBO J. 8, 2843-2848 (1989)), and one with (pGD-2)
25 the natural 25 amino acids signal sequence (Fig. lA). In one
case, a foreign oligopeptide, termed B3, was linked to the N-
terminal end of the toxin to yield the plasmid pB-B3-D1.
The constructs, which were placed behind T3 or SP6 RNA-
polymerase promotors, were transcribed and translated in vitro
30 (McGill, S., Stenmark, H., Sandvig, K. & Olsnes, S.:EMBO J. 8,
2843-2848 ~1989)). In each case a major band corresponding to
the full-length protein and only traces of material of lower
molecular weights were obtained (Fig. lB). Toxin with signal
sequence (lane 7) or with B3 (lane 1) migrated, as expected,
35 slightly more slowly than toxin as such (lanes 2 and 6).
Furthermore, toxin with B3 was selectively precipitated with
anti-B3 (lane 4), but not with a control serum (lane 5). ~roxin
without B3 was not precipitated with anti-B3 (lane 3).
The dialyzed translation products were bound to Ve:ro
' -: ' ' ' ~ ''
.
.
W092/0~99 PCT/NO91/00093
~ 7 2~3~2
cells, nicked on the cells with low concentrations of trypsin,
and then the cells were exposed to pH 4.8. Under these
conditions part of the bound toxin was translocated to the
cytosol and thereby became shielded against pronase added to
5 the medium (Moskaug, JØ, Sandvig, K. & Olsnes, S. J. Biol.
Chem. 263, 2518-2525 (1988)). In the case of diph-theria toxin
as such, two fragments (MW 21 kD and 25 kD) w~re protected
under these conditions (Fig. 2, lane 1), corresponding to the
whole A-fragment (21 kD) and part of the B fragment (25 kD out
o of total 37 kD). The interfragment disulfide was reduced,
apparently upon exposure to the cytosol (Moskaug, JØ,
Sandvig, K. & Olsnes, S. J. Biol. Chem. 262, 10339-10345
(1987)).
15 ExamPle 1
When the same experiment was carried out with toxin
containing B3, two major fragments (25 kD and 22.5 kD) were
protected in addition to small amounts of 21 kD fragment (lane
2). The latter probably represents A-fragment where B3 had
20 been cleaved off. When the exposure to low pH was omitted, no
fragments were protected (lane 3). The 22.5 kD fragment was
precipitated by anti-B3 (lane 9), but not with preimmune serum
(lane 10). Protected A-fragment without the oligopeptide was
not precipitated with anti-B3 (lane 8). The apparently higher
25 amount of protected A-fragment with B3 is due to more radio-
activity incorporated, as B3 contains 3 methionines and the A-
fragment alone 5.
When cells with translocated diphtheria toxin are
treated with low concentration of saponin allowing cytoplasmic
30 marker enzymes to leak out of the cells without dissolving the
membranes, the translocated A-fragment is released into the
medium, whereas the B-fragment-derived 25 kD polypeptide
remains associated with the membrane fraction (Moskaug, JØ,
Sandvig, K. & Olsnes, S. J. Biol. Chem. 263, 2518-2525 (1988~;
35 Moskaug, JØ, Sletten, K., Sandvig, K. & Olsnes~ S. J. Biol.
Chem. 264, 15709-15713 (1989); Moskaug, JØ, Sandvig, K. &
Olsnes, S. J. Biol. Chem, 264, 11367-11372 (1989)). This indi-
cates that the translocated A-fragment is fr~e in the cytosol,
whereas the 25 kD polypeptide is inserted into the membrane.
W092~00099 PCT/NO91/00093
Also most of ~e8A~ragment containing B3 was released wi-th
saponin ~lane 7) in the same way as normal A~fragment (lane
6), whereas the 25 kD fragment was associated with the
membranes (lanes 4 and 5). Therefore, it appears that diph- ,
5 theria toxin is able to translocate B3 (14 amino aclds) to the
cytosol.
Example 2
To test if also a larger oligopeptide could be trans-
o located, we chose toxin carrying its normal signal sequence
(25 amino acids). As shown in Fig. 3, lane 2, this protein was
nicked by trypsin into a 23O5 kD A-fragment and a 37 kD B-
fragment. (In this experiment the toxin was only partially
nicked. Partially nicked 12sI-labelled natural toxin is shown
5 for comparison in lane 1). When the toxin with signal sequence
was bound to cells, nicked, and then exposed to pH 4.8, two
fragments (23.5 kD and 25 kD) were protected against pronase
(lane 8). Protected A fragment with uncleaved signal seguence
is also shown in lane 3, where the material was precipitated
20 with an anti-diphtheria toxin serum which binds the whole
toxin, the A-fragment, as well as whole B-fragment (see lanes
1 and 2), but not the 25 kD-fragment. When the pronase-treated
cells were treated with saponin, the extended A-fragment was
released to the medium (lanes 4 and 12), whereas the 25 kD
25 fragment remained in thie membrane fraction (lane 10).
.
', . ..
;' ' ' ' ~
:
~ . .' ': ' . . ' . :
. '