Note: Descriptions are shown in the official language in which they were submitted.
~7~0
-- 1
59063.556
Benzimidazoles
The present invention relates to new
benzimidazoles, processes for their preparation and
pharmaceutical compositions containing them.
EP-A-392317 describes benzimidazoles which are
valuable angiotensin antagonists.
It has now been found that certain new
benzimidazoles are even more useful angiotensin
antagonists, particularly angiotensin-II-antagonists.
Thus, according to one aspect the present invention
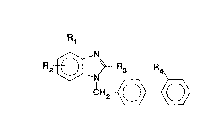
provides compounds of formula I:
Rl
R~- ~ ~ Rg R
2 ~ ~ ~
(I)
(wherein
R1 represents a C13-alkyl group, a hydrogen, fluorine,
chlorine or bromine atom;
R2 represents an oxazol-4-yl or thiazol-4-yl group
optionally substituted in the 2-position by a C16-alkyl
group or by a phenyl group,
or an imidazol-4-yl group optionally substituted in the
2-position by a C16-alkyl group or by a phenyl group and
substituted in the l-position
by a C17-alkyl group optionally substituted by a
carboxy, alkoxycarbonyl, aminocarbonyl,
,
.
2~7~0
alkylaminocarbonyl, dialkylaminocarbonyl,
morpholinocarbonyl, thiomorpholinocarbonyl or l-oxido-
thiomorpholinocarbonyl group,
by a C2~,-alkyl group substituted in the 2-, 3- or ~-
position by a hydroxy, alkoxy, alkoxyalkoxy,
dialXylamino, pyrrolidino, piperidino,
hexamethyleneimino, morpholino, thiomorpholino, l-oxido-
thiomorpholino or imidazol-l-yl group,
by a C13-alkyl group substituted by a trifluoromethyl
group, by a C37-cycloalkyl group or by a phenyl group
itself optionally mono- or disubstituted by fluorine or
chlorine atoms or by trifluoromethyl, methyl or methoxy
groups,
by a C13-alkyl group substituted by two phenyl groups,
or by a C37-cycloalkyl group;
where unless otherwise specified the above mentioned
alkyl and alkoxy moieties each contain 1 to 3 carbon
atoms;
R3 represents a C24-alkyl, C23-alkoxy, C23-alkylthio,
cyclopropyl or cyclobutyl group; and
R4 represents a carboxy, cyano, lH-tetrazolyl, 1-
triphenylmethyl-tetrazolyl or 2-triphenylmethyl-
tetrazolyl group or a group convertable in vivo into a
carboxy group);
and the salts thereof, particularly, for pharmaceutical
use, the physiologically acceptable salts thereof with
inorganic or organic acids or bases.
The term "a group convertable in vivo into a
'
2~7~0
-- 3
carboxy group" may denote, for example, carboxyl esters
such as those of formulae
- CO OR',
- CO - O - (HCR") - O - CO - R"' and
- CO - O - (HCR") - O - CO - OR"'
(wherein
R' denotes a straight-chained or branched C16-alkyl
group or C57-cycloalkyl, benzyll l-phenylethyl, 2-
phenylethyl, 3-phenylpropyl, methoxymethyl or cinnamyl
group,
~,
R" denotes a hydrogen atom or a methyl group, and
R"' denotes a straight-chained or branched C16-alkyl
group or a C57-cycloalkyl, phenyl, benzyl, l-
phenylethyl, 2-phenylethyl or 3-phenylpropyl group).
The ~ollowing are examples of the definitions o~
groups R1 to R4 given hereinbe~ore:
R1 may represent a hydrogen, fluorine or chlorlne atom, a
methyl, ethyl, n-propyl or isopropyl group;
R2 may represent an oxazol-4-yl, 2-methyl-oxazol-4-yl, 2-
ethyl-oxazol-4-yl, 2-n-propyl-oxazol-4-yl, 2-isopropyl-
oxazol-4-yl, 2-n-butyl-oxazol-4-yl, 2-isobutyl-oxazol-4-
yl, 2-n-pentyl-oxazol-4-yl, 2-isoamyl-oxazol-4-yl, 2-n-
hexyl-oxazol-4 yl, 2-phenyl-oxazol-4-yl, thiazol-4-yl,
2-methyl-thiazol-4-yl, 2-ethyl-thiazol-4-yl, 2-n-propyl-
thiazol-4-yl, 2-isopropyl-thiazol-4-yl, 2-n-butyl-
thiazol-4-yl, 2-isobutyl-thiazol-4-yl, 2-n-pentyl-
thiazol-4-yl, 2-isoamyl-thiazol-4-yl, 2-n-hexyl-thiazol-
4-yl, 2-phenyl-thiazol-4-yl, 1-methyl-imidazol-4-yl, 1-
ethyl-imidazol-4-yl, 1-n-propyl-imidazol-4-yl, 1-
isopropyl-imidazol-4-yl, 1-n-butyl-imidazol-4-yl, 1-
isobutyl-imidazol-4-yl, 1-n-pentyl-imidazol-4-yl-, l-
,
2087~0
- 4 - .
isoamyl-imidazol-4-yl, 1-n-hexyl-imidazol-4-yl, l-n-
hexyl-2-methyl-imidazol-4-yl, l-(l-methyl-n-pentyl)-
imidazol-4-yl, 1-(1-ethyl-n-butyl)-imidazol-4-yl, 1-(1-
methyl-n-hexyl)-imidazol-4-yl, l-(l-ethyl-n pentyl)-
imidazol-~-yl, l~ n-propyl-n-butyl)-imidazol-4-yl, 1-
n-heptyl-imidazol-4-yl, 1-ethyl-2-methyl-imidazol-4-yl,
l-n-propyl-2-methyl-imidazol-4-yl, 1-isopropyl-2~methyl-
imidazol-4-yl, 1-n-butyl-2-methyl-imidazol-4-yl, 1-
isobutyl-2-methyl-imidazol-4-yl, 1-n-pentyl-2-methyl-
imidazol-4-yl, 1-isoamyl-2-methyl~imidazol-4-yl, l-n-
hexyl-2-methyl-imidazol-4-yl, 1-n-heptyl-2-methyl-
imidazol-4-yl, 1-cyclopropylmethyl-imidazol-4-yl, 1-
cyclobutylmethyl-imidazol-4-yl, l-cyclopentylmethyl-
imidazol-4-yl, 1-cyclohexylmethyl-imidazol-4-yl, 1-
cycloheptylmethyl-imidazol-4-yl, 1-(2-cyclo-
propylethyl~-imidazol-4-yl, 1-(2-cyclobukylethyl)-
imidazol-4-yl, 1-(2-cyclopentylethyl)-imidazol-4-yl, 1-
(2-cyclohexylethyl~-imidazol-4-yl, 1-(2-cycloheptyl~
ethyl)-imidazol-4-yl, 1-(3-cyalopropylpropyl)~imidazol-
4-yl-, 1-(3-cyclobutylpropyl)-imidazol-4-yl, 1-(3-
cyclopentylpropyl)-imidazol-4-yl, 1-(3-cyclohexyl-
propyl)-imidazol-4-yl, 1-(3-cycloheptylpropyl)-imidazol-
4-yl, 1-(2,2,2-trifluoroethyl)-imidazol-4-yl, 1-(3,3,3-
~rifluoropropyl)-imidazol-4-yl, 1-benzyl-imidazol-4-yl,
1-(2-phenylethyl)-imidazol-4-yl, 1-(3-phenylpropyl)-
imidazol-4-yl, 1-(4-fluorobenzyl)-imidazol-4-yl, 1-(4-
chlorobenzyl)-imidazol-4-yl, l-(~-chlorobenzyl)-
imidazol-4-yl, 1-(4-trifluoromethyl-benzyl)-imidazol-4-
yl, 1-(3-methyl-benzyl)-imidazol-4-yl, 1-(4-methyl-
benY.yl)-imidazol-4-yl, 1-(3-methoxy-benzyl)-imidazol-4-
yl, 1-(4-methoxy-benzyl)-imidazol-4-yl, 1-(3,4-
dimethoxy-benzyl)-imidazol-4-yl, 1-(3,5-dimethoxy-
benzyl)-imidazol-4-yl, 1-cyclopropylmethyl-2-methyl-
imidazol-4-yl, 1-cyclobutylmethyl-2-methyl-imidazol-4-
yl, 1-cyclopentylmethyl-2-methyl-imidazol-4-yl, 1-
cyclohexylmethyl-2-methyl-imidazol-4-yl, l-cyclo-
heptylmethyl-2-methyl-imidazol-4-yl, 1-(2-
, . .
2~78~0
-- 5
cyclopropylethyl)-2-methyl-imidazol-4-yl, 1-(2-
cyclobutylethyl)-2-methyl-imidazol-4-yl, 1-(2-
cyclopentylethyl)-2-methyl-imidazol-4-yll 1-(2-
cyclohexylethyl)-2-methyl-imidazol-4-yl, 1-(2-
cyc~oheptylethyl)-2-methyl-imidazol-4-yl, 1-(3-
cyclopropylpropyl)-2-methyl-imidazol-4-yl, 1-(3-
cyclobutylpropyl~-2-methyl-imidazol-4-yl, 1-(3-
cyclopentylpropyl)-2-methyl-imidazol-4-yl, 1-(3-
cyclohexylpropyl)-2-methyl-imidazol~4-yl, 1-(3-
cycloheptylpropyl)-2-methyl-imidazol-4-yl, 1-(2,2,2-
trifluoroethyl)-2-methyl-imidazol-4-yl-, 1-(3,3,3-
trifluoropropyl)-2-methyl-imidazol-4-yl-, 1-benzyl-2-
methyl-imidazol-4-yl, 1-(2-phenylethyl)-2-methyl-
imidazol-4-yl, 1-(3-phenylpropyl)-2-methyl-imidazol-4-
yl, l-(4-fluorobenzyl)-2-methyl-imidazol-4-yl, 1-(4-
chlorobenzyl)-2-methyl-imidazol-4-yl, 1-(3-chloro-
benzyl)-2-methyl-imidazol-4-yl, 1-(4-trifluoromethyl-
benzyl)-2-methyl-imidazol-4-yl, 1-(3-methyl-benzyl)-2-
methyl-imidazol-4-yl, 1-(4-methyl-benzyl)-2-methyl-
imidazol-4-yl, 1-(3-methoxy-benzyl)-2-methyl-imidazol-4-
yl, 1-(4-methoxy-benzyl)-2-methyl-imidazol-4-yl, 1-(3,4-
dimethoxy-benzyl)-2-methyl-imidazol-4-yl, 1 (3,5-
dimethoxy-benzyl)-2-methyl-imidazol-4-yl, 1-
carboxymethyl-imidazol-4-yl, 1-(2-carboxyethyl)-
imidazol-4-yl, 1-~3-carboxypropyl)-imidazol-4-yl, 1-(4-
carboxybutyl)-imidazol-4-yl, 1-(5-carboxypentyl)-
imidazol-4-yl, 1-(6-carboxyhexyl)-imidazol-4-yl, 1-(7-
carboxyheptyl)-imidazol-4-yl, l-methoxycarbonylmethyl-
imidazol-4-yl, 1-(2-methoxycarbonylethyl)-imidazol-4-yl,
1-(3-methoxycarbonylpropyl)-imidazol-4-yl, 1-(4-
methoxycarbonylbutyl)-imidaæol-4-yl, 1-(5-
methoxycarbonylpentyl)-imidazol-4-yl, 1-(6-
methoxycarbonylhexyl)-imidazol-4-yl, 1-(7-methoxy-
carbonylheptyl)-imidazol-4-yl, l-ethoxycarbonylmethyl-
imidazol-4-yl, 1-(2-ethoxycarbonylethyl)-imidazol-4-yl,
1-(3-ethoxycarbonylpropyl)-imidazol-4-yl, 1-(4-
ethoxycarbonylbutyl)-imidazol-4-yl, 1-(5-ethoxycarbonyl-
20~78~D
-- 6
pentyl)-imidazol-4-yl, 1-(6-ethoxycarbonylhexyl)-
imidazol-4-yl, 1-(7-ethoxycarbonylheptyl)-imidazol-4-yl,
1-n-propoxycarbonylmethyl-imidazol-4-yl, 1-(2-n-
propoxycarbonylethyl)-imidazol-4-yl, 1-(3-n-
propoxycarbonylpropyl)-imidazol-4-yl, 1-(4-n-propoxy
carbonylbutyl)-imidazol-4-yl, 1-(5-n-propoxycarbonyl-
pentyl)-imidazol-4-yl, 1-(6-n-propoxycarbonylhexyl)-
imidazol-4-yl, 1-(7-n-propoxycarbonylheptyl)-lmidazol-4-
yl, l-isopropoxycarbonylmethyl-imidazol-4-yl, 1-(2-
isopropoxycarbonylethyl)-imidazol~4-yl, 1-(3-
isopropoxycarbonylpropyl)-imidazol-4-yl, 1-(4-
isopropoxycarbonylbutyl)-imidazol-4-yl, 1-(5-
isopropoxycarbonylpentyl)-imidazol-4-yl, 1-(6-
isopropoxycarbonylhexyl)-imidazol-4-yl, 1-(7-
isopropoxycarbonylheptyl)-imidazol-4-yl, 1-
aminocarbonylmethyl-imidazol 4-yl, 1-(2~aminocarbonyl-
ethyl)-imidazol-4-yl, 1-(3-aminocarbonylpropyl)-
imidazol-4-yl, 1-(4-aminocarbonylbutyl)-imidaæol-4-yl,
1-(5-aminocarbonylpentyl)-imidazol-4-yl, 1-(6-
aminocarbonylhexyl)-imidazol-4-yl, 1-(7-aminocarbonyl-
heptyl)-imidazol-4-yl, l-methylaminocarbonylmethyl-
imidazol-4-yl, 1-(2-methylaminocarbonyle~hyl)-imidazol-
4-yl, 1-(3-methylaminocarbonylpropyl)-imidazol-4-yl, 1-
(4-methylaminocarbonylbutyl)-imidazol-4-yl, 1-(5-
methylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-
methylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-
methylaminocarbonylheptyl)-imidazol-4-yl, 1-
ethylaminocarbonylmethyl-imidazol-4-yl, 1-(2-ethylamino-
carbonylethyl)-imidazol-4-yl, 1-(3-ethylaminocarbonyl-
propyl)-im.idazol-4-yl, 1-(4-ethylaminocarbonylbutyl)-
imidazol-4-yl, 1-(5-ethylaminocarbonylpentyl)-imidazol-
4-yl, 1-(6-ethylaminocarbonylhe~yl)-imidazol-4-yl, 1-(7-
ethylaminocarbonylheptyl)-imidazol-4-yl, l-n
propylaminocarbonylmethyl-imidazol-4-yl, 1-(2-n-
propylaminocarbonylethyl)-imidazol-4-yl, 1-(3-n-
propylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-n-
propylaminocarbonylbutyl)-imidazol-4-yl, 1-(5-n-
;
:
2~878~
-- 7
propylaminocarbonylpentyl)-imidazol-4-yl, 1 (6-n-
propylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-n-
propylaminocarbonylheptyl)-imidazol-4-yl, 1-
isopropylaminocarbonylmethyl-imidazol-4-yl, 1-(2-
isopropylaminocarbonylethyl)-imidazol-4-yl, 1-~3-
isopropylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-
isopropylaminocarbonylbutyl)~imidazol-4-yl, 1-(5-
isopropylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-
isopropylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-
isopropylaminocarbonylheptyl)-imidazol-4-yl, 1-
dimethylaminocarbonylmethyl-imidazol-4-yl, 1-(2-
dimethylaminocarbonylethyl)-imidazol-4-yl, 1-(3-di-
methylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-
dimethylaminocarbonylbutyl)-imidazol-4-yl, 1-(5-
dimethylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-
dimethylaminocarbonylhexyl) imidazol-4-yl, 1-(7-
dimethylaminocarbonylheptyl)-imidazol-4-yl, 1-
diethylaminocarbonylmethyl-imidazol-4-yl, 1-(2-
diethylaminocarbonylethyl)-imidazol-4 yl, 1-t3-
diethylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-
diethylaminocarbonylbutyl)-imidazol-4-yl, 1-(5-
diethylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-
diethylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-
diethylaminocarbonylheptyl)-imidazol-4-yl, l-di-n-
propylaminocarbonylmethyl imidazol-4-yl, 1-(2-di-n-
propylaminocarbonylethyl)-imidazol-4-yl, 1-(3-di-n-
propylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-di-n-
propylaminocarbonylbutyl)-imidazo~-4-yl, 1-(5-di-n-
propylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-di-n-
propylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-di-n-
propylaminocarbonylheptyl)-imidazol-4-yl, l-diiso- -
propylaminocarbonylmethyl-imidazol-4-yl, 1-(2-diiso-
propylaminocarbonylethyl)-imidazol-4-yl, 1-(3-diiso-
propylaminocarbonylpropyl)-imidazol-4-yl, 1-(4-diiso-
propylaminocarbonylbutyl)-imidazol-4-yl, 1-(5-diiso-
propylaminocarbonylpentyl)-imidazol-4-yl, 1-(6-
diisopropylaminocarbonylhexyl)-imidazol-4-yl, 1-(7-
20~78~
-- 8
diisopropylaminocarbonylheptyl)-imidazol-4-yl, 1-
morpholinocarbonylmethyl-imidazol-4-yl, 1-(2-
morpholinocarbonylethyl)-imidazol-4-yl, 1-(3-
morpholinocarbonylpropyl)-imidazol-4-yl, 1-t4-
morpholinocarbonylbutyl)-imidazol-4-yl, 1-(5-
morpholinocarbonylpentyl)-imidazol-4-yl, 1-(6-
morpholinocarbonylhexyl)-imidazol-4-yl, 1-(7-
morpholinocarbonylheptyl)-imidazol-4-yl, 1-
thiomorpholinocarbonylmethyl-imidazol-4-yl, 1-(2-
thiomorpholinocarbonylethyl)-imidazol-4-yl, 1-(3-
thiomorpholinocarbonylpropyl)-imidazol-4-yl, 1-(4-
thiomorpholinocarbonylbutyl)-imidazol-4-yl, 1-(S-
thiomorpholinocarbonylpentyl)-imidazol-~-yl, 1-(6-
thiomorpholinocarbonylhexyl)-imidazol-4-yl, 1-(7-
thiomorpholinocarbonylheptyl)-imidazol-4-yl, 1-
oxi~othiomorpholinocarbonylmethyl-imidazol-4-yl, 1--(2-
oxidothiomorpholinocarbonylethyl)-imidazol-4-yl, 1-(3
oxidothiomorpholinocarbonylpropyl)-imidazol-4-yl, 1-(4-
oxidothiomorpholinocarbonylbutyl)-imidazol-4-yl, 1-(5-
oxidothiomorpholinocarbonylpentyl)-imidazol-4-yl, 1-(6-
oxidothiomorpholinocarbonylhexyl)-imidazol-4-yl, 1-~7-
oxidothiomorpholinocarbonylheptyl)-imidazol-4-yl, 1-
carboxymethyl-2-methyl-imidazol-4-yl, 1-(2-
carboxyethyl)-2-methyl-imidazol-4-yl, 1-(3-
carboxypropyl)-2-methyl-imidazol-4-yl, 1-(4-
carboxybutyl)-2-methyl-imidazol-4-yl, 1-(5-
carboxypentyl)-2-methyl-imidazol-4-yl, 1-(6-
carboxyhexyl)-2-methyl-imidazol-4-yl, 1-(7-
carboxyheptyl)-2-methyl-imidazol-4-yl, 1-
methoxycarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
methoxycarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-
methoxycarbonylpropyl)-2-methyl-imidazol-4-yl, 1-(4-
methoxycarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
methoxycarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
methoxycarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
methoxycarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
ethoxycarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
2~87~0
ethoxycarbonylethyl)-2-methyl-imidazol-4~yl, 1 (3-
ethoxycarbonylpropyl~-2-methyl-imidazol-4-yl, 1-(4-
ethoxycarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
ethoxycarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
ethoxycarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
ethoxycarbonylheptyl)-2-methyl-imidazol-4-yl, l-n-
propoxycarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-n~
propoxycarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-n-
propoxycarbonylpropyl)-2-methyl-imidazol-4-yl, 1-(4-n-
propoxycarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-n-
propoxycarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-n-
propoxycarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-n-
propoxycarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
isopropoxycarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
isopropoxycarbonylethyl)-2-me-thyl-imidazol-4-yl, 1--(3-
isopropoxycarbonylpropyl)-2-methyl-imidazol~4-yl, 1-(4-
isopropoxycarbonylbutyl)-2-methyl-imidazol-4~yl, 1-(5-
isopropoxycarbonylpentyl)-2~methyl-imidazol-4-yl, 1-(6-
isopropoxycarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
isopropoxycarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
aminocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
aminocarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-
aminocarbonylpropyl)-2-me-thyl-imidazol-4-yl, 1-(4-
aminocarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
aminocarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
aminocarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
aminocarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
methylaminocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
methylaminocarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-
methylaminocarbonylpropyl)-2-methyl-imidazol-4-yl, 1-(4-
methylaminocarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
methylaminocarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
methylaminocarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
methylaminocarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
ethylaminocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
ethylaminocarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-
ethylaminocarbonylpropyl)-2-methyl-imidazol-4-yl, 1-(4-
- 2~78~0
-- 10 --
ethylaminocarbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
ethylaminocarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
ethylaminocarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
ethylaminocarbonylheptyl)-2-methyl-imidazol-4-yl, l-n-
propylaminocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
n-propylaminocarbonylethyl)-2-methyl-imidazol-4-yl, 1-
(3-n-propylaminocarbonylpropyl)-2-methyl-imidazol-4-yl,
1-(4-n-propylaminocarbonylbutyl)-2-methyl-~midazol-4-yl,
1-(5-n-propylaminocarbonylpentyl)-2-methyl-imidazol-4-
yl, l-(6-n-propylaminocarbonylhexyl)-2-methyl-imidazol-
4-yl, 1-(7-n-propylaminocarbonylheptyl)-2-methyl-
imidazol-4-yl, 1-isopropylaminocarbonylmethyl-2-methyl-
imidazol-4-yl, 1-(2-isopropylaminocarbonylethyl)-2-
methyl-imidazol-4-yl, 1-(3-isopropylaminocarbonyl-
propyl)-2-methyl-imidazol~4-yl, 1-(4-isopropylamino-
carbonylbutyl)-2-methyl-imidazol-4-yl, 1-(5-
isopropylaminocarbonylpentyl)-2-methyl-imidazol-4-yl, 1-
(6-isoprop~laminocarbonylhexyl)-2-methyl-imidazol-4-yl,
1-(7-isopropylaminocarbonylheptyl)-2-methyl-imidaæol-4-
yl, 1-dimethylaminocarbonylmethyl-2-methyl-imidazol-4-
yl, 1-~2-dimethylaminocarbonylethyl)-2-methyl-imidazol-
4-yl, 1-(3-dimethylaminocarbonylpropyl)-2-methyl-
imidazol-4-yl, 1-(4-dimethylaminocarbonylbutyl)-2-
methyl-imidazol-4-yl, 1-~5-dimethylaminocarbonylpentyl)-
2-methyl-imidazol-4-yl, 1-(6-dimethylaminocarbonyl-
hexyl)-2-methyl-imidazol-4-yl, 1-(7-dimethylamino-
carbonylheptyl)-2-methyl-imidazol-4-yl, 1-
diethylaminocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
diethylaminocarbonylethyl)-2-methyl-imidazol-4-yl, 1-(3-
diethylaminocarbonylpropyl)-2-methyl-imidazol-4-yl, 1-
(4-diethylaminocarbonylbutyl)-2-methyl-imidazol-4-yl, 1-
(5-diethylaminocarbonylpentyl)-2-methyl-imidazol-4-yl,
1-(6-diethylaminocarbonylhexyl)-2 methyl-imidazol-4-yl,
1-(7-diethylaminocarbonylheptyl)-2-methyl-imidazol-4-yl,
l-di-n-propylaminocarbonylmethyl-2-methyl-imidazol-4-yl,
1-(2-di-n-propylaminocarbonylethyl)-2-methyl-imidazol-4-
yl, l-(3-di-n-propylaminocarbonylpropyl)-2-methyl-
20~7~a
-- 11 --
imidazol-4-yl, 1-(4-di-n-propylaminocarbonylbutyl)-2-
methyl-imidazol-4~yl, 1-(5-di-n-propylaminocarhonyl-
pentyl)-2-methyl-imidazol-4-yl, 1-(6-di-n-propylamino-
carbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-di-n-
propylaminocarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
diisopropylaminocarbonylmethyl-2-methyl-imidazol-4-yl,
1-(2-diisopropylaminocarbonylethyl)-2--methyl-imidazol-4-
yl, l-(3-diisopropylaminocarbonylpropyl)-2-methyl-
imidazol-4-yl, 1-(4-diisopropylaminocarbonylbutyl)-2-
methyl-imidazol-4-yl, 1-(5-diisopropylaminocarbonyl-
pentyl)-2-methyl-imidazol-4-yl, 1 (6-diisopropylamino-
carbonylhexyl~-2-methyl-imidazol-4-yl, 1-(7-diisopropyl-
aminocarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
morpholinocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
morpholinocarbonylethyl)-2-methyl-imidazol-4-yl, 1--(3-
morpholinocarbonylpropyl)-2-methyl-imidazol-4-yl, 1-(4-
morpholinocarbonylbutyl)-2-methyl-imidazol-4-yl, 1--(5-
morpholinocarbonylpentyl)-2-methyl-imidazol-4-yl, 1-(6-
morpholinocarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
morpholinocarbonylheptyl)-2-methyl-imidazol-4-yl, 1-
thiomorpholinocarbonylmethyl-2-methyl-imidazol-4-yl, 1-
(2-thiomorpholinocarbonylethyl)-2-methyl-imidazol-4-yl,
1-(3-thiomorpholinocarbonylpropyl)-2-methyl-imidazol-4-
yl, l-(4-thiomorpholinocarbonyl-butyl)-2-methyl-
imidazol-4-yl, 1-(5-thiomorpholinocarbonylpentyl~-2-
methyl-imidazol-4-yl, 1-(6-thiomorpholinocarbonyl-
hexyl)-2-methyl-imidazol-4-yl, 1-(7-thiomorpholino-
carbonylheptyl)-2-methyl-imidazol-4-yl, l-oxidothio-
morpholinocarbonylmethyl-2-methyl-imidazol-4-yl, 1-(2-
oxidothiomorpholinocarbonylethyl)-2-methyl-imidazol-4-
yl, l-(3-oxidothiomorpholinocarbonylpropyl)-2-methyl-
imidazol-4-yl, 1-(4-oxidothiomorpholinocarbonylbutyl)-2-
methyl-imidazol-4-yl, 1-(5-oxidothiomorpholinocarbonyl-
pentyl)-2-methyl-imidazol-4-yl, 1-(6-oxidothio-
morpholinocarbonylhexyl)-2-methyl-imidazol-4-yl, 1-(7-
oxidothiomorpholinocarbonylheptyl)-2-methyl-imidazol-4-
yl, l-(2-hydroxyethyl)-imidazol-4-yl, 1-(3-
20~7~0~
- 12 -
hydroxypropyl)-imidazol-4-yl, 1-(4-hydroxybutyl)-
imidazol-4-yl, 1-(2-methoxyethyl)-imidazol-4-yl, 1-(3-
methoxypropyl)-imidazol-4-yl, 1-~4-methoxybutyl)-
imidazol-4-yl, 1-(2-ethoxyethyl)-imidazol-4-yl, 1-(3-
ethoxypropyl)-imidazol-4-yl, 1-(4-ethoxybutyl)-imidazol-
4-yl, 1-(2-n-propoxyethyl)-imidazol-4-yl, 1-(3-n-
propoxypropyl)-imidazol-4-yl, 1-(4-n-propoxybutyl)-
imidazol-4-yl, 1-~2-isopropoxyethyl)-imidazol-4-yl, 1-
(3-isopropoxypropyl)-imidazol-4-yl, 1-(4-isopropoxy-
butyl)-imidazol-4-yl, 1-(2-imidazol-1-yl-ethyl)-
imidazol-4-yl, 1-(3-Imidazol-l-yl-propyl)-imidazol-4-yl,
(4-imidazol-1-yl-butyl)-imidazol-4-yl, 1-(2,2-
diphenyl-ethyl)-imidazol-4-yl, 1-(3,3-diphenyl-propyl)-
imidazol-4-yl, 1-(4,4-diphenyl-butyl)-imidazol-4-yl, 1-
(2-hydroxyethyl)-2-methyl-imidazol-4-yl, 1-~3-hydroxy-
propyl)-2-methyl-imidazol-4-yl, 1-(4-hydroxybutyl)-2-
methyl-imidazol-4-yl, 1-(2-methoxyethyl)-2-methyl-
imidazol-4-yl, 1-(3-methoxypropyl)-2-methyl-imidazol-4-
yl, l-(4-methoxybutyl)-2-methyl-imidazol-4-yl, 1-(2-
ethoxyethyl)-2-methyl-imidazol-4-yl, 1-(3-ethoxypropyl)-
2-methyl-imidazol-4-yl, 1-(4-ethoxybutyl)-2-methyl-
imidazol-4-yl, 1-(2-n-propoxyethyl)-2-methyl-imidazol-4-
yl, l-(3-n-propoxypropyl)-2-methyl-imidazol-4-yl, 1-(4-
n-propoxybutyl)-2-methyl-imidazol-4-yl, 1-(2-
isopropoxyethyl)-2-methyl-imidazol-4-yl, 1-(3-
isopropoxypropyl)-2-methyl-imidazol-4-yl, 1-(4-
isopropoxybutyl)-2-methyl-imidazol-4-yl, 1-(2-imidazol-
l-yl-ethyl)-2-methyl-imidazol-4-yl, 1-~3-imidazol-1-yl-
propyl)-2-methyl-imidazol-4-yl, 1-(4-imidazol-1-yl-
butyl)-2-methyl-imidazol-4-yl, 1-(2,2-diphenyl-ethyl)-2-
methyl-imidazol-4-yl, 1-(3,3-diphenyl-propyl)-2-methyl-
imidazol-4-yl, 1-(4,4-diphenyl-butyl)-2-methyl-imidazol-
4-yl, 1-[2-(2-methoxyethoxy)-ethyl]-imidazol-4-yl, 1-[3-
(2-methoxyethoxy)-propyl]-imidazol-4-yl, 1-[4-(2-
methoxyethoxy)-butyl]-imidazol-4-yl, 1-[2-(2-
ethoxyethoxy)-ethyl]-imidazol-4-yl, 1-[3-(2-
ethoxyethoxy)-propyl]-imidazol-4-yl, 1-[4-(2-
20g7~0
- 13 -
ethoxyethoxy)-butyl]-imidazol-4-yl, 1-[2-(2-n-
propoxyethoxy)-ethyl]-imidazol-4-yl, 1-[3-(2-n-
propoxyethoxy)-propyl]-imidazol-4-yl, 1-[4-(2-n-
propoxyethoxy)-butyl]-imidazol-4-yl, 1-[2-(2-
isopropoxyethoxy)-ethyl]-imidazol-4-yl, 1-[3-(2-
isopropoxyethoxy)-propyl]-imidazol 4-yl, 1-[4-(2-
isopropoxyethoxy)-butyl]-imidazol-4-yl, 1-(2-
~imethylaminoethyl)-imidazol-4-yl, 1-(2-diethylamino-
ethyl)-imidazol-4-yl, 1-(2-di-n-propylamino-ethyl)-
imidazol-4-yl, 1-(2-diisopropylaminoethyl)-imidazol-4-
yl, 1-(3-dimethylaminopropyl)-imidazol-4-yl, 1-(3-
diethylaminopropyl)-imidazol-4-yl, 1-(3-di-n-
propylamino-propyl)-imidazol-4-yl, 1-(3-di-
isopropylamino-propyl)-imidazol-4-yl, 1-(4-
dimethylamino-butyl)-imidazol-4-yl, 1-(4-diethylamino-
butyl)-imidazol-4-yl, 1-(4-di-n-propylamino-butyl)-
imidazol-4-yl, 1-(4-diisopropylamino-butyl)-imidazol-4-
yl, l-(2-morpholino-ethyl)-imidazol-4-yl, 1-(3-
morpholino-propyl)-imidazol-4-yl, 1-(4-morpholino-
butyl)-imidazol-4-yl, 1-(2-pyrrolidino-ethyl)-imidazol-
4-yl, 1-(3-pyrrolidino-propyl)-imidazol-4-yl, 1-(4-
pyrrolidino-butyl)-imidazol-4-yl, 1-(2-piperidino-
ethyl)-imidazol-4-yl, 1-(3-piperidino-propyl)-imidazol-
4-yl, 1-(4-piperidino-butyl) imidazol-4-yl, 1-~2-
hexamethyleneimino-ethyl)-imidazol-4-yl, 1-(3-
hexamethyleneimino-propyl)-imidazol-4-yl, 1-(4-
hexamethyleneimino-butyl)-imidazol-4-yl, 1-(2-
thiomorpholino-ethyl)-imidazol-4-yl, 1-(3-thio-
morpholino-propyl)-imidazol-4-yl, 1-(4-thiomorpholino-
butyl)-imidazol-4-yl, 1-[2-(1-oxido-thiomorpholino)-
ethyl]-imidazol-4-yl, 1-[3-(1-oxido-thiomorpholino)-
propyl]-imidazol-4-yl or 1-[4-(1-oxido-thiomorpholino)-
butyl]-imidazol 4-yl group;
R3 may represent an ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, tert.butyl, cyclopropyl, cyclobutyl, ethoxy,
n-propoxy, isopropoxy, ethylthio, n-propylthio or
isopropylthio group; and
~78~0
R4 may represent a hydroxycarbonyl, methoxycarbonyl,
sthoxycarbonyl, n-propyloxycarbonyl,
isopropyloxycarbonyl, n-butyloxycarbonyl,
isobutyloxycarbonyl, tert.butyloxycarbonyl, n-
pentyloxycarbonyl, isoamyloxycarbonyl, n-hexyloxy-
carbonyl, cyclopentyloxycarbonyl, cyclohexyloxycarbonyl,
benzyloxycarbonyl, l-phenylethyloxycarbonyl, 2-
phenylethyloxycarbonyl, 3-phenylpropyloxycarbonyl,
methoxymethoxycarbonyl, cinnamyloxycarbonyl,
acetoxymethoxycarbonyl, propionyloxymethoxycarbonyl, n-
butyryloxymethoxycarbonyl, isobutyryloxymethoxycarbonyl,
n-pentanoyloxymethoxycarbonyl, isopentanoyloxy-
methoxycarbonyl, pivaloyloxymethoxycarbonyl, n-
hexanoyloxymethoxycarbonyl, cyclopentanoyloxy-
methoxycarbonyl, cyclohexanoyloxymethoxycarbonyl,
phenylacetoxymethoxycarbonyl, 2-phenylpropionyloxy-
methoxycarbonyl, 3-phenyl-propionyloxymethoxycarbonyl,
4-phenylbutyryloxymethoxycarbonyl, benzoyloxy-
methoxycarbonyl, 1-acetoxyethoxycarbonyl, 1-
propionyloxyeth~xycarbonyl, 1-n-butyryloxy-
ethoxycarbonyl, l~isobutyryloxyethoxycarbonyl, l-n-
pentanoyloxyethoxycarbonyl, l-isopentanGyloxy-
ethoxycarbonyl, l-pivaloyloxyethoxycarbonyl, l-n-
hexanoyloxyethoxycarbonyl, l-cyclopentanoyl-
oxyethoxycarbonyl, 1-cyclohexanoyloxyethoxycarbonyl, 1-
phenylacetoxyethoxycarbonyl, 1-(1-phenylpropionyloxy)-
ethoxycarbonyl, 1-~2-phenylpropionyloxy)-ethoxycarbonyl,
1-(3-phenylbutyryloxy)-ethoxycarbonyl, 1-
benzoyloxyethoxycarbonyl, methoxycarbonyloxymethoxy-
carbonyl, ethoxycarbonyloxymethoxycarbonyl, n-
propyloxycarbonyloxymethoxycarbonyl, isopropyloxy-
carbonyloxymethoxycarbonyl, n-butyloxycarbonyloxy-
methoxycarbonyl, isobutyloxycarbonyloxymethoxy-
carbonyl, tert.butyloxycarbonyloxymethoxycarbonyl, n-
pentyloxycarbonyloxymethoxycarbonyl, isoamyloxycarbonyl-
oxymethoxycarbonyl, n-hexyloxycarbonyloxymethoxy-
carbonyl, cyclopentyloxycarbonyloxymethoxycarbonyl,
2 0 ~ 0
- 15 -
cyclohexyloxycarbonyloxymethoxycarbonyl, benzyloxy-
carbonyloxymethoxycarbonyl, l-phenylethoxycarbonyloxy-
methoxycarbonyl, 2-phenylethoxycarbonyloxy-
methoxycarbonyl, 3-phenylpropyloxycarbonyloxy-
methoxycarbonyl, cinnamyloxycarbonyloxymethoxycarbonyl,
l-(methoxycarbonyloxy)-ethoxycarbonyl, 1-.
(ethoxycarbonyloxy)-ethoxycarbonyl, l-(n-
propyloxycarbonyloxy)-ethoxycarbonyl, 1-
(isopropyloxycarbonyloxy)-ethoxycarbonyl, l-(n-
butyloxycarbonyloxy)-ethoxycarbonyl, 1-(isobutyloxy-
carbonyloxy)-ethoxycarbonyl, l-(tert.butyloxy-
carbonyloxy)-ethoxycarbonyl, l-(n-pentyloxycarbonyloxy)-
ethoxycarbonyl, 1-(isoamyloxycarbonyloxy)-
ethoxycar~onyl, l-(n-hexyloxycarbonyloxy)-
ethoxycarbonyl, l-(cyclopentyloxycarbonyloxy)-ethoxy-
carbonyl, l-(cyclohexyloxycarbonyloxy)-ethoxycarbonyl,
cyclopentylcarbonyloxymethoxycarbonyl, 1-
(benzyloxycarbonyloxy)-ethoxycarbonyl, 1-(1-
phanylethoxycarbonyloxy)-ethoxycarbonyl, 1-(2-
phenylethoxycarbonyloxy)-ethoxycarbonyl, 1-(3-
phenylpropyloxycarbonyloxy)-ethoxycarbonyl, 1-
(cinnamyloxycarbonyloxy)-ethoxycarbonyl, cyano, lH-
tetrazolyl, l-triphenylmethyl-tetrazolyl or 2-
. triphenylmethyl-tetrazolyl group.
`:
Preferred compounds according to the invention
include those of formula I wherein R1, R2, R3 and R4 are
as hereinbefore defined with R2 in the 6-position of the
benzimidazole ring,
and the salts thereof.
Particularly preferred compounds according to the
invention include those of formula I wherein
R1 represents a hydrogen or chlorine atom or a methyl
group;
2~'~78~0
- 16 -
R2 represents an oxazol-4-yl or thiazol-4-yl group
optionally substituted in the 2-position by a methyl or
phenyl group
or an imidazol-4-yl group optionally substituted in the
2-position by a methyl group and substituted in the
l-position
by a C17-alkyl group optionally substituted in the 1-,
2-, 3-, 4-, 5-, 6- or 7-position by a carboxy,
methoxycarbonyl, aminocarbonyl, methylaminocarbonyl,
ethylaminocarbonyl, n-propylaminocarbonyl,
dimethylaminocarbonyl, morpholinocarbonyl,
thiomorpholinocarbonyl or l-oxido-thiomorpholino-
carbonyl group,
by a C2,4-alkyl group substituted in the 2-, 3- or
4-position by a hydroxy, methoxy, 2-methoxyethoxy,
dimethylamino, diethylamino, pyrrolidino, piperidino,
morpholino or imidazol-l-yl group,
or by a 2,2,2-trifluoroethyl, 3,3,3-trifluoropropyl,
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl,
cyclohexylmethyl, benzyl, 2~phenylethyl, 4-fluorobenæyl,
3-chlorobenzyl, 4-methylbenzyl, ~ trifluoromethylbenzyl,
3,5-dimethoxybenzyl or 2,2-diphenylethyl group;
K3 represents a C24-alkyl, C23~alkoxy, C23-alkylthio
cyclopropyl or cyclobutyl group; and
R4 represents a carboxy or lH-tetrazolyl group or a group
convertable in vivo into a carboxy group;
especially those compounds wherein R2 is in the 6-
positlon;
and the salts thereof.
- .:
. .
2~7~V
- 17 -
; More particularly preferred compounds of formula I include those wherein
Rl represents a hydrogen atom or a methyl group;
,
R2 represents an oxazol-4-yl or thiazol-4-yl group
optionally substituted in the 2-position by a methyl or
phenyl group
or an imidazol-4-yl group optionally substituted in the
2-pasition by a methyl group and substituted in the 1-
position
by a C17-alkyI group which may be substituted in the 1-,
2-, 3-, 4-, 5-, 6- or 7-position by a carboxy,
methoxycarbonyl, dimethylaminocarbonyl or
morpholinocarbonyl group,
~ .
by a C24-alkyl group substituted in the 2-, 3- or 4-
: position by a hydroxy, methoxy, 2-methoxyethoxy,
dimethylamino, diethylamino, pyrrolidino, piperidino,
morpholino or imidazol-l-yl group,
,
or by a 2,2,2-trifluoroethyl, 3,3,3-trifluoropropyl,
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl,
cyclohexylmethyl, benzyl or 4-fluoro-benzyl group;
R3 represents a C24-alkyl, ethoxy~ ethylthio, cyclopropyl
or cyclobutyl group; and
R4 represents a carboxy or lH-tetrazolyl group;
particularly those compounds of formula I above wherein
R2 is in the 6-position;
and the salts thereof.
~ : .
- ,
:~ .,
.' ~ ' ,' ' '' . ' .
2~1~78~
- 18 -
The present invention particularly relates to the
following compounds of formula I:
(a) 4'-~(2-n-propyl-4-methyl-6-~1-methyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl~-biphenyl-2-carboxylic
acid;
(b) 4'-[(2-n-propyl-4-methyl-6-(1-isopropyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl;
(c) 4'-[(2-n-propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid;
(d) 4'-[(2-ethoxy-6-(1-isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid;
(e) 4'-[(2-n-propyl-4-methyl-6-(1-cycloheptyl-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid;
(f) 4'-[(2-n-propyl-4-methyl-6-(1-aminocarbonylmethyl-
- imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-
2-carboxylic acid; and
(g) 4'-[(2-n-propyl-4-methyl-6-(1-(3-dimethylamino-
propyl)-imidazol-4-yl)-benzimidazol-l-yl)-methyl]
biphenyl-2-carboxylic acid;
and the salts thereof.
According to a further aspect the invention also
provides a process for the preparation of compounds of
the invention, said process comprising at least one of
the following steps:
::
`::
2087~
-- 19
a) cyclising a compound of formula II
~, X;
(II)
(wherein
one of the groups X1 and Y1 represents a group of ~ormula
~; --NR6--~H2~
and the other group X1 or Yl represents a group of
~ormula
,
1 ,~ ~ Z2
- NH - C - R3
Rs represents a hydrogen atom or an R3CO group,
R1, R2, R3 and R4 are as hereinbefore defined,
Z1 and Z2t which may be identical or different, represent
optionally substituted amino groups or hydroxy or
mercapto groups optionally substituted by C16-alkyl
groups or
Z1 and Zz together represent an oxygen or sulphur atom,
an imino group optionally substituted by a C13-alkyl
group, or an alkylenedioxy or alkylenedithio group each
having 2 or 3 carbon atoms) or an N-oxide thereof and
optionally subsequently reducing a cyclized N-oxide thus
~ obtained;
:
. ~ .
2~7~1D0
- 20 -
b) reacting a benzimidazole of formula III
R2 ~ ~ R~
(III)
(wherein
R1, R2 and R3 are as hereinbefore defined) with a
biphenyl compound of formula IV
R
Z3--CH~ ~ ~
(IV)
(wherein
R4 is as hereinbefore defined and
Z3 represents a nucleophilic leaving group such as a
~:~ halogen atom, e.g. a chlorine, bromine or iodine atom,
or a substituted sulphonylaxy group, e.g. a
methanesulphonyloxy, phenylsulphonyloxy or p-
toluenesulphonyloxy group);
c) (to prepare a compound of formula I wherein R4
represents a carboxy group) converting a compound of
formula V
~:;
- ~ .
, ~ ' ' , .
~ ~ . . . .
2~878~
R
~ V)
(wherein
R1, R2 and R3 are as hereinbefore defined, and
R4' represents a group which may be converted into a
carboxy group by hydrolysis, thermolysis or
hydrogenolysis) into a corresponding carboxy compound by
hydrolysis, thermolysis csr hydrogenolysis;
d) (to prepare a compound of formula I wherein R4
represents a lH-tetrazolyl group) cleaving a protec:ting
group from a compound of formula VI
R
R2~ R~, 4
CH2~( ~
(VI)
(wherein
R1, R2 and R3 are as hereinbefore defined and
R4" represents a lH-tetrazolyl group protected in the 1-
or 3-position by a protecting group);
e) (to prepare a compound of formula I wherein R4
represents a lH-tetrazolyl group) reacting a compound of
formula VII
,
2~8780~
- 22 -
R1
R~ 3 CN
CH~ ~ O } /~
(VII~
twherein
R1, R2 and R3 are as hereinbefore defined) with hydrazoic
acid or a salt thereof:
f) converting a compound of formula I wherein R4 denotes
a carboxy group, by esterification into a corresponding
compound of formula I wherein R4 denotes a group
convertable in vivo into a carboxy group;
q) converting a compound of formula I wherein R2 denotes
an imidazol-4-yl qroup subst.ituted in the 1- ~osition by
a C24-alkyl group itself substituted in the 2-, 3- or ~-
position by an alkoxy or alkoxyalkoxy group, by ether
splitting into a corresponding compound of formula I
wherein Rz denotes an imidazol-4-yl group substituted in
the l-position by a C24-alkyl group substituted in the
2-, 3- or 4-position by a hydroxy group;
h) separating the l-isomer from a 1-, 3-isomer mixture
of a compound of formula I by isomer separation;
.
i) converting a compound of formula I into a salt
thereof, more particularly into a physiologically -:.
acceptable addition salt thereof with an inorganic or
organic acid or base, or converting a salt of a compound
of formula I into the free compound; and
j) performing a process as defined in any one of steps
(a) to ti) above on a corresponding protected compound
'
.,
"; ' ,
.
~ . '
20~3~0
and subsequently removing the protecting groups used.
The cyclisation of step (a~ is conveniently carried
out in a solvent or mixture of solvents such as ethanol,
isopropanol, glacial acetic acid, ben~ene,
chlorobenzene, toluene, xylene, glycol, glycolmono-
methylether, diethyleneglycol dimethylether, sulpholane,
dimethylformamide, tetraline or in an excess of the
acylating agent used to prepare the compound of formula
II, e.g. in the corresponding nitrile, anhydride, acid
halide, ester or amide, e.g. at temperatures between O
and 250C, but preferably at the boiling temperature of
the reaction mixture, optionally in the presence of a
condensing agent such as phosphorusoxychloride,
thionylchloride, sulphuryl-chloride, sulphuric acid, p-
toluenesulphonic acid, methanesulphonic acid,
hydrochloric acid, phosphoric acid, polyphosphoric acid
or acetic anhydride, or optionally in the presence of a
base such as potassium ethoxide or potassium tert.-
butoxide. However, ~he cyclisation may also be carried
out without a solvent and/or condensing agent.
It i5 particularly advantageous to carry out the
reaction of step (a) by preparing a compound of formula
II in the reaction mixture by reducing a corresponding
o-nitro-amino compound, optionally in the presence of a
carboxylic acid of formula R3COOH, or by acylation of a
corresponding o-diamino compound. When the reduction of
the nitro group is broken off at the hydroxylamine
stage, the N-oxide of a compound of formula I is
obtained in the subsequent cyclisation. The resulting
N-oxide is then converted by reduction into a
corresponding compound of formula I.
The subsequent reduction of the N-oxide of formula
I obtained is preferably carried out in a solvent such
as water, water/ethanol, methanol, glacial acetic acid,
ethyl acetate or dimethylformamide with hydrogen in the
presence of a hydrogenation catalyst such as Raney
20~78~0
- 24 -
nickel, platinum or palladium/charcoal, with metals such
as iron, tin or zinc in the presence of an acid such as
acetic acid, hydrochloric acid or sulphuric acid, with
salts such as iron(II)sulphate, tin(II)chloride or
sodium dithionite, or with hydrazine in the presence of
Raney nickel at temperatures between 0 and 50C, but
preferably at ambient temperature.
The reaction of step (b) is conveniently carried
out in a solvent or mixture of solvents such as
methylene chloride, diethylether, tetrahydrofuran,
dioxane, dimethyl-sulphoxide, dimethylformamide or
benzene, optionally in the presence of an acid binding
agent such as sodium carbonate, potassium carbonate,
sodium hydroxide, potassium terk.-butoxide, sodium
hydride, triethylamine or pyridine, whilst the latter
two may simultaneously also be used as solvent,
preferably at temperatures between 0 and 100C, e.g. at
temperatures between ambient temperature and 50C.
In the reaction of step tb~, a mixture of the 1-
and 3- isomers is preferably obtained from which
subsequently the l-isomer is isolated by crystallisation
or chromatography using a substrate such as silica gel
or aluminium oxide.
In step (c), functional derivatives of the carboxy
group such as optionally substituted amides, esters,
thiolesters, orthoesters, iminoethers, amidines and
anhydrides, and nitrile and tetrazolyl groups may be
converted by hydrolysis into a carboxy group, esters
with tertiary alcohols, e.g. tert.butylesters, may be
co~verted by thermolysis into a carboxy group and esters
with aralkanols, e.g. benzylesters, may be converted by
hydrogenolysis into a carboxy group.
The hydrolysis of step (c) is conveniently carried
out either in the presence of an acid such as
hydrochloric acid, sulphuric acid, phosphoric acid,
trichloroacetic acid or trifluoroacetic acid or in the
presence of a base such as sodium hydroxide or potassium
2~878~0
- 25 -
hydroxide in a suitable solvent such as water,
water/methanol, ethanol, water/ethanol,
water/isopropanol, water/dioxane, methylene chloride or
chloroform at temperatures between -10C and 120C, e.g.
at temperatures between ambient temperature and the
boiling temperature of the reaction mixture. When
hydrolysis is carried out in the presence of an organic
acid such as trichloroacetic acid or trifluoroacetic
acid, any alcoholic hydroxy groups present may
simultaneously be converted into a corresponding acyloxy
group such as a trifluoroacetoxy group.
If R4' in a compound of formula V represents a cyano
or aminocarbonyl group, such a group may also be
converted into a carboxy group with a nitrite r e.g.
sodium nitrite, in the presence of an acid such as
sulphuric acid, which may simultaneously also be used as
solvent, at temperatures between 0 and 50C.
If R4' in a compound of formula V represents ~or
example a tert.-butyloxycarbony]. group, the tert.-butyl
group may also be thermally cleaved, optionally in an
inert solvent such as methylene chloride, chloroform,
henzene, toluene, tetrahydrofuran or dioxana and
preferably in the presence of a catalytic amount o~ an
acid such as p-toluenesulphonic acid, sulphuri.c acid,
phosphoric acid or polyphosphoric acid, conveniently at
temperatures between 40C and 100C, preferably at the
boiling temperature of the solvent used.
If R4' in a compound of formula V represents, for
example, a benzyloxycarbonyl group, the benzyl group may
also be hydrogenolytically cleaved in the presence of a
hydrogenation catalyst such as palladium/charcoal in a
suitable solvent such as methanol, ethanol,
ethanol/water, glacial acetic acid, ethyl acetate,
dioxane or dimethylformamide, preferably at temperatures
between 0 and 50C, e.g. at ambient temperature, under a
hydrogen pressure of 1 to 5 bar. During hydrogenolysis,
other groups may be reduced at the same time, e.g. a
208~
- 26 -
nitro group may be reduced to the amino group, a
benzyloxy group to the hydroxy group, a vinylidene group
to the corresponding alkylidene group or a cinnamic acid
group to the corresponding phenyl-propionic acid group,
or they may be replaced by hydrogen atoms, e.g. a
halogen atom may be replaced by a hydrogen atom.
Suitable protecting groups for use in step (d)
incl~lde, for example, triphenylmethyl/ tributyl tin and
triphenyl tin groups.
The cleaving of a protecting group used is
preferably carried out in the presence of a hydrohalic
-~ acid, more preferably in the presence of hydrochloric `-
acid, in the presence of a base such as sodium hydroxide
or alcoholic ammonia, in a suitable solvent such as
methylene chloride, methanol, methanol/ammonia, ethanol
or isopropanol, at temperatures between 0 and 100C, but
preferably at ambient temperature or, if the react:ion is
carried out in the presence of alcoholic ammonia, at
elevated temperatures, e.g. at temperatures between lO0
and 150C, preferably at temperatures between 120 and
140C.
The reaction of step (e) is preferably carried out
in a solvent such as benzene, toluene or
dimethylformamide, at temperatures between 80 and 150C,
preferably at 125C. Advantageously, either the
hydrazoic acid is liberated during the reaction from an
alkali metal azide, e.g. from sodium azide, in the
presence of a weak acid such as ammonium chloride, or
the tetrazolide salt obtained in the reaction mixture by
reacting with a salt of hydrazoic acid, preferably with
aluminium azide or tributyl tin azide, which are also
conveniently prepared in the reaction mixture by
reacting aluminium chloride or tributyl tin chloride
with an alkali metal azide such as sodium azide, is
subsequently liberated by acidification with a dilute
acid such as 2N hydrochloric acid or 2N sulphuric acid.
The conversion in step ~f) of a carboxyl group into
: `
20~78~
- 27 -
a group which is metabolically convertable in vivo into
a carboxy group is expediently carried out by
esterifiGation with a corresponding alcohol or with a
corresponding reactive acyl derivative, conveniently in
a solvent or mixture of solvents such as water,
methylene chloride, chloroform, ether, tetrahydrofuran,
dioxane or dimethylformamide or in an excess of an
acylating agent as solvent, optionally in the presence
of an acid activating or dehydrating agent such as
thionyl chloride, with the anhydrides, esters or halides
thereof, optionally in the presence of an inorganic or
tertiary organic base, such as sodium hydroxide,
: potassium carbonate, triethylamine or pyridine, whilst
the latter two may simultaneously be used as solvent, at
temperatures between -25 and 100C, but preferably at
temperatures between -10 and 80C.
The subsequent ether splitting is conveniently
carried out hydrolytically in an aqueous solvent, e.g.
in water, isopropanol/water, tetrahydrofuran/water or
dioxane~water, in the presence of an acid such as
hydriodic acid or hydrobromic acid, but preferably under
the effect of a Lewis acid such as boron trifluoride,
boron tribromide, boron trichloride, dimethyl
borobromide or aluminium trichloride in a suitable
solvent such as dichloromethane or chloroform, or with
the aid of bromotrimethylsilane, iodotrimethylsilane or
chlorotrimethylsilane/sodium iodide in a suitable
; solvent such as acetonitrile, dichloromethane or
chloro~orm, at temperatures between 0 and 100C,
preferably at 20C, with subsequent aqueous working up.
In the reactions described above, any reactive
groups present such as hydroxy, amino or alkylamino
groups may be protected during the reaction by means of
con~entional protecting groups which are split off again
after the reaction.
Examples of suitable protecting groups for a
hydroxy group include trimethylsilyl, acetyl, benzoyl,
~:'
~ .
- 2~ -
methyl, ethyl, tert.butyl, benzyl and tetrahydropyranyl
groups and suitable protecting groups for an amino,
alkylamino or imino group may include acetyl, benzoyl,
ethoxycarbonyl and benzyl groups.
The optional subsequent cleaving of a protecting
group is preerably carried out by hydrolysis in an
aqueous solvent, e.g. in water, isopropanol/water,
tetrahydrouran/water or dioxane/water, in the presence
of an acid such hydrochloric acid or sulphuric acid or
in the presence of an alkali metal base such as sodium
hydroxide or potassium hydroxide, at temperatures
between O and lOO~C, preferably at the boiling
temperature of the reaction mixture. However, a benzyl
group is pre~erably cleaved by hydrogenolysis, e.g. with
hydrogen in the presence of a catalyst such as
palladium/charcoal, in a solvent such as methanol,
ethanol, ethyl acetate or glacial acetic acid,
optionally with the addition of an acid such as
h~drochloric acid, at temperatures between O and 50C,
but preferably at ambien~ temperature, and under a
hydrogen pressure of from 1 to 7 bar, but preferably
from 3 to 5 bar.
An isomer mixture of a compound of formula I thus
obtained may if desired be resolved by chromatography
using a substrate such as silica gel or aluminium oxide.
The compounds of formula I obtained may, if
desired, be converted into the acid addition salts
thereof, more particularly for pharmaceutical use into
the physiologically acceptable salts thereof with
inorganic or organic acids. Suitable acids for this
purpose includ~ hydrochloric acid, hydrobromic acid,
sulphuric acid, phosphoric acid, fumaric acid, succinic
acid, lactic acid, citric acid, tartaric acid and maleic
acid.
Furthermore, the new compounds of formula I thus
obtained, if they contain a carboxy or lH-tetrazolyl
group, may if desired subse~uently be converted into the
- ,
2n~78~0
- 29 -
addition salts thereof with inorganic or organic bases,
more particularly for pharmaceutical use into the
physiologically acceptable salts thereof. Suitable
bases for this purpose include for example sodium
hydroxide, potassium hydroxide, cyclohexylamine,
ethanolamine, diethanolamine and triethanolamine.
Some of the compounds of formulae II to VII used as
starting materials are known from the literature.
Otherwise those compounds may be obtained by methods
known from the literature.
Thus, for example, a compound of formula I~ may be
obtained by alkylation of a corresponding o-amino-
acylamino compound with a compound of formula IV. The
o-amino-acylamino compound re~uired for this may be
obtained by reduction of a corresponding o-nitro-
acylamino compound which in turn may be obtained by
nikration of a corresponding acylamino-acetophenone,
subsequent conversion of the resulting corresponding o-
nitro-acylamino-acetophenone into the corresponding ~-
bromo-acetophenone, subsequent cyclisation of the ~-
bromo-acetophenone with a corresponding acid amide and
subsequent reduction of the nitro group. Before the
reduction of the nitro group an oxazol~4-yl compound
thus obtained may be converted into the corresponding
imidazol-4-yl compound by means of a corresponding
amine, preferably with ammonia, under pressure, or an
imidazol-~-yl compound unsubstituted in the l-position
obtained in this way may be con~erted by alkylation into
a corresponding imidazol-4-yl compound alkylated in the
l-position.
A starting compound of formula III may be obtained
by reduction and cyclisation of an o-nitro-acylamino
compound as described hereinbefore.
Starting compounds of formulae V, VI and VII may be
obtained by reacting a compound of formula III with a
corresponding compound of formula IV.
The new compounds of formula I and the
.
2~7~0
- 30 -
physiologically acceptable salts thereof have valuable
pharmacological properties. They are angiotensin-
antagonists, in particular, angiotensin-II antagonists.
The compounds of formula I wherein R4 represents a
group convertable in vivo into a carboxy group, a
carboxy or lH-tetrazolyl group have, in particular,
useful pharmacological properties since they are
angiotensin-antagonists, particularly angiotensin-II-
antagonists. The other compounds of formula I are
valuable as intermediate products for preparing the
compounds mentioned above.
By way of example, the ~ollowing compounds:
A = 4'-[(2-n-propyl-4-methyl-6-(1-methyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl~-biphenyl-2-carboxylic
acid;
B = 4'-[(2-n-propyl-4-methyl-6-(1-isopropyl-imidazol-4-
yl)-benzimidazol-1-yl~-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl;
c - 4 ' - E ( 2-n-propyl 4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxyli~
acid;
D = 4'-[(2-ethoxy-6-(1-isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid;
E = 4'-[(2-n-propyl-4-methyl-6-(1-cycloheptyl-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
;~ carboxylic acid;
F = 4'- E t 2-n-propyl-4-methyl-6-(1-aminocarbonylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-
2-carboxylic acid; and
~ '
2~78~0
- 31 -
G = 4'-[(2-n-propyl-4-methyl 6~ 3-dimethylamino-
propyl)-imidazol-4-yl)-benzimidazol-1-yl)-methyl]-
biphenyl-2-carboxylic acid-dihydrochloride-
pentahydrate
were investigated for their biological activities as
follows:
Description of method: an~iotensin-II-receptor bondinq
The tissue (rat's lung) is homogenised in tris
bu~fer (50 mMol Tris, 150 mMol NaCl, 5 mMol EDTA, pH
7.40) and centrifuged twice for 20 minutes each time at
20,000 x g. The ~inished pellet is resuspended in
incubation buffer (50 mMol Tris, 5 mMol MgC12, 0. 2% BSA,
pH 7.40) 1:75, based on the moist weight of the tissue.
Each Q.l ml of homogenate is incubated for 60 minutes at
37C with 50 pM t125~]-angiotensin-II (NEN, Dreieich,
~RG) and increasing concentrations of the test substance
in a total volume of 0.25 ml. The incubation i~ ended
by rapid filtration through glass fibre filter mats.
The filters are each washed with 4 ml of ice cold buffer
(25 mMol Tris, 2.5 mMol MgC12, ~ BSA, pH 7.40). The
bound radioactivity is measured in a gamma-counter. The
corresponding IC50 value is determined ~rom the dosage-
activity curve.
Substances A to G show the following IC50 values in
the test described:
_
Substance ICso [nM]
A 1.2
B 1.5
C 40
D 15
F 3.4
G 1.3
208781)0
- 32 -
~ n view of their pharmacological properties, namely
a hypotensive effect with a diureticJsaluretic
component, the new compounds and the physiologically
acceptable addition salts thereof are suitable for the
treatment of hypertension and cardiac insu~ficiency and
also for treating chronic renal insufficiency, ischaemic
peripheral circulatory disorders (e.g. Raynaud's
syndrome) and cerebovascular circulatory disorders,
myocardial ischaemia (angina), for the prevention of the
progression of cardiac insufficiency after myocardial
infarct and for treating diabetic nephropathy and
retinopathy, glaucoma, gastrointestinal diseases and
bladder diseases.
Furthermore, the new compounds and the
physiologically acceptable salts thereof are suitable
for treating pulmonary diseases, e.g. lung oedema and
chronic bronchitis, for preventing arterial re-stenosis
after angioplasty, for preventing thickening of the
~ascular walls aPter vascular operations, and for
treating arteriosclerosis, diabetic angiopathy,
hyperuricaemia and gout.
Bec~use of the effect of angiotensin on the releasa
of acetylcholine and dopamine in the brain, the new
an~iotensin antagonists are also suitable for
alleviating central nervous system disorders, e.g.
depression, Alzheimer's disease, Parkinson's syndrome,
as well as disorders of cognitive functions.
Thus viewed from a further aspect the present
invention provides a pharmaceutical composition
comprising a compound of formula I or a physiologically
acceptable salt thereof together with at least one
physiologically acceptable carrier or excipient.
Viewe~ from a still further aspect the present
invention provides the use of a compound of formula I or
a physiologically acceptable salt thereof for the
manufacture of a therapeutic agent for the treatment of
hypertension and cardiac insufficiency and also for
20878~0
~ 33 -
treating chronic renal insufficiency, ischaemic
peripheral circulatory disorders (e.g. Raynaud's
syndrome) and cerebovascular circulatory disorders,
myocardial ischaemia (angina), for the prevention of the
progression of cardiac insufficiency after myocardial
infarct and for treatin~ diabetic nephropathy and
retinopathy, glaucoma, gastrointestinal diseases and
bladder diseases.
In particular, the present invention provides the
use of a compound of formula I or a physiologically
acceptable salt thereof for the manufacture of a
therapeutic agent for the treatment of pulmonary
diseasesl for preventing arterial re-sterosis after
angioplasty, for preventing thickening of the vascular
walls after vascular operations, and for treating
arteriosclerosis, diabetic angiopathy, hyperuricaemia
and gout.
More particularly, the present invention provides
the use of a compound of formula I or a physiologically
acceptable salt thereof for the manufacture of a
therapeutic agent for alleviating central nervous system
disorders.
Viewed from a yet still further aspect the present
invention provides a method of treatment of the human or
non-human animal body to combat hypertension and cardiac
insufficiency and also for treating chronic renal
insufficiency, ischaemic peripheral circulatory
disorders and cerebovascular circulatory disorders,
myocardial ischaemia ~angina), for the prevention of the
progression of cardiac insufficiency after myocardial
infarct and for treating diabetic nephropathy and
retinopathy, glaucoma, gastrointestinal diseases and
bladder diseases, said method comprising administering
to said body a compound of formula I or a
physiologically acceptable salt thereof.
In particular, the present invention provides a
method of treatment of the human or non-human animal
.
208~8~0
- 34 -
body to combat pulmonary diseases, for preventing
arterial re-sterosis after angioplasty, for preventing
thickening of the vascular walls after vascular
operations, and for treating arteriosclerosis, diabetic
angiopathy, hyperuricaemia and gout, said method
comprising administering to said body a compound of
formula I or a physiologically acceptable salt thereof.
More particulary, the present invention provides a
method of treatment o~ the human or non-human animal
body to combat central nervous system disorders, said
method comprising administering to said body a compound
of formula I or a physiologically acceptahle salt
thereof.
The dosage required to achieve these effects in
adults is appropriately, when administered
intravenously, 0.5 to 100 mg, preferably 1 to 70 mg,
and, when administered orally, 0.1 to 200 mg~ preferably
1 to 100 mg, 1 to 3 times a day. For this purpose, the
compounds of formula I prepared according to the
invention, optionally in conjunction with other active
substances such as, for example, hypotensive agents~ ACE
inhibitors, diuretics and/or calcium antagonists, may be
inaorporated together with one or more inert
conventional carriers and/or diluents, e.g. with corn
starch, lactose, glucose, micro-crystalline cellulose,
magnesium stearate, polyvinyl-pyrrolidone, citric acid,
tartaric acid, water, water/ethanol, water/glycerol,
waterjsorbitol, water/polyethyleneglycol, propylene-
glycol, cetylstearyl alcohol, carboxymethylcellulose or
fatty substances such as hard fat or ~suitable mixtures
thereof, in conventional galenic preparations such as
plain or coated tablets, capsules, powders, suspensions
or suppositories.
Examples of additional active substances which may
be used in the combinations mentioned above include
bendroflumethiazide, chlorothiazide,
hydrochlorothiazide, spironolactone, benzothiazide,
20~7~0
- 35
cyclothiazide, ethacrinic acid, furosemide, metoprolol,
prazosin, atenolol, propranolol, ~di)hydralazine-
hydrochloride, diltiazem, felodipine, nicardipine,
nifedipine, nisoldipine, nitrendipine, captopril,
enalàpril, lisinopril, cilazapril, quinapril, fosinopril
and ramipril. The dosage of these active substances is
conveniently 1/5 of the lowest dose normally recommended
up to 1/1 or the normally recommended dosage, that is
for example 15 to 200 mg of hydrochlorothiazide, 125 to
2000 mg of chlorothiazide, 15 to 200 mg of ethacrinic
acid, 5 to 80 mg of furosemide, 20 to 480 mg of
propranolol, 5 to 60 mg of felodipine, 5 to 60 mg of
nifedipine or 5 to 60 mg of nitrendipine.
The following non-limiting Examples are provided to
illustrate the invention. All percentages and ratios
given are by weight, other than eluant or solvent ratios
which are by volume.
-, :
.
.
2087~0
Example 1
4'-[(2-n-Propyl-4-methyl-6-(1-methyl-imidazol-4-yl)-
ben7imidazol-l-yl)-methyl]-biphenyl-2-carboxylic acid
a) 3-Methyl 4-but~ylamino-5-nitro-acetophenone
32.6 g (148 mMol) of 3-methyl-4-butyrylamino-
acetophenone are added in batches to 300 ml of fuming
nitric acid, with stirring, at -15C and stirred for a
further 30 minutes at -15C. The reaction mixture is
then poured onto 3 litres of ice with stirring, the
crude product precipitated is suction filtered, washed
with 400 ml of water, dried and purified by
recrystallisation from ethanol/diethylether ~1:1).
Yield: 23.~ g (61.0% of theory),
Rf value: 0.32 (~ilica gel; methylene chloride~
R~ value: 0.48 ~silica gel; methylene chloride~methanol =
50:1)
b) 3-MethYl-4-butyrvlamino-5-nitro-~-bromoaceto~-enone
At ambient temperatura, with stirring, a solution of
16.0 g (200 mMol) of bromine in 140 ml of dioxane is
slowly added dropwise to a solution of 23.8 g (90 mMol)
of 3-methyl-4-butyrylamino-5-nitro-acetophenone in
900 ml of dichloromethane so that the reaction mixture
is constantly completely decolorised. It is then
stirred for a further two hours, then the reaction
mixture is evaporated to dryness in vacuo, the residue
thus obtained is triturated with about 20 ml of
dichloromethane/diethylether (1:1), suction filtered and
then dried. 23 g (74% of theory) of 3-methyl-4-
butyrylamino-5-nitro-~-bromoacetophenone are thus
obtained, containing about 10% of starting material.
The product is further reacted without any more
purification.
Rf value: 0.69 (silica gel; methylene chloride/methanol =
50:1)
~: :
:` `
,,
2087800
-- 37 --
Rf value~ 0.84 (silica gel; methylene chloride/methanol =
g : 1 )
c) 2-Butyx~lamino-3-nitro-5-(imidazol-4-yl) toluene
A solution of 6.8 g (20 mMol) of 3-methyl-4-
butyrylamino-5-nitro-~bromoacetophenone in 20 ml of
formamide is heated to 140C for 2 hours. The cooled
solution is then poured into about 50 ml of 1 N ammonia
and stirred for about 15 minutes. The crude product
precipitated is suction filtered, washed with about
50 ml of water and dried. In this way, 4.4 g (75% of
theory) of the product are obtained, which is further
reacted without any more purification.
Rf value: 0.29 (silica gel; methylene chloride/methanol =
9 1)
d) 2-Butyrylamino-3 nitro-5~ methyl-imidazol-4-yl)-
toluenQ _ _
1.3 g (9.5 mMol) of methyl iodide are added dropwise at
ambient temperature to a solution of 2.5 g (8~7 mMol) of
Z-butyrylamino-3-nitro-5-(imida 2 ol-4-yl)-toluene and
5.2 g (30 mMol) of potassium carbonate dihydrate in
30 ml of dimethylsulphoxide at ambient temperature and
~hen stirred for 2 hours. The reaction mixture is then
stirred into about 150 ml of water and then extracted
four times with 25 ml of ethyl acetate. The organic
extracts are washed with about 30 ml of water, dried and
concentrated by evaporation. The crude product thus
obtained is purified by column chromatography (300 g
silica gel, eluant: methylene chloride/methanol = 30:1).
Yield: 640 mg (24% of theory),
Rf value: 0.54 (silica gel; methylene chloride/methanol -
9:1)
e) 2-Butyrylamino-3-amino-5-(1-methyl-imidazol-4-yl)-
toluene
640 mg (2.1 mMol) of 2-butyrylamino-3-nitro-5-(1-methyl-
2~7~0
- 38 -
imidazol-4-yl)-toluene are hydrogenated at ambient
temperature under a hydrogen pressure of 5 bar in 30 ml
of methanol after the addition of about 200 mg of 20%
palladium/charcoal. After all the water has bean
absorbed the catalyst is filtered off and the filtrate
is evaporated down. The crude product thus obtained is
further reacted without any more purification.
Yield: 600 mg (100% o theory),
Rf value~ 0.23 tsilica gel; methylene chloride/methanol =
9 : 1 )
f) 2-n-Propyl-4-methyl-6-(1-methyl-imida~ol-4-yl)-
benzimidazole
600 mg (2.1 mMol) of 2-butyrylamino-3-amino-5~ methyl-
imidazol-4-yl)-toluene are refluxed in 10 ml of glacial
acetic acid for one hour. Then the mixture is
e~aporated to dryness ln vacuo, the residue is mixed
with about 15 ml of water, made alkaline with ammonia
and extracted four times with about 10 ml of ethyl
acetate. The or~anic extracts are washed with about
15 ml of water, dried and finally evaporated down. The
crude produc~ thus obtained is further reacted without
any more purification.
Yield: 420 mg (79% of theory),
R~ value: 0.37 (silica gel; methylene chloride/methanol -
9:1)
g~ Tert.butyl 4'-[(2-n-propyl-4-methyl-6-(1-methyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate
280 mg (0.8 mMol) of tert.butyl 4'-bromomethyl-biphenyl-
2-carboxylate are added to a solution of 200 mg
(0.79 mMol) of 2-n-propyl-4-methyl-6-(1-methyl-imidazol-
4-yl)-benzimidazole and 90 mg (0.8 mMol) of potassium
tert.butoxide in 5 ml of dimethylsulphoxide and the
mixture is stirred for 90 minutes at ambient
,~
2l1~78VO
- 39 -
temperature, then stirred into about 40 ml of water,
extracted four times with about 10 ml of ethyl acetate,
then the organic extracts are washed with lo ml of
water, dried and evaporated to dryness. The crude
product thus obtained is purified by column
chromatography (100 g silica gel; eluant;
dichloromethane/methanol = 30:1).
Yield: 230 mg (56% of theory),
Rf value: 0.61 (silica gel; methylene chloride/methanol =
9 : 1 )
h) 4'-[(2-n-Propyl-4-methyl-6-~1-methyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl1-biphenyl-~-carboxylic acid
A solution of 230 mg (0.44 mMol) of tert.butyl 4'-[(2-n-
propyl-4-methyl~6-(1-methyl-imidazol-4~yl)-benzimidazol-
l-yl)-methyl]-biphenyl-2-carboxylate and 2 ml of
tri~luoroacetic acid in 10 ml of dichloromethane is
stirred overnight at ambient temperature and then
evaporated to dryness. The residue is dissolved in
about 5 ml of dilute sodium hydroxide solution, the
solution is neutralised with acetic acid, the
precipitate ~ormed is suction filtered, washed with
water and ~ried.
Yield: 120 mg ~59% of theory),
Melting point: 293-295C
R~ value: 0.39 ~silica gel; methylene chloride~methanol =
9:1)
4'-[(2-n-Propyl-4-methyl-6-(1-methyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl-hydrate
a) 4'-[(2-n-Propyl-4-methyl-6-(1-methylimidazol-4-yl)-
benzimidazol-l-Yl)-methvll-2-cYano-biphenyl
: .
2~87g~0
- 40 -
218 mg (0.8 mMol) of 4'-bromomethyl-2-cyano-biphenyl are
added to a solution of 200 mg (0.79 mMol) of 2-n-propyl-
4-methyl-6~ methyl-imidazol-4-yl~-benzimidazole and
90 mg (0.8 mMol) of potassium tert.butoxide in 6 ml of
dimethylsulphoxide and the mixture is stirred for 14
hours at ambient temperature. Then it is stirred into
about 40 ml of water, extracted four times with about
10 ml of ethyl acetate, the oryanic extracts are washed
with about 10 ml of water, dried and evaporated to
dryness. The crude product thus obtained is purified by
column chromatography (100 g silica gel; eluant:
dichloromethane/ethanol = 50:1).
Yield: 240 mg (67% of theory),
Rf value: 0.38 (silica gel; methylene chloride/ethanol =
19 : 1 )
'~
b) 4' [(2-n-Propyl-~-methyl-6-(1-methyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetraæol-5 yl)-
biphenvl-hvdrate
A solution of 222 mg (0.5 mMol) of 4'-[(2-n-propyl-4-
methyl-6-(1-methyl-imidazol-4-yl)-benzimidazol-1-yl)-
methyl]-2-cyano-biphenyl, 660 mg (10 mMol) of sodium
azide and 540 mg (10 mMol) of ammonium chloride in 12 ml
of pure dimethylformamide is heated to 140C for 18
hours. The solution is then evaporated substantially to
dryness and the product is isolated by column
chromatography (60 g of silica gel, eluant:
dichloromethane with 10% ethanol). The product thus
obtained is taken up in about 10 ml of dilute ammonia
solution and the solution is then adjusted to pH 6 with
acetic acid. A greasy residue is formed which becomes
crystalline after the addition of a little ethyl acetate
and several hours' stirring. The crystalline product is
suction filtered, washed with about 5 ml of water and
dried.
Yield: 61.0 mg (24.0% of theory),
~`
:~ `
2087~6~
- 41 -
Melting point: 255-257C
C29H28N~ x H20 (506.62)
Calculated: C 68.75 H 5.97 N 22.12
Found: 68.90 5.97 22.03
Rf value: 0.24 ~silica gel; methylene chloride/methanol =
9 : 1 ~
Example 3
4'-[(2-n-Propyl-4-methyl-6-~1-isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic acid
_ _ _
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-tl-isopropyl-imidazol~4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 84.0% o~ theory,
Melting po~nt: 285-286C
Rf value: 0.55 (silica gel; methylene chloride/methanol =
9 : 1 )
Example 4
,
41-[(2-n~Propyl-4-methyl-6-(l-n-hexyl-imidazol-4-yl)-
benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic acid
.
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-n-hexyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 74.0% of theory,
Melting point: 258-259C
C34H3gN402 (534-71)
Rf value: 0.48 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 534
.
.~ '
.
2~g~
- 42 -
Example 5
4'-[(2-n-Propyl-4-methyl-6-(l-cyclopentylmethyl-
imidazol-4-yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example l from tert.butyl 4'-
[(2-n-propyl-4-mekhyl-6-(l-cyclopentylmethyl-imidazol-4-
yl)-benzimidazol-1-yl)-methyl]-bipheny:L-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Example 6
4'-[(2-n-Propyl~4-methyl-6-(1-cyclohexylmethyl-imidazol~
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic
acid
_
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(l-cyclohexylmethyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Exam~le 7
4'-[(2-n-Propyl-4-methyl-6-(1-benzyl-imidazol-4-yl)-
benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-benzyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 44.0% of theory,
Melting point: 226-227C
C35H32N402 (540.68)
Rf value: 0.51 (silica gel; methyiene chloride/methanol =
9 : 1 )
7~
- 43 -
Mass spectrum: m/e = 540
Example 8
4'-[(2-n-Propyl-4-methyl-6-(1-(4-fluorobenzyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic
acid
Prepared analogously to Example 1 ~rom tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-(4-fluorobenzyl)-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
The following compounds may be obtained analogously to
Example 8:
4'-[(2-n-propyl-4-methyl-6-(1-(3-chlorobenzyl)-imidazol-
4-yl)-benzimidazol-l~yl)-methyl]-biphenyl-2-carboxylic
acid
4'-~(2-n-propyl-4-methyl-6-(1-(3,5-dimethoxybenzyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
4'-~(2-n-propyl-4-methyl-6~ (4-methylbenzyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic
acid
4'-[(2-n-propyl-4-methyl-6-(1-(4-trifluoromethyl-
benzyl)-imidazol-4-yl)-benzimidazol-1-yl)-methyl~-
biphenyl-2-carboxylic acid
; ' , ~ .
2~87~0~
- 44 -
Example 9
4'-[(2-n-Propyl-4-methyl-6-(1-(2-phenylethyl)-imidazol-
4-yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6~ (2-phenylethyl)-imidazol-4-
yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Exam ~e lO
4'-[(2-n-Propyl-4-methyl-6-(1-(2,2,2-trifluoroethyl)-
imidazol-4-yl)-benzimidazol-l-yl)-methyl~-biphenyl-2-
carboxylic acid
Prepared analogously to Example l from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-(2,2,2-trifluoroethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and tri~luoroacetic acid in methylene
chloride.
Example 11
4'-[(2-n-Propyl-4-methyl-6-(1-(3,3,3-trifluoroprop~l)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
~(2-n-propyl-4-methyl-6-(1-(3,3,3-trifluoropropyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
~ . . .
;~
2~7~
- 45 -
Example 12
4'-[(2-n-Propyl-4-methyl-6~ aminocarbonylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert~butyl 4'-
[(2-n-propyl-4-methyl-6-(1-aminocarbonylmethyl-imidazol
4-yl)-banzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Example 13
4'-[(2-n-Propyl-4-methyl-6~ cyclobutylmethyl~imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic
acid
Prepared analogously to Example 1 ~rom tert.butyl 4'-
~(2-n-propyl-4-methyl-6-(1-cyclobutylmethyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Example 14
4' [(2-n-Propyl-4-methyl-6-(1-cyclopropylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-~1-cyclopropylmethyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl~-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Yield: 59.0% of theory,
Melting point: 279-280C
C32H32N40z (504-64)
Calculated: C 76.16 H 6.39 N 11.10
208~8~
- 46 -
~ound: 76.41 6.37 11.20
Rf value: 0.44 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 504
ExamPle 15
4'-[(2-n-Propyl-4-methyl-6-(1-isopropyl-imidazol-4-yl)~
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
.
Prepared analogously to Example 2 from 4'-[~2-n-propyl-
4-methyl-6-(1-isopropyl-imidazol-4-yl)-benzimidazol-1-
yl~-methyl]-2-cyano-biphenyl and sodium azide in
dimethylformamide.
Yield: 18.0% of theory,
Melting point: amorphous
C3~H32N8 (516.66)
Rf value: 0.2~ (silica gel; methylene chloride/methanol =
9:1)
Mass spectrum: m/e = 516
Exam~le 16 . .
4'-[(2-n-Propyl-4-methyl-6-(1-cyclopropylmethyl-
imidaæol 4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-yl)-biphenyl
a) 4'-[(2-n-Propyl-4-methyl-6-(1-cyclopropylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-2-(2-
triphenylmethyl-tetrazol-5-yl)-biPhenyl
To a solution of 300 mg (1.0 mMol) of 2-n-propyl-4-
methyl-6-(1-cyclopropylmethyl-imidazol-~-yl)-
benzimidazole and 110 mg (1.0 mMol) potassium
tert.butoxide in 20 ml of dimethylsulphoxide are added
560 mg (1.0 mMol) of ~'-bromomethyl-2-(2-
triphenylmethyl-tetrazol-5-yl)-biphenyl and the mixture
, '
2~7~0
- ~7
is stirred for 16 hours at ambient temperature, then
stirred into about 120 ml of water and extracted four
times with 15 ml of ethyl acetate. The organic extracts
are washed with about 30 ml of water, dried and then
evaporated to dryness. The crude product thus obtained
is purified by column chromatography (:L00 g silica gel,
eluant: methylene chloride/methanol = 30:1).
Yield: 460 mg ~60 % of theory),
Rf value: 0.78 (silica gel; methylene chloride/methanol =
9 ~
b) 4'-L(2-n-Propyl-4-methyl-6-(1-cyclopropylmethyl-
imidazol~4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-vl)-biphenvl
A mixture of 460 mg (0.6 mMol) of 4'-~(2-n-propyl-4-
methyl-6~ cyclopropylmethyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl~-2-(2-triphenylmethyl-
tetraæol-5-yl)~biphenyl and 10 ml of saturated
methanolic hydrochloric acid is stirred Eor one hour at
ambient temperature. The mixtura is then evaporated to
dryness, the residue is dissolved in dilute ammonia
solution and washed with ether. The aqueous phase is
adjusted to pH 5 to 6 with acetic acid and subsequently
the solid precipitate is suction filtered. The crude
product thus obtained is purified by column
chromatography (100 g silica gel, eluant: methylene
chloride/methanol = 15:1)
Yield: 130 mg (41% of theory),
Melting point: amorphous
C32H32N8 (528-67)
Rf value: 0.32 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 528
20~8~0
- 48 -
Example 17
4'-[(2-n-Propyl-4-methyl-6-(1-cyclobutylmethyl-imidazol-
4-yl)-benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)
bip~enyl
Prepar~d analogously to Example 16 from ~'-[(2-n-propyl-
4-methyl-6-(1-cyclobutylmethyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]~2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl and sodium azide in
dimethylformamide.
Exam~e 18
4'-t(2-n-Propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-1-yl)~methyl]-biphenyl-2-carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[~2-n-propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 67.0~ of theory,
Melting point: 241-243C
C29H27N303 (465.56)
Calculated: C 74.82 H 5.85 N 9.03
Found: 74.65 5.98 8.85
Rf value: 0.27 (silica gel; methylene chloride~ethanol =
19 : 1 )
Exam~le 19
4'-t(2-n-Propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-l-yl)-methylJ-2-(lH-tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-n-propyl-
4-methyl-6-(2-methyl-oxazol-4-yl)-benzimidazol-1-yl)-
78~0
~,9
methyl]-2-(2-triphenylmethyl-tetrazol-s-yl)-biphenyl and
sodium azide in dimethylformamide.
Example O
4i-[(2-n-Propyl-4-methyl-6-(2~phenyl-oxazol-4-yl)-
benzimidaæol-l-yl)-methyl]-biphenyl-2 carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(2-phenyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 87.0~ of theory,
Melting point: 281-283C
C34H2sN303 (527-63)
Calculated: C 77.40 H 5.54 N 7.96
Found: 77.09 5.71 7.76
Rf value: 0.18 (silica gel; methylene chloride~ethanol =
19 : 1 )
Example 21
4'-[(2-n-Propyl-4-methyl-6-(2-phenyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-n-propyl-
4-methyl-6-(2-phenyl-oxazol-4-yl)-benzimidazol-1-yl)-
methyl]-2-(2-triphenylmethyl-tetrazol-5-yl)-biphenyl and
sodium azide in dimethylformamide.
2~73~0
- 50 -
ExamPle 22
4'-[(2-n-Propyl-4-methyl-6-(1-benzyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
.. . .... _ . _ _ . _ .. . _ _ . . .
Prepared analogously to Example 16 from 4'-[(2-n-propyl-
4-methyl-6-(1-benzyl-imidazol-4-yl)-benzimidazol-1-yl)-
methyl]-2-(2-triphe,nylmethyl-tetrazol-5-yl)-biphenyl.
Yield: 45~0~ of theory,
Melting point: 168-170C
C3$H32Ng (564.70)
Rf value: 0.37 (silica gel; methylene chloride/methanol =
9 1)
Mass spectrum: m/e = 564
Example 23
-
4'-[(2-n-Propyl-4-methyl-6-(1-n-hexyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-n-Propyl-
4-methyl-6-(l~n-hexyl-imidazol-4-yl)-benzimidazol-1-yl)-
methyl~-2-(2-triphenylmethyl-tetrazol-5-yl)-biphenyl.
Yield: 61.0% of theory,
Melting point: 126-128C
C34H38N8 (558.74)
Rf value: 0.31 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 558
Example 24
4'-~(2-Ethoxy-6-(1-isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-ethoxy-6-
2~781D0
(l-isopropyl-imidazol-4-yl)-benzimidazol-l~yl)-methyl]-
2-(2-triphenylmethyl-tetrazol-5-yl)-biphenyl.
Yield: 69.0% of theory,
Melting point: 175-178C
C2~H28N8O (504.61)
Calculated: C 69.03 H 5.59 N 22.21
Found: 68.85 5.58 21.97
R~ value: 0.27 (silica gel; methylene chloride/ethanol =
9 : 1 )
Mass spectrum: m/e = 504
~ .
Exam~le 25
~ (2-Ethoxy-6-(1-isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
C(2-ethoxy-6~(1-isopropyl-imidazol-4-yl)-benzimidazol-1-
yl)~methyl]-biphenyl-2-carboxylate and trifluoroacetic
acid in methylene chloride.
Yield: 38.0% of theory,
Melting point: 220-223C
c2~28N403 (480-58)
Calculated: C 72.48 H 5.87 N 11.66
Found: 72.36 6.05 11.41
R~ value: 0.26 (silica gel; methylene chloride/methanol =
9:1)
Mass spectrum: m/e = 480
Example 26
4~-[(2-Ethoxy-5~ isopropyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-~(2-ethoxy-5-
(l-isopropyl-imidazol-4-yl)-benzimidazol-1-yl)-methyl]-
' - .
2~7~
- 52 -
2-(2-triphenylmethyl-tetrazol-5-yl)-biphenyl.
Yield: 44.0% of theory,
Melting point: amorphous
C29H28N8 (504.61)
Rf value: 0.24 (silica gel; methylene chloride/ethanol =
9 : 1 )
Mass spectrum: m/e = 504
xample 27
,.
4'-[(2-n-Propyl-4-methyl-6-(1-cycloheptyl-imidazol-4-
yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carbcxylic
acid
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4~methyl-6-(1-cycloheptyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylata
and tri~luoroacetic acid in methylene chloride.
Yield: 79.0% of theory,
Melting point: from 190C (decomp.)
C35H38N4O2 (546.71)
Rf value: 0.36 (silica gel; methylene chloride~methanol =
9:1)
Mass spectrum: m/e = 546
Exam~le 28
4'-[(2-n-Propyl-4-methyl-6-(1-cycloheptyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl
Prepared analogously to Example 16 Erom 4'-[(2-n-propyl-
4-methyl-6-(1-cycloheptyl-imidazol-4-yl)-benzimidazol-1-
yl)-methyl]-2-(2-triphenylmethyl-tetrazol-5-yl)-
biphenyl.
Yield: 27.0% of theory,
- -
,
~ .
2~7~00
- 53 -
Melting point: 198-201C
C35H38N~ (570~75)
Rf value: 0.48 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 570
~mEL~
4'-[(2-n-Propyl-4-methyl-6-(1-~1-n-propyl-n-butyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl~-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-(1-n-propyl-n-butyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 28.0% of theory,
Melting point: 236-238C
C35H40N402 (548.73)
Rf value: 0.61 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 548
Example 30
4'-[~2-~thoxy-4-methyl-6-(1-cyclopropylmethyl-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic
acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethoxy-4-methyl-6-(1-cyclopropylmethyl-imidazol-4-
yl)-henzimidazol-l-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 52.0~ of theory,
'
- : '
~0~78~0
- 54 -
Melting point: 172-173C
C3l~30N4O3 (506-61)
Calculated: C 73.50 H 5.97 N 11.06
Found: 73.36 5,94 11.30
Rf value: 0.52 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 506
Example 31
4'-[(2-Ethoxy-4-methyl-6-(1-cyclopropylmethyl-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl
Prepared analogously to Example 16 from 4'-~2-ethoxy-4-
methyl-6~ cyclopropylmethyl-imidazol-4-yl)-
benzimidazol-1-yl)-methyl]-2-(2-triphenylmethyl
tetrazol 5-yl)-biphenyl.
Yield: 42.0% o~ theory,
Melting point: amorphous
C31H30N8 (53~.64)
Rf value: 0.50 (silica gel; methylene chloride/methanol =
9:1)
. Mass spectrum: m/e = 530
Exam~le 32
4'-[(2-n-Propyl-4-methyl-6-(1-(l~n-propyl-n-butyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-n-propyl-
4-methyl-6-(1-(1-n-propyl-n-butyl)-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl.
Yield: 12.0% of theory,
,
2 ~ 0
Melting point: from 150C (sintering)
C35H40N~ (572,76)
Rf value: 0.34 (silica gel; methylene chloride/methanol =
9 : 1 )
Mass spectrum: m/e = 572
Example 33
4'-[(2-n-Propyl-4-methyl-6-(1,2-dimethyl-imidazol-4-yl)-
benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic acid
hydrate
a) tert. Butyl ~ (2-n-propyl 4-methyl-6-(2-methyl-
~oxazol-4-yl)-benzimidazol-l-yl)-methyl]-biphenyl--2-
carboxylate _ _
A solution of 2.8 g (11 mMol) of 2-n-propyl-4-methyl-6-
(2-methyl-oxazol-4-yl~-benzimidazole and 1.7 g (15 mMol)
of potassium tert.butoxide in 60 ml of dimethyl-
sulphoxide is stirred for 15 minutes at ambient
temperature. Then 5.2 g (15 mMol) of tert.butyl 4'-
bromomethyl-biphenyl-2-carboxylate are added and the
mixture is stirred for a further 14 hours at ambient
temperature. Then the solution is stirred into about
150 ml of saturated sodium chloride solution, the crude
product precipitated is suction filtered and purified by
column chromatography (400 g of silica gel; eluant:
methylene chloride with l to 2% ethanol).
Yield: 3.5 g (61.4% of theory),
Melting point: amorphous
Rf value: 0.90 (silica gel; methylene chloride/ethanol =
4:1)
20~78~0
- 56 -
b) 4'-[(2-n-Propyl-4-methyl-6-(1,2-dimethyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid hydrate
A mixture of 1.5 g (3 mMol) of tert.butyl 4'-~(2-n-
propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-benzimidazol-1-
yl)-methyl]-biphenyl-2-carboxylate, 10 ml of 40~ N-
methylamine solution and 15 ml of N-methylformamide is
heated for 10 hours to 200C in an autoclave. ~fter
cooling, the contents of the autoclave are stirred with
about 40 ml of water, this suspension is adjusted to pH
6.5 with glacial acetic acid, then the crude product
precipitated is suction filtered and dissolved in lN
sodium hydroxide solution. This solution is washed
successively with 25 ml of acetic acid and diethylether,
th~n adjusted to pH 6 with 20~ citric acid. The product
precipitated is suction filtered, washed with about
30 ml of water and dried, then triturated with
diethylether and dried in a high vacuum.
Yield: 950 mg (68% of theory),
Melting point: 239-240C
C30H30N4O2 x H2O (496.62)
Calculated: C 72.55 H 6.49 N 11.28
Found: 72.62 6.62 11.54
Rf value: 0.70 (silica gel; methylene chloride/ethanol =
4:1)
Example 34
4'-[(2-n-Propyl-4-methyl-6-(1,2-dimethyl-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl-hydrate
a) 4'-[(2-n-Propyl-4-methyl-6-(2-methyl-oxazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenYl
2~7~
- 57 -
A solution of 2.8 g (11 mMol) of 2-n-propyl-4-methyl-6-
(2-methyl-oxazol-4-yl)-benzimidazole and 1.7 g (15 mMol)
of potassium tert.butoxide in 60 ml of
dimethylsulphoxide is stirred for 15 minutes at ambient
temperature. Then 6.0 g of (11 mMol) o~ 4'-bromomethyl-
2-~2-triphenylmethyl-tetrazol-5-yl)-biphenyl are added
and the mixture is stirred for a further 3 hours at
ambient temperature. Then the solution is stirred into
about 150 ml of saturated sodium chloride solution, the
crude product precipitated is suction filtered and
purified by column chromatography (500 g of silica gel;
eluant: petroleum ether/ethyl acetate = 1:1)
Yield: 3.6 g (45% o~ theory)
b) 4'-[(2-n-Propyl-4-methyl-6-(1,2-dimethyl-imidazol-4-
yl)-benzimidazol-l-yl)-methyl]-2-(lH-tetra201-5-yl)-
biphenyl-hYdrate
A mixture of 3.6 g (4,9 mMol) of 4'~(2-n-propyl-4-
methyl-6-(2-methyl-oxazol-4-yl)-benzimidazol-1-yl)-
methyl]-2-(2-triphenylmethyl-tetrazol-5-yl)-biphenyl,
20 ml of 40% N-methylamine solution and 30 ml of N-
methylformamide is heated to 200C for 10 hours in an
autoclave. After cooling, the contants of the autoclave
are stirred with about 50 ml of water, this suspension
is adjusted to pH 6.5 with 20% citric acid, then the
crude product precipitated is suction filtered and
purified by column chromatography (200 g silica gel;
eluant: methylene chloride with 5 to 20% ethanol).
Yield: 1.0 g (41% of theory),
Melting point: from 195C sintering
C30H30N8 x H20 (520.6)
Calculated: C 69.21 H 6.19 N 21.52
Found: 68.99 6.26 21.37
Mass spectrum: m/e = 502
,
.
208780~
- 58 -
Example 35
4'-[(2-Ethyl-4-methyl-6-~1-t2-methoxyethyl)-imidazol-4-
yl)-banzimidazol-l-yl)-methyl]-biphenyl-2-carboxylic
acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethyl-~-methyl-6-(1-(2-methoxyethyl)-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chlorid~.
Yield: 49% of theory,
Melting point: 165-167C
C30H30N4O3 (494.60)
Calculated: C 72.85 H 6.11 N 11.33
Found: 72.62 6.27 11.35
Mass spectrum: m/e = 494
Example 36
4'-[(2-Cyclopropyl-4-methyl-6-(1-(2-methoxyethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
" Prepared analogously to Example 1 from tert. butyl 4'-
[(2-cyclopropyl-4-methyl-6~ 2-methoxyethyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 78% of theory,
Melting point: 179-181C
C31H30N4O3 (506.61)
Calculated: C 73.50 H 5.g7 N 11.06
Found: 73.37 6.02 11.02
Mass spectrum: m/e = 506
' ~
~0~78~0
- 59 -
Example 37
4'-[(2~n-Propyl-4~methyl-6-(1-aminocarbonylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert. butyl 4'-
[(2-n-propyl-4-methyl-6-(1-aminocarbonylmethyl-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Yield: 26% of theory,
Melting point: 190-192C
C30H29N503 (507.60)
Rf value: 0.44 (silica gel; methylene chloride/methanol =
8:2)
Example 38
4'-C(2-n-Propyl-4-methyl-6-(l-ethoxycarbonylmethyl-
imidazol-4-yl)-henzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4 methyl-6-(1-ethoxycarbonylmethyl-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 18% of theory,
Melting point: 223-224C
C32H32N404 (536.63)
Rf value: 0.69 (silica gel; methylene chloride/methanol =
8:2)
Mass spectrum: m/e = 536
2087~0
6~ -
Example 39
4'-[(2-Cyclapropyl-4-methyl-6-(1-(2-hydroxyethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
A solution of 500 mg (1.0 mMol) of 4'-[(2-cyclopropyl-4-
methyl-6-(1-(2-methoxyethyl)-imidazol-4-yl)-
benzimidazol-1-yl)-methyl] biphenyl-2-carboxylic acid
and 1.5 g (6.0 mMol) of boron tribromide in 50 ml of
methylene chl~ride is stirred for 16 hours at ambient
temperature, then mixed with about 30 ml of water and
stirred vigorously for another 10 minutes. This mixture
is evaporated to dryness and the residue is refluxed in
about 40 ml of ethanol for 10 minutes. The mixture is
evaporated to dryness once more, the residue is
dissolved in about 30 ml of 2N ammonia solution and this
solution is adjusted to pH 5-6 with ~N acetic acid. The
crude product precipitated is suction filtered and
purified by column chromatography (80 g silica gel;
eluant: methylene chloride/methanol = 4:1).
Yield: 150 mg (30% of theory),
Melting point: 220-222C
C30H28N4O3 (492.58)
Rf value: 0.20 (silica gel; methylene chloride/methanol =
9:1)
Mass spectrum: m/e = 492
Exam~le 40
4'-[(2-n-Propyl-4-methyl-6-(1-(2-N-morpholinoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert. butyl 4'-
20~7~1D0
- 61 -
[(2-n-propyl-4-methyl-6-(1-(2-N-morpholinoethyl)-
imidazol-4-yl)~benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 54% of theory,
Melting point: 259-261~C
C34H37Ns3 (563-70)
Calculated: C 72.44 H 6.62 N 12.42
Found: 72.68 6.65 12.53
Mass spectrum: m/e = 563
Example 41
4 ' - L ( 2-n-Propyl-4-methyl-6-(1-(2-methoxyethoxy-2-ethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-hiphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from te,rt.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-(2-methoxyethoxy-2-ethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 49% of theory,
Melting point: 192-194C
C33H36N404 (552.67)
Calculated: C 71.72 H 6.57 N 10.14
Found: 71.52 6.36 10.25
Rf value: 0.36 (silica gel; dichloromethane/methanol =
9 : 1 )
Mass spectrum: m/e = 552
20~78~
- 62 -
Example 42
4'-[(2-n-Propyl-4-methyl-6-(}-(3-dimethylaminopropyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid dihydroch-oride-pentahydrate
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(1-(3-dimethylaminopropyl)-
imidazol-4-yl) benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylate and trifluoroacetic acid in methylene
chloride.
Yield: 12% of theory,
Melting point: from 128~C (decomp.)
C33H37NsO2 x 2 HCl x 5 H20 (535 70)
Rf value: 0.20 (silica gel; dichloromethane/methanol =
9 : 1 )
Mass spectrum: m/e = 535
Example 43
4'-[(2-n-Propyl-4-methyl-6-(2-methyl-thiazol-4-yl)-
benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-n-propyl-4-methyl-6-(2-methyl-thiazol-4-yl)-
benzimidazol-l-yl)-methyll-biphenyl-2-carboxylate and
trifluoroacetic acid in methylene chloride.
Yield: 32% of theory,
Melting point: 248-250C
Cz9H27N32S (481.62)
Calculated: C 72.32 H 5.65 N 8.72
Found: 72.21 5.83 8.67
R~ value: 0.26 (silica gel; dichloromethane/methanol =
9 : 1 )
~78~
- 63 -
Mass spectrum: m/e = 481
Example 44
4'-[~2-n-Propyl-4-methyl-6-(2-methyl-thiazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(lH-tetrazol-5-yl)-
biphenyl-dihydrochloride
Prepared analogously to Example 16 from 4'-[(2-n-propyl-
4-methyl-6-(2-methyl-thiazol-4-yl)-benzimidazol-1-yl)-
methyl]-2-~2-triphenylmethyl-tetrazol-5-yl)-biphenyl.
Yield: 91% of theory,
Melting point: from 219C (decomp.)
C29Hz9C12N7s (578.58)
Calculated: C 60.20 H 5.05 N 16.95 Cl 12.25
Found: 59.96 5.19 16.63 12.42
Rf value: 0.32 (silica ~el; dichloromethane/methanol =
9 : 1 )
Mass spectrum: m/e = 505
Example 45
4'-[(2-Ethyl-4-methyl-6-(1-(2-N-morpholinoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethyl-4-methyl-6-(1-(2-N-morpholinoethyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Yield: 27 % of theory,
Melting point: 201-202C
C33H35NsO3 (549.65)
Calculated: C 72.11 H 6.42 N 12.74
20~7~00
- 64 -
Found: 72.00 6.48 12.62
Rf value: 0.36 (silica gel; methylene chloride/methanol =
9 : 1 )
mass spectrum: m/e = 549
Example 46
4'-[(2-Ethyl-4-methyl-6-(1-(2-N-morpholinoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-yl)-biphenyl-hydrate
r
Prepared analogously to Example 16 from 4'-[(2-ethyl-4-
methyl-6-(1-(2-N-morpholinoethyl)-imidazol-4-yl)-
benzimida~ol-l-yl)-methyl~-2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl.
Yield: 14 % of theory,
Melting point: above 180C (decomp.)
C33H35N9o x H2O (573.68)
Calculated: C 66.98 H 6.30 N 21.31
Found: 66.87 6.36 21.22
Rf value: 0.31 (silica gel; methylene chloride/methanol =
9 : 1 )
mass sp ctrum: mJe _ 573
Example 47
4'-[(2-Ethyl-4-methyl-6-(1-(2-aminocarbonylethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
E ( 2-ethyl-4-methyl-6-(1-(2-aminocarbonylethyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Yield: 66 % of theory,
20~780~
- 65 -
Melting point: above 185C (decomp.)
C30H2sNs3 (507.59)
Calculated: C 70.99 H 5.76 N 13.80
Found: 70.73 5.72 13.66
mass spectrum: m/e = 507
Example 48
4'-[t2-Ethyl-4-methyl-6-tl-(2-aminocarbonylethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-yl~-biphenyl
Prepared analogously to Example 16 Erom 4'-~(2-ethyl-4-
methyl-6-~1-(2-aminocarbonylethyl)-imidazol-4-yl)-
benzimidazol-l-yl?-methyl]~2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl.
Yield: 42 ~ of theory,
Melting point: above 191C (decomp.)
C30H29N90 (531.63)
Calculated: C 67.78 H 5.50 N 23.71
Found: 67.7g 5.4Q 23.66
Rf value: 0.20 (silica gel; methylene chloride~methanol =
8:2)
mass spectrum: m/e = 531
Example 49
4'-[(2-Ethyl-4-methyl-6-(1-(2-N-pyrrolidinoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethyl-4-methyl-6-(1-(2-N-pyrrolidinoethyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
.
~ . '
: .
' ,
20~78~
- 66 -
Yield: 60 % of theory,
Melting point: 215-217C
C33H35N5O2 (533.67)
Calculated: C 74.27 H 6.61 N 13.12
Found: 74.03 6.~5 13.11
Rf value: 0.30 tsilica gel; methylene chloride/methanol =
8:2)
mass spectrum: m/e = 533
Example 50
4'-[(2-Ethyl-4-methyl-6-(1-(2-N-pyrrolidinoethyl)-
imidazol-4-yl~-benzimidazol-1-yl)-methyl~-2-(lH-
tetrazol-5-yl)-biphenyl
__ __
Prepared analogously to Example 16 from 4'-[(2-ethyl-4-
methyl-6~ (2-N-pyrrolidinoethyl)-imidazol-4-yl)-
benzimidazol-l-yl)-methyl]-2-(2-triphenylmethyl~
tetrazol-5-yl)-biphenyl.
Yield: 38 % of theory,
Melting point: above 128C (sintering)
C33H3sN9 (551.71)
Calculated: C 71.84 H 6.39 N 22.85
Found: 71.63 6.20 22.49
Rf value: 0.23 (silica gel; methylene chloride/methanol =
8:2~
Example 51
4'-[(2-Ethyl-4-methyl-6-(1-(2-diethylaminoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid-dihydrochloride
-
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethyl-4-methyl-6-(1-(2-diethylaminoethyl)-imidazol-
20~7~00
- 67 -
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
and trifluoroacetic acid in methylene chloride.
Yield: 32 ~ of theory,
Melting point: 255-257C (decomp.)
C33H37Ns2 x 2 HCl (608.60)
Rf value: 0.24 (silica gel; methylene chloride/methanol =
9:1)
mass spectrum: m/e = 535
Example 52
4'-[(2-Ethyl-4-methyl-6-(1-(2-diethylaminoethyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-2-(lH-
tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-ethyl-4-
methyl-6-(1-(2-diethylaminoethyl)-imidazol-4-yl)-
benzimidazol~l-yl)-methyl]-2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl.
Yield: 51 % o_ theory,
Melting point: 191-193C
C33H37N9 (559.70)
Calculated: C 70.81 H 6.66 N 22.52
Found: 70.59 6.66 22.58
Rf value: 0.30 (silica gel; methylene chloride/methanol =
8:2)
Example 53
4'-[(2-Ethyl-4-methyl-6-(1-(3-N-piperidinopropyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-
carboxylic acid
Prepared analogously to Example 1 from tert.butyl 4'-
[(2-ethyl-4-methyl-6-(1-(3-N-piperidinopropyl)-imidazol-
4-yl)-benzimidazol-1-yl)-methyl]-biphenyl-2-carboxylate
20~78~0
- 6~ -
and trifluoroacetic acid in methylene chloride.
Yield: 19 % of theory r
Melting point: amorphous
C3sH39N502 (561.73)
Cal~ulated: C 74.84 H 7.00 N 12.47
Found: 7~.61 6.92 12.31
Rf value: 0.34 (silica gel; methylene chloride/methanol =
8:2)
Example 54
4~ [(2-Ethyl-4-mQthyl-6-~1-t3-N-piperidinopropyl)-
imidazol-4-yl)-benzimidazol-1-yl)-methyll-2-(lH-
tetrazol-5-yl)-biphenyl
Prepared analogously to Example 16 from 4'-[(2-ethyl-4-
methyl-6~ (3-N-piperidinopropyl~-imidazol-4-yl)-
~ben2imidazol-l-yl)-methyl]-2-(2-triphenylmethyl-
tetrazol-5-yl)-biphenyl.
Yield: 71 ~ of theory,
Melting point: above 140C (decomp.)
C3sH39N9 (585.76)
Calculated: C 71.77 H 6.71 N 21.52
Found: 71.58 6.68 21.44
Rf value: 0.22 (silica gel; methylene chloride/methanol =
8:2)
2~7300
- 69 -
In the Examples of Pharmaceutical Formulations which
follow, any suitable compound of formula I including the
physiologically acceptable salts thereof, particularly
those wherein ~4 represents a carboxy or lH-tetrazolyl
group, may be used as the active substance:
Example I
Ampoules containing 50 mg of active substance per 5 ml
Active substance 50 mg
KH2P04 2 mg
Na2HP04 x 2HzO 50 mg
NaCl 12 mg
Water for injections ad 5 ml
PreParation:
The buffer substances and isotonic substance are
dissolved in some o~ the water. The active substance is
added and, once it has been completely dissolved, water
is added to make up the required volume.
.
Example II
Ampoules containing 100 mg of active substance per 5 ml
Active substance 100 mg
Methyl glucamine 35 mg
Glycofurol 1000 mg
Polyethyleneglycol-polypropylene-
glycol block polymer 250 mg
Water for injections ad 5 ml
,
.
~87800
- 70 -
Preparakion:
Methyl glucamine is dissolved in some of the water and
the active substance is dissolved with stirring and
heating. After the addition of solvents, water is added
to make up the desired volume.
ExamE~Le III
Tablets containing 50 mg of active substance
_
Active substance 50.0 mg
Calcium phosphate 70.0 mg
Lactose 4C.0 mg
Corn starch 35.0 mg
Polyvinylpyrrolidone 3.5 mg
Magnesium stearate 1 5 mq
200.0 mg
Preparation:
The active substance, CaHPO4, lactose and corn starch are
uniformly moistened with an aqueous PVP solution. The
mass is passed through a 2 mm screen, dried at 50C in a
circulating air dryer and screened again.
After the lubricant has been added, the granules are
compressed in a tablet making machine.
Example IV
Coated tablets containing 50 mg of active substance
Active substance 50.0 mg
Lysine 25.0 mg
Lactose 60.0 mg
:~ '
20~7~
- 71 -
Corn starch 34.0 mg
Gelatin 10.0 mg
Magnesium stearate 1.0 mq
180.0 mg
Preparation:
The active substance is mixed with the excipients and
moistened with an aqueous gelatin solution. After
screening and drying, the granules are mixed with
magnesium stearate and compressed to form tablet cores.
The cores thus produced are covered with a coating by
known methods. A colouring may be added to the coating
suspension or solution.
Exa_ple V
Coated tablets containing 100 mg of active substance
Active substance 100.0 mg
Lysine 50.0 mg
Lactose 86.0 mg
Corn starch 50.0 mg
Polyvinylpyrrolidone 2.8 mg
Microcrystalline cellulose60.0 mg
Magnesium stearate 1.2 mq
350.0 mg
Preparation:
The active substance is mixed with the excipients and
moistened with an aqueous PVP solution. The moist mass
is passed through a 1.5 mm screen and dried at 45C.
After drying, it is screened again and the magnesium
stearate is added. This mixture is compressed into
cores.
20~78~
- 72 -
The cores thus producad are covered with a coating by
known methods. Colourings may be added to the coating
suspension or solution.
Example VI
Capsules containing 250 mg of active substance
Active substance 250.0 mg
Corn starch 68.5 mg
Magnesium stearate 1.5 mq
320.0 mg
Preparation:
The active substance and corn starch are mixed together
and moistened with water. The moist mass is screened
and dried. The dry granules are screened and mixed with
ma~nesium stearate. The final mixture is packed into
size 1 hard gelatin capsules.
Example VII
Oral suspension containing 50 mg of active substance per
5 ml
.
Active substance 50.0 mg
Hydroxyethylcellulose 50.0 mg
Sorbic acid 5.0 mg
70% sorbitol 600.0 mg
Glycerol 200.0 mg
Flavouring 15.0 mg
Water ad 5.0 ml
.
,
,
2~87~
- 73 -
Preparation:
Distilled water is heated to 70~C. Hydroxyethyl-
cellulose is dissolved therein with stirring. By the
addition of sorbitol solution and glycerol the mixture
is cooled to ambient temperature. At ambient
temperature, sorbic acid, flavouring and active
substance are added. The suspension is evacuated with
stirring to remove any air. One dose of 50 mg is
contained in 5.0 ml.
Example VIII
Suppositories containing 100 mg of active substarlce
Active sub~tance 100.0 mg
Solid fat 1600.0 m~
1700.0 mg
Th~ hard fat is melted. At 40C the ground active
substance is homogeneously dispersed in the melt. It is
cooled to 38C and poured into slightly chilled
suppository moulds.