Note: Descriptions are shown in the official language in which they were submitted.
2~g~82
A- 189921A
_bpxYlic acid esters of hy~y~h~nols as stabilisers
The present invention relates to novel carboxylic acid esters of
(D-(3,5-dialkyl-4-hydroxyphenyl)alkanols, the organic materials stabilised against thermal,
oxidative and actinic degradation using these compounds, the use of the novel compounds
as stabilisers and novel Cd-(3,5 dialkyl-4-hydroxyphenyl)alkanols.
The use of the esters of ld-hydroxyphenyl-o~-carboxylic acid esters as stabilisers for
organic n1aterials is known.
A number of publications relate to corresponding inverse esters: Chem. Abstr. 84 32016k,
DE-A-2 147 544, EP-B-171 139, EP-A-349 380, NL-A-79-05000, GB-A-l 509 876,
Chem. Abstr. 84 106614c, Chem. Abstr. 106 156001u, US-A-4 910 286, US-A-4 311 637,
US-A-4 104 252 and US-A-3 919 097 describe carboxylic acid esters of
3-(3,5-di~tert-butyl-4-hydroxyphenyl)alkan- l-ols,
3-~3-tert-butyl-5-methyl-4-hydroxyphenyl)alkan- l-ols and individual
4-(3,5-dialkyl-4-hydroxyphenyl)alkan-1-ols, as well as their use as polymer stabilisers.
Several novel carboxylic acid esters of ~-(3,5-dialkyl-4-hydroxyphenyl)alkanols have now
been found which have par~icularly good stabiliser properties.
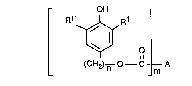
One subject of the present invention ~erefore comprises compounds of the formula I
OH
R1~
L (CH2) n --C ~;~ A
in which n is an integer from the range from 4 to 8 and m is an integer from the range from
2~99~2
I to4;
it` m = l, A is Cl-C2salkyl, whicll is llnsllbstilllted or sllbs~itllte(l by C5-C8cycloalkyl; or is
C2-C2salkyl which is intelrllpte(l by C5-C8cycloalkyl or one or more of the grollps -S-, -C)-
nn-l/or -NR2-; or, ir m = ~ is Cs-C8cyclo~11kyl, which is unsllbstillltecl or sllbstitllted by
Cl-CI2alkyl or C2-CI~llkenyl; C~,-Cxcycloalkellyl, whicll is ~Insllbslilllle(l or sllbstillltecl by
Cl-CI2alkyl or C'2-CI2c~lkellyl; pllellyl, which is llnsllhstilllted or sllbstitllted by
C`l-CI2alkyl or C2-CI2alkellyl; naphtllyl, which is llnsubstitlltecl or sllbstitllte(l by
Cl-Cl2alkyl or C2-CI2alkenyl; biphenyl, which is llnsubstitllted or substituted by
Cl-Cl2alkyl or C2-Cl~alkenyl; or C2-C2salkenyl; C6-CI0bicycloalkenyl;
C7-CI2phenylalkyl; C8-Cl2phenylalkenyl; Cll-Cl6naphthylalkyl; Cl2-Cl6naphthylalkenyl;
Cl3-CI8biphenylalkyl; Cl4-Cl8biphenylalkenyl; or a group of the formula Il
R4
- C~12 CH2~ OH (:lI);
R3
if m = 2, A is a direct boncl; Cl-Cl2alkylene; C2-Cl2alkenylene; Cs-C8cycloalkylene,
which is unsubstituted or substituted by Cl-Cl2alkyl or C2-Cl2alkenyl;
C6-C8cycloalkenylene, which is unsubstituted or substituted by Cl-Cl2alkyl or
C2-CI2alkenyl; C6-Cl0bicycloalkenylene; phenylene; naphthylene; a divalent heterocyclic
radical from the group comprising furan, thiophene or pyrrole, which is saturated on the
nitrogen atorn by hydrogen or the substituent -R2; or, if m = 2, A is C2- C36alkylene which
is interrupted by C5-C8cycloalkylene or phenylene or at least one of the groups -S-, -O- or
-NR2-;
if m = 3, A is Cl-C8alkanetriyl; C2-Cgalkenetriyl; benzenetriyl; naphthalenetriyl; a
R6
trivalent group of the formula _ R5--N--R7_; or C2-Cl8aLkanetxiyl, which is intexrupted
by at least one of the groups -S-, -O- or -NR2-;
or, if m = 4, A is a benzene radical, naphthyl radical, tetrahydrofuryl radical or cyclohexyl
radical having 4 free valencies;
Rl and Rl, independently of one another, are Cl-C24alkyl or Cs-C8cycloaLkyl;
R2 is H or Cl-C4alkyl;
R3 and E~4, independently of one another, are Cl-C4alkyl; and
Rs, R6 and R7, independently of one another, are Cl-C3alkylene.
Cl-C24Alkyl or Cs-C8cycloalkyl Rl and Rl axe for example, independently of one
20~0082
another, melhyl, ethyl, propyl, blllyl, pen~yl, hexyl, heplyl, oc~yl, nonyl, decyl,
cyclopentyl, cyclohe~yl, cycloheptyl or eyclooctyl.
1~1 is preterllbly secoîldary or ~er~iary C3-C2~alkyl, t`or example 2-bll~yl (sec-bll~yl),
~ert-bll~yl, 2-1~ell~yl, 2-hexyl ol~ 3-he,Yyl, or Rl is Cs-C8cycloalkyl, for example cyclohexyl,
an(l Rl is Cl-C'I(~alkyl or C~-Cgcycloalkyl.
Rl is par~iclllarly preler(~n~ially secondary or tertklry C3-Cl8alkyl or Cs-CBcycloalkyl, for
example isopropyl, sec-blltyl, tert-blltyl or cyclohexyl, and Rl ;s C3-C8alkyl or
Cs-C8cycloalkyl or is methyl.
Partieuku1y preferred compounds of the formula I are those in whieh Rl is methyl,
tert-butyl or eyelohexyl, in partieular methyl, and Rl is tert-blltyl or eyelohexyl, in
partieular tert-butyl.
C1-C4~lkyl R2, R3 und R'~ are methyl, ethyl, l-propyl (n-propyl), 2-propyl (isopropyl),
l-butyl (n-blltyl), 2-blltyl (sec-butyl), 2-methylpropyl (isobutyl) or l,l-dimethylethyl
(tert-butyl); methyl or tert-butyl is preferred.
Cl-C3Alkylene Rs, R6 and R7 are methylene, 1,1- or 1,2-ethylene, or l,1-, 1,2-, 2,2- or
1,3-propylene. Methylene is preferred.
If A is C1-C2salkyl, it is a branched or straight-ehain radieal, for example methyl, ethyl,
propyl, isopropyl, n-butyl, see-butyl, isobutyl, t-butyl, 2-ethylbutyl, n-pentyl, isopentyl,
1 methylpentyl, 1,3 dimethylbutyl, n-hexyl, l-methylhexyl, n-heptyl, isoheptyl,
1,1,3,3-tetramethylbutyl, l-methylheptyl, 3-methylheptyl, n-oetyl, 2-ethylhexyl,1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, deeyl, undecyl, 1-methylundecyl,
dodeeyl, 1,1,3,3,5,5-hexamethylhexyl, tridecyl, tetradeeyl, pentadecyl, hexadecyl,
heptadeeyl, oetadeeyl, eieosyl, doeosyl or pentaeosyl; straight-chain aLkyl is preferred and
straight-chain C6-Cl8alkyl is particularly preferred.
As Cl-C2salkyl substituted by Cs-C8eyeloalkyl, A is, for example, eyclopentylmethyl,
cyclohexylmethyl, cycloheptylmethyl, cyclooctylmethyl, cyclohexylethyl,
2-eyelohexyl-n-propyl, 3-cyclohexyl-n-propyl or 4-cyclohexyl-n-butyl.
As C2-C2salkyl interrupted by Cs-CBcycloalkyl or one or more of the groups -S-, -O-
and/or -NR2-, A is one of the alkyl radieals described above, with the exception of methyl,
2090~82
in the chain of which cyclopentylene or cyclopentylidene, cyclohexylene or
cyclohexylidene, cycloheptylene or cycloheptylidene, cyclooctylene or cyclooctylidene is
inserted at one point or the abovemetltiolled groups containing hetero atoms are inserted at
one or more points. A thcrefore, for example, has the folmulae
-C6~ll~C~I3 ,-c~l2-s-c4H9~-c2H4-o-c2H~l-o-cl2H~s~-cl8H36-N(c'~H9)2
As Cs-C8cycloalkyl or C6-C8cycloalkenyl which is unsubstituted or substituted byCl-CI~alkyl or C2-CI2alkenyl, A can be, for example: cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl, 2- or 4-methylcyclohexyl, dimethylcyclohexyl,
trimethylcyclohexyl, t-butylcyclohexyl, 2-cyclohexenyl, 3-cycloheptenyl,
cyclooctatetraenyl or 4-tert-butylcyclohex-2-enyl. Cyclohexyl and cyclohexenyl,
especially cyclohexyl, are preferred.
As phenyl, naphthyl or biphenyl substituted by Cl-Cl2alkyl or C2-CI2alkenyl, A is, inter
alia, methylphenyl, dimethylphenyl, trimethylphenyl, ethylphenyl, vinylphenyl,
diethylphenyl, isopropylphenyl, tert-butylphenyl, di-tert-butylphenyl,
methyl-di-t-butylphenyl, 1,1,3,3-tetramethylbutylphenyl and
1,1,3,3,5,5-hexamethylhexylphenyl, dodecenylphenyl, 1-methylnaphthyl,
2-methylnaphthyl, l-ethylnaphthyl, 2-propylbiphenyl-4-yl or
4-(1-he~c-3-enyl)biphenyl-8-yl. Phenyl, which is unsubstitllted or substituted by 1-3, for
example 1-2 and in particular 1 Cl-C4alkyl group(s), in particular methyl, is preferred.
C2-C2sAlkenyl A is, for example, vinyl, propenyl, isopropenyl, 2-butenyl, 3-butenyl,
isobutenyl, n-penta- 2,4-dienyl, 3-methylbut-2-enyl, n-oct-2-enyl, n-dodec-2-enyl,
iso-dodecenyl, n-octadec-2-enyl or n-octadec-4-enyl.
C7-CI2Phenylalkyl, C7-CI2phenylalkenyl, Cll-CI6naphthylalkyl, Cll-Cl6naphthylalkenyl,
Cl3-Cl8biphenylalkyl or Cl3-Cl8biphenylalkenyl A is Cl-C6alkyl or Cl-C6alkenyl
substituted by phenyl, naphthyl or biphenyl, for example benzyl, phenethyl,
3-phenylpropyl, c~-methylbenzyl, a,a-dimethylbenzyl, 2-phenylethenyl,
1-phenylprop-2-enyl, 6-phenylhexyl, 1-naphthylmethyl, 2-naphthylmethyl,
l-naphthyleth-1-yl, 2-(4-biphenylyl)prop-2-yl or 1-(4-biphenylyl)pent-3-en-1-yl.
C6-CIOBicycloalkenyl or C6-CIObicycloalkenylene A is an unsaturated bicyclic,
monovalent or divalent radical of 6 to 10 carbon atoms which as a monovalent radical can
2~9Q0~2
be, for example, bicyclo[2.2.1]hepta-5-en-2-yl and as a divalent radical can have, for
oxample, the folmulac ~ or ~\ .
I`lle divalent (for m - 2), trivalent (for m = 3) and tetrnvnlent radicnls A (for m = 4) are
derived from the monovalent radicals mentioned above. A divalent radical differs from the
corresponding monovalent radical ;n that it contains an open bond in place of a hydrogen
atom and a trivalent radical differs from the corresponding monovalent radical in that it
contains two open bonds in place of two hydrogen atoms; a tetravalent radical differs from
the corresponding monovalent radical in that it contains three open bonds in place of three
hydrogen atoms. Thus, within the fMmework of the indicated meanirlgs, for example, A,
as a divalent radical, can also be methylene, ethylene, -CH2-C(CH3)2-CH2-,
prop-2 enylidene, 1,3-propylene, 1,4-butylene, I,S-pentylene, 1,6-hexylene, o-, m- or
p-phenylene, CH2~CH2-, CH2 CH2~CH2-CH2-, -C2H4-S-C2H4- OI'
-C2H4-~O-C2H4-, as a trivalent radical can be, ~or example, -CH2-CH-CH2~
CH2
~3 or -CH2~ and as a trivalen~ radical can be, for example,
or~--.
The novel esters of the forrnula I can be prepared by conventional esterification or
transesterification methods from c~-hydroxyphenylalkanols of the formula IV
OH
R~ (IV~ and organic carboxylic acids of the forrmula V
~CH2)n OH
2090~8
r ol
L~l - C ~ A (V) or derivatives ot` such carboxylic acids, t`or example by methods such
as are described in Telrahedron 36, ~09 (1980). The symbols R~ , A, n and m in the
fonnulac IV ancl V have the mennings indicated above ~or folTnula 1. Suitable carboxylic
aci~l derivatives are, for example, anhydrides, chlorides or lower alkyl esters, for example
methyl or ethyl esters. Suitllble esterification catalysts, such as mineral acids (for example
sulfuric acid) or sulfonic acids (t`or example p-toluenesulfonic acid) can be used in the
syntheses if appropriate.
The reaction can be cal~ied out in a manner known per se, expediently by adding one of
the two educts to the second educt and mixing the two reactants thoroughly, preferably
with the exclusion of oxygen. The reaction can be carried out in the presence of a solvent,
for example toluene, or also without a solvent. The temperature control is not critical; the
temperature can be between the melting point and the boiling point of the reaction
mixture, for example between -50 and 10~C, preferably between 0 and S0CC. The
purification of the resulting product can also be carried Ollt in accordance with known
methods, for example by washing with water/HCI, extraction with an organic solvent,
crystallisation and/or chromatography. Preferred solvents for the extraction and for the
chromatographic purification step are hexane, ethyl acetate or mixtures thereof.
If the derivative of the carboxylic acid of the ~ormula V used in the reaction is the acid
chloride, an acid acceptor is expediently added to the reaction mixture. Suitable acid
acceptors are, for example, amines, such as pyridine or triethylamine. Preferably the
amount of the acid acceptor is at least equivalent to the amount of the acid chloride and is,
for example, 1 to 2 equivalents, in particular 1.2 to 1.7 equivalents, with respect to the acid
chloride.
If the reaction is carried out as a transesteri~lcation of an alcohol of the formula IV with
the ester of a carboxylic acid of the forrnula V, conventional transesterification catalysts
are expediently added to the reaction mixture. Such catalysts are, for example, organic or
inorganic bases (for example lithium amide, lithium methoxide, potassium hydroxide and
the like) or Lewis acids (for example dibutyltin oxide).
If the carboxylic acid of the formula V and the alcohol of the formula IV aIe used directly
as educts, the reaction is expediently carried out using a water separator, distilling off
209~082
water an(l/or ~Ising rc.lgen~s which abs()rb libcr(llc(l w~ltcr, ror cxalllple,
dicyclohcxylcarho(liillli(tc.
'[`he use Or mi,~ lres ol` ~arboxylic aci(ls Or Ihe l`orm~lla V or tlleir der;vativcs can be of
par~iCIIIar prac~ical illtel'CS~ ill thc prcpnrn~ioll Or compollnds of the formulll 1 Sucil
mixlllros call be usc(l l`or the synthesis in the manner described above, correspollding
mixtllres of compolln(ls of the formula I being oblaine(l. Mixtures of compolmds of the
formula I can be use(l as sllch as stabilisers.
Alcohols of the formula IV are themselves also effective as stabilisers. Compositions
containing (a) an organic material sensitive to oxidative, thermal and/or actinic
degradation and (b) a compound of the formula IV, and also the use of compounds of the
formula IV for stabilising organic material against o~idative, thermal or actinic
degradation, are thereEore further subjects of the invention.
The compositions according to the invention pret`erably contain those compounds of the
formula IV in which ~I has the meaning of seconclary or tertiary C3-C24alkyl or
Cs-C8cycloalkyl, and Rl has the meaning Cl-CIOalkyl or C5-C8cycloalkyl. Particularly
preferentially, Rl has the meaning of secondary or tertiary C3-CI8alkyl or
Cs-C8cycloalkyl, for example isopropyl, sec-butyl, tert-butyl or cyclohexyl, and R1 is
C3-C8alkyl or Cs-C8cycloalkyl or is methyl.
Compounds of the formula IV in which ~1 is methyl, tert-butyl or cyclohexyl, in particular
methyl, and Rl is tert-butyl or cyclohexyl, in particular tert-butyl, are particularly
preferred in the compositions according to the invention.
The compositions according to the invention preferably contain those compounds of the
forrnula IV in which n is a number from the range 5 to 8, for example 6 to 8 and in
particular the number 6.
The alcohols of the formula III described further below are also particularly preferred in
the compositions according to the invention.
The cd-(hydroxyphenyl)alkanols of the formula IV can be prepared by known processes or
analogously to such processes. Thus, the compounds of the formula IV are obtainable, for
example, by the process described in US-A-4 260 832 or by a process analogous to this.
- 2090082
The correspondillg 2,6-disllbslitute(l phenols are then reac~ed wi~h o~,~-alkancliols in lhe
presence of alkali mctal, alkali mctal hyd~ lcs or alkali metal alkoxidcs a~ ~empela~llres
Or 20() to 300C wi~h rem()val of ~he wa~er rorll~ed dllring ~he reac~i()n.
Tllc l~ro(lllc~ e.ln lhell l~e isoklle(l by el)llvellli()n.ll me~ll()(l~s nll(l use(l for rcac~ioll with
carhoxylic aci(l dcriv~llives ot` the l`orlnlllll V; h()wevcr, it is IlISO possible to use lhe crude
prodllc~ direc~ly wi~h(~ furlller pllrificll~i()ll t`or ~he prepllrlltioll ol` compoullds of ~he
~ormulà [.
If a 2,6-disubs~ituted phenol in which none or at most one of the alkyl or cycloalkyl
substituents is tèrt-butyl is used in place of the starting phenols described inUS-A-4 260 832, novel compolmds of the formula III
OH
G1~
(CH2)n OH
in which Gl and Gl have the meanings indicated further above for Rl and R1, but with
the exception of tert-butyl for Gl, are obtained. As defined further above for formula I, n is
an integer from the range 4 to 8.
A compound of the forrnula III is also a subject of the invention.
Preferred compounds of the formula III are those in which Gl has the meaning of
secondary or tertiary C3-C24alkyl or C5-C8cycloalkyl; and Gl has the meaning Cl-ClOalkyl
or Cs-C8cycloalkyl. G1 particularly preferentially has the meaning of secondary or tertiary
C3-CI8alkyl or Cs-C8cycloalkyl, for example isopropyl, sec-butyl, tert-butyl or
cyclohexyl, and G' is C3-C8alkyl with the exception of tert-butyl, or is Cs-C8cycloalkyl or
methyl.
Compounds of the formula III in which G1 is methyl or cyclohexyl, in particular methyl,
and G1 is tert-butyl or cyclohexyl, in particular tert-butyl, are preferred in particular.
2~900~2
Those compounds of the fo mula III in whieh n is a number from the range S to 8, for
exnmple 6 to 8 and in particulllr the nutnber 6, are prefe~ed.
Further proeesses by which, or analogo~lsly to whicll, compounds of the fo rnula III and
compounds of the t`o mul/l IV can be prepa~ed are described or .~ferred to in, for example,
DE-~-2 147 544 and in NL-A-79-()5000.
As deseribed above, the radieal A in formula I is derived from a m-basie earboxylie aeid.
The aeids ean be aromatie, aliphatie, mixed aromatie-aliphatie, eyeloaliphatie or
bieyeloaliphatie acids or unsaturated derivatives thereof, for example the following aeids:
eaprylic acid, aeetic acid, stearic aeid, polyisobutenyls~leeinie aeid, n-hexaeosanoie aeid,
trimethylaeetie acid, propionic aeid, iso~ialeric acid, laurie acid, oleie aeid, aerylie aeid,
methaerylie acid, sorbie aeid, linolellie acid, maleie acid, itaconie aeid, glutaeonie aeid,
dibasie aeids~ sueh as oxalic aeid, sueeinie aeid, iso-dodeeylsueeinie aeid, glutarie aeid,
adipie aeid, pimelie acid, suberic acid, azelic acid or sebacic aeid, tlle polymers of fatty
aeids, sueh as the dimers and trimers thereof of the ~ype deseribed, for example, in Ind.
and Eng. Chem. 33, 86-89 (1941), hetero atom-eontaining aeids, for example,
nitrilot;iaeetie aeid, eyeloaliphatie aeids, sueh as eyelohexaneearboxylie aeid, 1,2- and
1,~-eyelohexanediearboxylie aeid and naphthenie acids, ineluding, for example,
eyelopentaneearboxylie aeid, eyelopentylaeetie aeid, 3-methyleyelopentylaeetie aeid,
eamphor aeid, 4-methylcyclohexanecarboxylic aeid and
2,4,6-trimethylcyclohexanecarboxylic aeid, bieyelo[2.2.2]octa-5-ene-2,3-dicarboxylie aeid
and bieyelo[2.2.1]hepta-5-ene-2-earboxylie aeid, aromatic earboxylie acids, sueh as
ben~oie aeid, o-, m- and p-toluie aeid, phthalie aeid, terephthalic acid, trimellitie aeid,
trimesie aeid, 1,2,4,5-benzenetetraearboxylie aeid, diphenie aeid, 1-naphthoie aeid~
2-naphthoie aeid, naphthalene-1,8-diearboxylie aeid, naphthalene-1,4-diearboxylie aeid,
naphthalene-1,4,5-triearboxylie aeid and the like, paraffinie (d-aryl aeids, sueh as
phenylaeetie aeid, hydroeinnamie aeid, phenylbuty~ie aeid, y-(1-naphthyl)butyrie aeid,
~-phenylene-n-valerie aeid, ~-phenyl-n-eaproie aeid, o-, m- or p-phenylenediaeetie aeid or
o-phenyleneaeetie-~-propionie aeid, and also unsaturated phenyl aeids, for example,
einnamie aeid.
Compounds of the formula Ia
~` 2~9~82
- 10-
¦ ~C~3)3C~R1 ~_
(C~2) ~ O--C A
in which n is lm integer from the range from 4 lo ~ and m is an inteuer from the range from
1 to 3,
if m = 1, A is Cl-C2salkyl, which is unsubstiluted or substituted by Cs-C8cyclo~1kyl, or is
C2-C2salkyl, whiçh is interrupted by Cs-C8cycloalkyl or one or more of the groups -S-, -O-
and/or -NR2-, or, if m = 1, A is Cs-C8cycloalkyl, which is unsubstituted or substituted by
Cl-CI2alkyl or C2-CI2allcenyl, C6-C8cyclonlkenyl, which is unsubstitu~ed or substituted by
Cl-CI2alkyl or CrCI2aLkenyl, phenyl, which is unsubstituted or substituted by
Cl Cl2alkyl or C2-CI2alkenyl, naphthyl, which is unsubstituted or substituted byCl-CI2alkyl or C2-CI2aLkenyl, biphenyl, which is unsubstituted or substituted byCl-CI2alkyl or C2-CI2a1kenyl, or A is C2-C2salkenyl, C6-C IObicycloalkenyl,
C7-CI2phenylalkyl, C~CI2phenylalkcnyl, Cll-Cl6naphthylalkyl, Cll-Cl6naphthylalkenyl,
Cl3-CI8biphenylalkyl, Cl3-CI8biphenylalkenyl, or a group of the lormula II
-CH2CHt~OH (Il),
R3
if m - 2, A is a direct bond, Cl-Cl2alkylene, C2-CI2alkenylene, C5-C8cycloalkylene,
which is unsubstitutçd or substituted by Cl-CI2alkyl or C2-C~2alkenyl, or A ts
C6-C8cycloalkenylene, which is unsubstituted or substituted by Cl-CI2alkyl or~
C2-C12alkenyl, or A is C6-ClObicycloalkenylene, phenylene, naphthylene or
C2-CI8alkylene which.is interrupted by Cs-C8cycloalkylene or phenylene or at least one of
the groups -S-7 -O- or-1~2-,
if m = 3, A is Cl-C8alkanetriyl, C2-C8alkenetriyl, benzenetriyl, naphthalenetriyl, a
R6
trivalent group of the formula _ R~--N _ p~7 , or C2-CI8alkanetriyl which is interrupted
by at least one of the groups -S-, -{)- or -NR2-,
Rl is methyl or tert-butyl,
~90082
R2 is H or Cl-C4alkyl,
R3 an(: R4, indcpendcntly of one another, are Cl-C4alkyl, and
Rs, R6 and R7, independently o~ one another, are Cl-C3alkylene,
are a prefcrred subjcct of the inVent;Otl.
Preferred compollnds of the formula I are those ill which n is an integel from the range
frorn 4 to 8 and m is an integer from the range from 1 to 4; if m = 1, A is Cl-C25alkyl,
which is unsubstituted or substituted by Cs-Cacycloalkyl; or C2-C2salkyl, which is
interrupted by Cs^C8cycloaL~cyl or one or more of the groups -S-, -O- andlor -NR2-; or if m
= 1, A is C5-C8cycloalkyl, which is unsubstituted or substituted by Cl-CI2alkyl;C6-C8cycloaLlcenyl, which is unsubstituted or substituted by C1-C~2alkyl; phenyl, which is
unsubstituted or substituted by CI~CI2aLlcyl; or C2-C2 jalkenyl; C7-CI2phenylalkyl; or a
group of the formula 11
R4
- CH2 CH2~ OH (II),
R3
if m = 2, A is a direct bond; Cl-CI2alkylene; C2-CI2nlkenylene; Cs-C8cycloallcylene,
which is unsubstituted or substituted by Cl-CI2alkyl; C6-C8cycloalkenylene, which is
unsubstituted or substituted by Cl-CI2alkyl; phenylene; a divalent heterocyclic radical
from the group comprising furan, thiophene or pyrrole, which is saturated on the nitrogen
atom by hydrogen or the substituent R2; or, if m = 2~ A is C~-CI8alkylene which is
interrupted by Cs-C8cycloalkylene or phenylene or at least one of the ;,roups -S-, -O- or
-NR2-; and
if m = 3, A is Cl-C8aLkanetriyl; C2-C8alkenetriyl; benzenetriyl; a trivalent group of the
R6
formula R5--N--R7_; or C2-CI8alkanetriyl, which ls interrupted by at least one of the
groups -S-, -O- or-NR2-;
if m - 4, A is a benzene or cyclohexyl radical having 4 free valencies;
Rl and Rl, independently of one another, are Cl-ClOalkyl or Cs-C8cycloaLkyl;
R2 is H or Cl-C4aL~cyl;
R3 and R4, independently of one ano~her, are Cl-C4alkyl; and
Rs, R6 and R7, independently of one another, are Cl-C3aLkylene.
2~082
- 12-
Compounds of the formula I in whicll Rl is tert-butyl or cyclohexyl and Rl is methyl,
tert-butyl or cyclohexyl;
n is n number from the range 4 to 6; ~md
if m = 1, A is C6-Cl8alkyl or C2-Cl2alkenyl;
if m - 2, A is n clirect bond; Cl-CI2alkylene; phenylene; ~; or C2-C36alkylene
inten~lpted by I to S oxygen or sulfur atoms;
CH2 -
if m = 3, A is benzenetriyl or a trivalent group of the formula _ CH - N C~ ; and
if m = 4, A is a benzene radical having 4 free valencies, are a particularly preferred
subject.
Amongst these compounds, compounds of outstanding importance are those in which, if m
= 1, A is C2-CI2alkenyl; and
if m = 2, A is phenylene; ~, C2-C36alkylene intermpted by 1 to 5 oxygen
atoms; or a group -(CH2)k-S-(CH2)k-; and k is an integer from the range from 1 to 3; and
in particular the compounds in which m is 1 or 2.
Compounds of the formula I which are also particularly preferred are those in which n is
an integer from the range from S to 8, in particular from the range 6 to 8 and especially 6.
Amongst the compounds of the formula I, compounds of the formula
HO OH
CH3 ~C(CH3)3 CHa ~C(CH3)3
~CH2)6- O--C A C--O--(CH2)6
in which A is -(CH2)j-; -(CH2)k-S-(OEI2)k-; ~5~ or
-(CH2)2-O-C(CH2)2-O-]j-~CH2)2- hat; j is O or an integer from the range from 1 to 4; and k
is an integer from the range from 1 to 3, are particularly outstanding.
The following cornpounds in particular are of outstanding importance:
20~0082
a) 6-(3-tert-butyl-4-hydroxy-5-methylplletlyl)llexyl stearate;
b) fi-(3,5-di-tert-butyl-4-llydroxypllellyl)hexyl stearate;
c) bis[6-(3-tert-butyl-4-llydroxy-5-methylptlellyl)hexyl~ succillate;
d) bis[6-(3-tert-butyl-4-hydroxy-5-1llethylphenyl)hcxyll adipat¢;
e) bis[6-(3-tert-blltyl-4-hy(lroxy-5-metllylpllenyl)tlexyll suberate;
f) bis[6-(3-tert-butyl-4-hydroxy-5-metllylpllenyl)hexylJ isophthalate;
bis[6-(3,5-di-tert-butyl-4-hydroxyphenyl)hexyl~ isophthalate;
h) tris[6-(3-tert-butyl-4-hydroxy-5-methylphenyl)hexyl] trimellitate;
j) tris[6-(3,5-di-tert-butyl-4-hydroxyphenyl)hexyl] trimellitate;
k) tris[6-(3-tert-butyl-4-hydroxy-5-methylphenyl)hexyl] trimesate; and
1) tris[6-(3,5-di-tert-butyl-4-hydroxyphenyl)hexyl] trimesate
OH
(CH3)3C~ CH3
of the forrnulae I~J (a);
(CH2) 6 --C--C17H35
OH
(CH3)3C~C(C~13)3
I~J o (b);
(CH2) 6 --C--C~7H35
HO ~
CH3 _~C(CH3)3 CH3 ~,C(CH3)3
(CH2)6 0--C--(CH2)2--C-- -(CH2)6
HO OH
CH3 ~C(CHo)3 CH3 ~_C(CH3)3
(CH2~¢ 0--C--(cH2)4--C O -(ctl2)6
HO OH
CH3 _~C(CH3)3 CH3 ~ C(CH3)3
(CH2)6 0--C--(CH2)6--C--O -(CH2)6
2~9~0~2
- 14 -
OH OH
(CH3)3C~3,CH30 (CH3~3C~l,CH3
)6- 0--C--0--C--O (CH2)6
OH OH
(CH3)3C~c(cHa)3 (CH3)3C~,C(C~13)3
(CH2)6- 0--C ~ C--O (CH2~6
OH OH
(CH3)3C~,CH3 CH3 ~ C(CH3)3
(CH2)6-0--C,~ C--(CH2)6
\=< (h);
,C--0-~
CH3 J~C(CH3)3
OH
OH OH
(CH3)3C~3,C(CH3)3 (CH3)3C ~,C(CH3)3
(CH2)6- --C ~C-- '(CH2)6
\=~ (j); :
,C--O-~6
(CH3)3C J~C(CH3~3
OH
2~9~82
- 15-
OH
OH CH3 ~J~ C(GH3)3
(CH3)3C~3,CH3
o ,~ C--O (CH2)6
(C~12)6- 0--C ~/ \~
\==< (k); nnd
C--O -(CH2)6
O ~
CH3 ~C(CH3)3
OH
OH
OH (CH3)3C J~,C(CH3)3
(CH3)3C~[~, C(CH3)3 1.~
o J ~ c--o (CH2)6
(CH2)6- 0--C ~
\=( (1,).
C ~ O -(CH2)6
(GH3)3C ~C(CH3)3
OH
Compounds of the formula I in which n is an integer from the range 4 to 6; in particular
those in which n is an integer from the range from 4 to 6 and in which, if m = 1, A is
C6-C20alkyl, if m = 2, A is a direct bond, Cl-CIOalkylene, phenylene, or C2-C6alkylene
interrupted by -S- and if m = 3, A is benzenetriyl are of particular Industrial importance.
A preferred subject of the invention also comprises cornpounds of the fo~mula I in which
m is 2 and A is C4-C36alkylene Interrupted by at least one oxygen atom.
The compounds of the forrnula I in which, if m = 1, A is C6-C20alkyl, C3-CI8alkenyl,
C6-C36alkyl interrupted by one or more -S- or -O- groups, cyclohexyl, cyclohexylsubstituted by Cl-C4alkyl, phenyl, phenyl substituted by Cl-Cl2alkyl, or phenylalkyl
having a total of 7 to 9 carbon atoms,
if m - 2, A is a direct bond, C1-CI2alkylene, C2-CI2alkenylene, phenylene, or
C2-C36alkylene interrupted by a -S- or -O- group, and
20~82
- 16 -
if m = 3, A is Cl-C8alkanetriyl, benzenetriyl, alkylbcnzenetriyl of 7 to 10 carbon atoms, or
C~2-
CH2--N - Cl-l2
arc also importallt.
The compounds of thc formula I are suitable for stabilising organic materials against
thermal, oxidative and actinic degradation, Reference is made in particular to their
outstanding action as antioxidants in the stabilisation of organic materials.
Examples of such materials are:
1. polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut- 1-ene, poly-4-methylpent- l-ene, polyisoprene or polybutadiene and also polymers
of cycloolefins, for example, of cyclopentene or norbornene; and also polyethylene (which
can be crosslinked, if desired), for example high density polyethylene (HDPE), low
density polyethylene (Ll)PE), linear low density polyethylene (LLDPE) or branched low
density polyethylene (BLDPE).
Polyolefins, i.e. polymers of monoolefins, such as are mentioned by way of example in the
preceding paragraph, in particular polyethylene and polypropylene, can be prepared by
diverse processes, in particular by the following methods:
a) free radical polymerisation (usually at high pressure and high temperature).
b) by means of a catalyst, the catalyst usually containing one or more metals ofgroups IVb, Vb, VIb or VIII. These metals usually have one or more ligands,
such as oxides, halides, alcoholates, esters, ethers, amines, alkyls, alkenyls and/or
aryls, which can be either ~- or ~-coordinated. These metal complexes can be
free or fixed on a support, for example on activated magnesium chloride,
titanium(II) chloride, aluminium oxide or silicon oxide. These catalysts can be
soluble or insoluble in the polymerisation medium. The catalysts can be active as
such in the polymerisation or further activators can be used, ~or example metal
alkyls, metal hydrides, metal alkyl halides, metal alkyl oxides or metal alkyl
oxanes, the metals being elements of groups Ia, IIa and/or IIIa. The activators
can, for example, be modified by further ester, ether, amine or silyl ether groups.
2 ~
- 17 -
These catalyst systems are llsually desi~nated Phillips, Standard Oil Indiana,
Ziegler (-Nattn), TNZ (DuPont), metallncene or sin~le site catalysts (SSC).
2. Mixtllres of the polymers mcntione(l llnclcr 1), for ex~lmple mixtures of polypropylene
with polyisoblltylcne, polypropylene with polyethylene (for ex~m1ple PP/~IDPE,
PP/LDl'E~) nl)d mixt~lres of variolls types of polyethylene tfor example LDPF~ PE).
3. Copolymers of monoolefins and diolefins with one another or with other vinyl
monomers, for example ethylene-propylene copolymers, linear low density polyethylene
(LLDPE) and mixtures thereof with low density polyethylene (LDPE),
propylene-but~l-ene copolymers, propylene-isobutylene copolymers, ethylene-but-1-ene
copolymers, ethylene~hexene copolymers, ethylene-methylpentene copolymers,
ethylene-heptene copolymers, ethylene-octene copolymers, propylene-b~tadiene
copolymers, isobutylene-isoprene copolymers, ethylene-alkyl acrylate copolymers,ethylene-alkyl methacrylate copolymers, ethylene-vinyl acetate copolymers and their
copolymers with carbon monoxide, or ethylene-acrylic acid copolymers and their salts
(ionomers), and also terpolymers o.f ethylene with propylene and a diene, such as
hexadiene, dicyclopentadiene or ethylidenenorbornene; and also mixtures of such
copolymers with one another and with polymers mentioned under 1), for example
polypropylene/ethylene-prnpylene copolymers, LDPE/ethylene-vinyl acetate copolymers,
LDPE/ethylene acrylic acid copolymers, LLDPE/ethylene-vinyl acetate copolymers,
LLDPE/ethylene-acrylic acid copolyrners and alternating or randomly composed
polyalkylene/carbon monoxide copolymers and mixtures thereof with other polymers, for
example polyamides.
4. Hydrocarbon resins (for example Cs-Cg) including hydrogenated modifi~ations thereof
(for example tacklfier resins) and mixtures of polyalkylenes and starch.
5. Polystyrene, poly(p-methylstyrene),and poly(a-methylstyrene).
6. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for
example styrene-butadiene, styrene-acrylonitrile, styrene-alkyl methacrylate,
styrene-butadiene-alkyl acrylate and-alkyl methacrylate, styrene-maleic anhyclride and
styrene-acrylonitrile-rnethyl acrylate; mixtures of high impact strength composed of
styrene copolymers and another polymer, for example a polyacrylate, a diene polymer or
an ethylene-propylene-diene terpolymer; and also block copolymers of styrene, for
2~90~82
- 18 -
example styrene-blltacliene-styrene, styrene-isoprene-styrerle,
styrene-ethylene/butylene-styrene or styrene-ethylene/propylene-styrene.
7. ara~t copolymers of styrene or (x-methylstyrene, for example styrene on polybutadiene,
styrene on polyblltadicne~styrene or polyb~ltadiene-acrylonitrile copolymers, styrene and
ncrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile and methyl
methacrylate on polybutadiene; styrene and maleic anhydride on polybutadiene; styrene,
acrylonitrile and maleic anhydride or maleimide on polybutadiene; styrene and maleimide
on polybutadiene, styrene and alkyl acrylates or alkyl methacrylates on polybutadiene,
styrene and acrylonitrile on ethylene-propylene-diene terpolymers, styrene and
acrylonierile on polyalkyl acrylates or polyalkyl methacrylates, styrene and acrylonitrile
on acrylate-butadiene copolymers, and also their mixtures with the copolymers mentioned
under 6), as are known, for example, as so-called ABS, MBS, ~SA or AES polymers.
8. ~alogen-containing polymers, for example polychloroprene, chlorinated rubber,chlorinated or chlorosulfonated polyethylene, copolymers of ethylene and chlorinated
ethylene, epichlorohydrin homopolymers and copolymers, in particular polymers ofhalogen-containing vinyl compounds, for example polyvinyl chloride, polyvinylidene
chloride, polyvinyl fluoride or polyvinylidene fluoride; and also tbeir copolymers, such as
vinyl chloride-vinylidene chloride, vinyl chloride-vinyl acetate or vinylidene
chloride-vinyl acetate.
9. Polymers which are derived from a,~-unsaturated acids and their derivatives, such as
polyacrylates and polymethacrylates, polymethyl methacrylates impact-modi~led with
butyl acrylate, polyacrylamide and polyacrylonitrile.
10. Copolymers of the monomers mentioned under 9~, with one another or with other
unsaturated monomers, for example acrylonitrile-butadiene copolymers,
acrylonitrile-alkyl acrylate copolymers, acrylonitrile-alkoxyalkyl acrylate copolymers,
acrylonitrile-vinyl halide copolymers or acrylonitrile-alkyl methacrylate-butadiene
terpolymers.
11. Polymers which are derived from unsaturated alcohols and amines or their acyl
derivatives or acetals, such as polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate,
polyvinyl ben~oate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate and polyallyl
rnelamine; and also their copolymers with olefins mentioned under point i.
2~90~82
,()
12. Homopolymers and copolymers of cyclic ethers, sllch as polyalkylelle glycols,
polyethylelle oxide, polypropylene oxide or thcir copolymers with bisglycidyl ethers.
13. Polyacetals, sncll as polyoxymethylelle"lnd also those polyoxymethylenes which
contnin comonomers, for examplc ethylene oxide; polyacetals which are modified with
thertnoplastic polyurethanes, acrylates or M~S.
14. Polyphenylene oxides and polyphenylene sulfides and their mixtures Wit}l styrene
polymers or polyamides.
15. Polyurethanes which are derived from polyethers, polyesters and polybutadienes
having terminal hydroxyl groups on the one hand and aliphatic or aromatic
polyisocyanates on the other hand, and their precursors.
16. Polyamides and copolyamides which are derived from diamines and dicarboxylic acids
and/or from aminocarboxylic acids or the corresponding lactams, such as polyamide 4,
polyamide 6, polyamide 6/6, 6/10, 619, 6/12, 4/6 and 12/12, polyamide 11, polyamide 12
and aromatic polyamides based on m-xylene, diamine and adipic acid; polyamides
prepared from hexamethylenediamine and isophthalic acid and/or terephthalic acid and, if
desired, an elastomer as modi~ler, for example
poly-2,4,4-tlimethylhexamethyleneterephthalamide or poly-m-phenyleneisophthalamide.
Block copolymers of the abovementioned polyamides with polyolefins, olef1n copolymers,
ionomers or chemically bonded or grafted elastomers; or with polyethers, for example
with polyethylene glycol, polypropylene glycol or polytetramethylene glycol. And also
polyamides or copolyamides modified with EPDM or ABS; and also polyamides
condensed during processing ("RIM polyamide systems").
17. Polyureas, polyimides, polyamide-imidçs and polybenzimidazoles.
18. Polyesters which are derived from dicarboxylic acids and dialcohols and/or from
hydroxycarboxylic acids or the corresponding lactones, such as polyethylene
terephthalate, polybutylene terephthalate, poly-1,4-dimethylolcyclohexane terephthala~e,
and polyhydroxybenzoates, and also block polyether-esters which are derived frompolyethers having hydroxyl end groups; and also polyesters modified with polycarbonates
or MBS.
209~2
- 2~) -
19. Polycarbonates and polyester-carbonates.
2~). Polyslllfones, polyether-slllfones an(l polyether-ketones.
21. Crosslinked polymers wllich are derived from nldehydes on the one hand and phenols,
ure~l or melamine on tlle other hand, sllch as phenol-formaldehyde resins,
urea-formaldehyde resins and melamine-forrnaldehyde resins.
22. Drying and non-clrying alkyd resins.
23. Unsaturated polyester resins which are denved from copolyesters of satllrated and
unsaturated dicarboxylic acids with polyhydric alcohols and also vinyl compounds as
crosslinking agents, and also their halogen containing modifications of low ~lammability.
24. Crosslinkable acrylic resins which are derived from sllbstituted acrylic acid esters, for
example from epoxy acrylates, urethane acrylates or polyester acrylates.
25. Alkyd resins, polyester resins and acrylate resins which are crosslinked with melamine
resins, urea resins, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins which are derived *om polyepoxides, for example from
bis-glycidyl ethers or from cycloaliphatic diepoxides.
27. Naturally occurring polymers, such as cellulose, natural rubber, gelatin, and also the~r
polymer-homologous chemically modified derivatives, such as cellulose acetates,
cellulose propionates and celllllose butyrates, or cellulose ethers, such as methylcellulose;
and also colophony resins and derivatives.
28. Mixtures (polyblends) of the abovementioned polymers, for example PP/EPDM,
polyamide/EPDM or AB~, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylate, POM/thermoplastic PUR, Pc!thermoplastic
PUR, POM/a~rylate, POM/MBS, PPO/~IPS, PPO/PA 6.6 and copolymers, PA/~IDPE,
PA/PP, PA/PPO.
29. Naturally occurring and synthetic organic substances which are pure monomer
20go08~
- 21 -
compoun(ls or mixtures of such comyounds, for example mineral oils, animal or vegetable
fats, oils and wa.Yes, or oils, waxcs nnd fnts bascd on synthetic esters (for exnmple
phthalntes, ndipates, phospllntes or trimellitates), nnd nlso ndmixtures of synthetic esters
with minernl oils in nrbitrnry proportions by wcight, such as are used, for example, as
spinnillg prepnrntions, and also aqlleolls emulsions thereof.
30~ Aqueous emulsions of naturally occurring or synthetic rubbers, for example natural
rubber latex or latexes of carboxylated styrene-butadiene copolymers~
Further subjects of the invention are, therefore, cornpositions containing an organic
material sensitive to oxidative, thermal an(Vor actinic degradation and at least one
compound of the forrnula 1, as well as the use of cornpounds of the ~ormula I for
stabilising organic material against oxidative, thermal or actinic degradation~
The invendon therefore also comprises a method for stabilising organic material against
therrnal, oxidative and/or actinic degradation, which method comprises adding at least one
compound of the formula I to said material~
The use of compounds of the formula I as antioxidants in synthetic organic polymers is of
particular interest~
Preferred organic materials are polymers, for example synthetic organic polymers or
mixtures of such polymers, in particular thermoplastic polymers. Particularly preferred
organic materials are polyolefins and styrene copolymers, for example those indicated
above under points 1 to 3 and under points 5 and 6, in particular polyethylene and
polypropylene and also ABS and styrene-butadiene copolymers. Composi~ions in which
the organic material is a synthetic organic polymer or a mixture of such polymers, in
particular a polyole~in or a styrenc copolymer, are therefore a preferred subject of the
invention.
In general, the compounds of the ~ormula I are added to the material to be stabilised in
amounts of from 0.01 to 10 %, preferably 0.01 to 5 % and in particular 0.01 to 2 %, with
respect to the total weight of the material to be stabilised. The use of the compounds
according to the invention in amounts of from 0.01 to 0.5 %, in particular 0.05 to 0.3 %, is
particularly preferred.
209~082
In addition to the compoun~ls of tlle ~ormul~l 1, the compositions according to the invention
can additionally ColltLlill conventional additives, for example those indicated below.
1~ Antioxi(lants
I.1. a!kylntecl-!n~ for example 2,6-di-tert-blltyl-4-methylphenol, 2-tert-butyl-4,6-dimethylphenol, 2,6-di-tert-blltyl-4-etllylpllenol, 2,6-di tert-butyl-4-n-butylphenol,
2,6-di-tert-butyl-4-isobutylphenol, 2,6-di-cyclopentyl-4-methylphenol, 2-(a-methylcyclo-
hexyl)-4,6-dimethylphenol, 2,6-di-octadecyl-4-methylphenol, 2,4,6-t~i-cyclohexylphenol,
2,6-di-tert-butyl-4-methoxymethylphenol, 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-
(l'-methyl-undec-l'-yl)phenol, 2,4-dimethyl-6-(1'-methyl-heptadec-1'-yl)phenol, 2,4-di-
methyl-6-(1'-methyl-tridec-1'-yl)phenol and mixtures the;eof~
1.2, Alkylthiomethylphenols, for example 2,4-di-octylthiomethyl-6-tert-butylphenol,
2,4-di-octylthiomethyl-6-methylphenol, 2,4-di-octylthiomethyl-6-ethylphenol, 2,6-di-do-
decylthiomethyl-4-nonylphenol.
1.3. Hydroquinones and alkvlated hYdroquinones, for example 2,6-di-tert-butyl-4-rilethoxyphenol, 2,5-di-tert-butyl-hydroquinone, 2,5-di-tert-amyl-'n~ydroquinone,
2,6-diphenyl-4-octadecyloxyphenol, 2,6-di-tert-butyl-hydroquinone,
2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyanisole,
3,5-di-tert-butyl-4-hy~roxyphenyl stearate, and bis(3,5-di-tert-butyl-4-hydroxyphenyl)
adipate.
1.4. HydroxYlated thiodiphenvl ethers, for example 2,2'-thio-
bis(6-tert-butyl-4-methylphenol), 2,2'-thio-bis(4-octylphenol),
4,4'-thio-bis(6-tert-butyl-3-methylphenol), 4,4'-thio-bis(6-tert-butyl-2-methylphenol),
4,4'-thio-bis(3,6-di-sec-amylphenol) and 4,4'-bis(2,6-dimethyl-4-hydroxyphenyl) disulffde.
1.5. AlkYlidene-bis~,ahenols, for example 2,2'-methylene-bis(6-tert-butyl-4-methylphenol),
2,2'-methylene-bis(6-tert-butyl-4-ethylphenol), 2,2'-methylene-bis[4-methyl-6-(a-methyl-
cyclohexyl)phenol], 2,2'-methylene-bis(4-methyl-6-cyclohexylphenol), 2,2' methylene-
bis(6-nonyl-4-methylphenol), 2,2'-methylene-bis(4,6-di-tert-butylphenol), 2,2'-
ethylidene-bis(4,6-di-tert-butylphenol), 2,2'-ethylidene-bis(6-tert-butyl-4-isobutylphenol),
2,2'-methylene-bis[6-(a-methylbenzyl)-4-nonylphenol], 2,2'-methylene-bis~6-(a,a-di-
methylbenzyl)-4-nonylphenol], 4,4'-methylene-bis(2,6-di-tert-butylphenol), 4,4'-
~0082
- 23 -
methylene-bis(6-tert-butyl-2-methylphcnol), 1,1-bis(S-tert-butyl-4-hydroxy-2-methyl-
phellyl)butane, 2,6-bis(3-tert-blltyl-S-methyl-2-hydroxybenzyl)-4-metllylphenol, 1,1,3-
tris(S-~ert-blltyl-4-llydroxy-2-metllylptlellyl)blltane~ 1,1-bis(5-tert-butyl-4-hydroxy-2-
methylphellyl)-3-n-clodecylmercaptobutlllle, ethylene ~Iycol bisL3,3-bis(3'-tert-butyl-4'-
hydroxypllenyl)blltyr.lte], bis(3-tert-butyl-4-hydroxy-5-methylphenyl)dicyclopentadiene,
bis[2-(3'-tert-butyl-2'-hydroxy-57-llletllylbenzyl)-6-tert-butyl-4-methylphenyll tere-
phthalate, t,1-bis(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2-bis(3,5-di-tert-butyl-4-
hydroxyphenyl)propflne, 2,2-bis(S-tert-butyl-4-hydroxy-2-methylphenyl)-4-n-dodecyl-
mercaptobutane and l,1,5,5-tetra(S-tert-butyl-4-hydroxy-2-methylphenyl)pentane .
1.6. O-, N- and S-benzyl compounds, for example 3,5,3',5'-tetra-tert-butyl-4,4'-dihydroxy-
dibenzyl ether, octadecyl 4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tris(3,5-di-
tert-butyl-4-hydroxybenzyl)amine, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-butyl-4-hydroxybenzyl) sulfide, and isooctyl
3 ,5-di-tert-butyl-4-hydroxybenzylmercaptoacetate.
1.7. HYdroxvbenzYlated malonates, for example dioctadecyl
2,2-bis(3,5-di-tert-butyl-2-hydroxybenzyl)malonate, di-octadecyl
2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)malonate, di-dodecylmercaptoethyl
2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate, di[4-
(1,1,3,3-tetramethylbutyl)phenyl~ 2,2-bis(3,S-di-tert-butyl-4-hydroxybenzyl)malonate.
1.8. HYdroxybenzyl-aromatic compounds? for example
1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-
tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetramethylbenzene, 2,4,6-tris(3,5-di-tert-
butyl-4-hydroxybenzyl)phenol .
1.9. Triazine compounds, for example
2,4-bis-octylmercapto-6-(3,5-di-tert-butyl-4-hydroxyanilino)- 1 ,3,5-triazine,
2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)- 1 ,3,5-triazine,
2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)- 1 ,3,5-triazine,
2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris(3,5-di-
tert-butyl-4-hydroxybenzyl) isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-di-
methylbenzyl) isocyanurate, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-
1,3,5-triazine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexahydro-1,3,5-triazine and 1,3,5-tris(3,5-dicyclohexyl-4-hydroxybenzyl) isocyanurate.
2 ~ 2
- 24 -
,~,~lospholultes~ for example dim~thyl
2,~-di-~ert-blltyl-4-hydro~ybenzylpllosphollate, diethyl
3,5-di-tert-butyl-4-hydroxybellzylpl-osphonatc, dioctadecyl 3,5-di-tert-butyl-4-
hydroxybenzylpllosphollate, dioctadecyl S-tert-blltyl-4-hydroxy-3-methylbenzylphos-
phollate and the calcium salt of monoethyl 3,5-di-tert-butyl-4-hydroxyben~ylphosphonate.
1.11. AcYlaminophenols, for example 4-hydroxylauric acid anilide, 4-hydroxystearic acid
anilide and octyl N-(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
1.12 Esters of ,B-(3~5-di-tert-butyl-4-hYdroxvphenYl)propionic acid with monohydric or
polyhydric alcohols, for example with methanol, ethanol, octadecanol, 1,6-hexanediol,
1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaelythritol, tris(hydroxyethyl) isocyanurate,
N,N'-bis(hydroxyethyl)oxalic acid diamide, 3-thiallndecanol, 3-thiapentadecanol, tri-
methylhexanediol, trimethylolpropane and 4-hydroxymethyl- 1-phospha-2,6,7-tri-
oxabicyclo[2.2.2]octane.
1.13. Esters of ~-~S-tert-butYI-4-hYdroxY-3-methvlPhenyl)propionic acid with monohydric
or polyhydric alcohols, for example methanol, ethanol, octadecanol, 1,6-hexanediol,
1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxy)ethyl isocyanurate,
N,N'-bis(hydroxyethyl)oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, tri-
methylhexanediol, trimethylolpropane and 4-hydroxymethyl- 1 -phospha-2,6,7-tri-
oxabicyclo[2.2.2]octane.
1.14. Esters of ~-(3~5-dicYclohexvl-4-hYdroxyphenYl)propionic ac d with monohydric or
polyhydric alcohols, for example with methanol, ethanol, octadecanol, 1,6-hexanediol,
1 ,9-nonanediol, ethylene glycol, 1 ,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxy)ethyl isocyanurate,
N,N'-bis(hydroxyethyl)oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, tri-
methylhexanediol, trimethylolpropane and 4-hydroxyrne~hyl-1-phospha-2,6,7-tri-
oxabicyclo[2.2.2]octane.
1.15. Esters of 3,5-di-tert-butyl-4-hydroxyphenylacetic acid with monohydric or
polyhydric alcohols, for example methanol, ethanol, octadecanol, 1,6-hexanediol,
209~082
1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopcntyl glycol, thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxy)etllyl isocyanurate,
N,N'-bis(hydroxyethyl)oxalic acid diamide, 3-thiaunclecallol, 3-thiapentadecanol, tri-
methylllex~ ediol, trirnethylolpropane and 4-hydroxymetllyl- 1-phospha-2,6,7-t~i-
oxabicyclo~2.2.2:1octane.
1.16. Amides of ~ (3~$-di-tert-butvl-4-hvdroxyphenvl)propionic acid, for example
N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexamethylenediamine,
N,N'-bis(3,5-di-tert-b~ltyl-4-hydroxyphenylpropionyl)trimethylenediamine and
N,N'-bis(3,5-di-tert-b~ltyl-4-hydroxyphenylpropionyl)hydrazine.
2. UV-absorbers and li~ht stabilisers
2.1. 2-(2'-HydroxYPhenvl)benzotriazoles, for example
2-(2'-hydroxy-5 '-methylphenyl)benzotriazole,
2-(3 ' ,5 '-di-tert-butyl-2'-hydroxyphenyl)benzotriazole,
2-(5' tert-butyl-2'-hydroxyphenyl)benzotriazole,
2-(2'-hydroxy-5 '-(1,1 ,3,3-tetramethylbutyl)phenyl)benzotriazole,
2-(3 ' ,5 ' -di-tert-butyl-2 ' -hydroxyphenyl)-5-chlorobenzotriazole,
2-(3'-tert-butyl-2'-hydroxy-5 '-methylphenyl)-5-chlorobenzotriazole,
2-(3 '-sec-butyl-5 '-tert-butyl-2'-hydroxyphenyl)benzotriazole,
2-(2'-hydroxy-4'-octoxyphenyl~benzotriazole,
2-(3' ,5 '-di-tert-amyl-2'-hydroxyphenyl)benzotriazole,
2-(3',5'-bis(a,o~-dimethylbenzyl)-2'-hydroxyphenyl)benzotriazole, a mixture of
2-(3'-tert-butyl-2'-hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)-5-chlorobenzotriazole,
2-(3'-tert-butyl-5'-[2-(2-ethylhexyloxy)carbonylethyl]-2' -hydroxyphenyl)-5-chlorobenzo-
triazole,
2-(3'-tert-butyl-2'-hydroxy-5'-(2-methoxycarbonylethyl)phenyl)-5-chlorobenzotriazole,
2-(3'-tert-butyl-2'-hydroxy-5'-(2-methoxycarbonylethyl)phenyl)benzotriazole,
2-(3'-tert-butyl-2' -hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)benzotriazole,
2-(3'-tert-butyl-5'-[2-(2-ethylhexyloxy)carbonylethyl]-2'-hydroxyphenyl)benzotriazole,
2-(3'-dodecyl-2'-hydroxy-5'-methylphenyl)benzotriazole, and
2-(3'-tert-butyl-2'-hydroxy-5 '-(2-isooctyloxycarbonylethyl)phenylbenzotriazole,2,2'-methylene-bis[4-(1,1,3,3-tetramethylbutyl)-6-benzotriazol-2-ylphenol]; the
transesteri~lcation product of 2-[3'-tert-butyl-5'-(2-methoxycarbonylethyl)-2'-
2~9~0~2
- 26 -
hydroxypllenyllbenzotriazole with polyethylellc ~Iycol 300; [~-CH2CH2-COO(C~12)3~,
whcrc R=3'-tert-bu~yl-4'-hydroxy-5'-21-1-bcllzotrinzol-2-ylphenyl.
hellolle, for example the 4-hydroxy, 4-methoxy, 4-octoxy, 4-decyl-
oxy, 4-do(lecyloxy, ~l-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-dimethoxy
delivative.
2.3. Esters of substituted or unsubstitute_benzoic acids, for example 4-ter~butyl-phenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoylresorcinol, bis~4-tert-butyl-
benzoyl)resorcinol, benzoylresorcinol, 3,4-di-tert-butylphenyl 3,5-di-tert-butyl-4-
hydroxybenzoate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-
butyl-4-hydroxyben~oate and 2-me~hyl-4,6-di-tert-butylphenyl
3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrylates, for example ethyl and isooctyl ~-cyano-~"~-diphenylacrylate, methyl
oc-carbomethoxycinnamate, methyl and butyl a-cyano-,B-methyl-p-methoxycinnamate,methyl cc-carbomethoxy-p-methoxycinnamate and N-~,B-carbomethoxy-~-
cyanovinyl)-2-methylindoline.
2.5. Nickel compounds, for example nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex, if desired with additional ligands,
such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel
dibutyldithiocarbamate, nickel salts of 4-hydroxy-3,5-di-tert-butylbenzylphosphGnic ~cid
monoalkyl esters, such as of the methyl or ethyl ester, nickel complexes of ketoximes,
such as of 2-hydroxy-4-methylphenylundecylketoxime and nickel cGmplexes of 1-
phenyl-4-lauroyl-5-hydroxypyrazole, if desired with additional ligands.
2.6. Sterically hindered amines, for example bis(2,2,6,6-~etramethylpiperidyl) sebacate,
bis(2,2,6,6-tetramethylpiperidyl) succinate, bis(1,2,2,6,6-pentame~hylpiperidyl) sebacate,
bis(1,2,2,6,6-pentamethylpiperidyl) n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate,
the condensation product of 1-hydroxyethyl-2,2,6,6-tetramethyl-4-hydroxypiperidine and
succinic acid, the condensation product of N,N'-bis(2,2,6,6-
tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-dichloro-1,3,5-
s-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl) nitrilotriacetate, tetrakis(2,2,6,6-tetra-
methyl-4-piperidyl) 1,2,3,4-butanetetraoate,
2090082
I, I '-( I ,2-ethanediyl)bis(3,3,5,5-tetr.ullcthylpipcr~zinone),
4-bcn;~oyl-2,2,6,6-tetratnetllylpiperidinc, 4-stc~ryloxy-2,2,6,6-tetramethylpiperidine,
bis(l,2,2,6,6-pent.lmethylpiperidyl) 2-n-blltyl-2-(2-llydroxy-3,5-cli-tert-butyl-
benzyl)malonnte, 3-n-octyl-7,7,9,9-tetrnmethyl-1,3,8-trinzaspiro[4.51decane-2,4-dione,
bis(l-octyloxy-2,2,6,6-tetrametllylpiperidyl) sebncnte, bis(l-octyloxy-2,2,6,6-tetramethyl-
piperidyl) succinate, the condensation product of N,N'-bis~2,2,6,6-tetramethyl-
4- piperidyl)llexametllylenediamine and 4-morpholino-2,6-dichloro- l ,3,5-triazine, the
condensation product of 2-chloro-4,6-di(4-n-butylamino-2,2,6,6-tetramethylpiperidyl)-
1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, the condensation product of
2-chloro-4,6-di(4-n-butylamino-1,2,2,6,6-pentamethylpiperidyl)-1,3,5-triazine and
1,2-bis(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-(2,2,6,6-tetramethyl-4-
piperidyl)pyrrolidine-2,5-dione and 3-dodecyl-1-(1,2,2,6,6-penta-
methyl-4-piperidyl)pyrrolidine-2,5 -dione .
2.7. Oxalic acid diamides, for example 4,4'-dioctyloxyoxanilide, 2,2'-dioctyloxy-S,S'-di-
tert-butyloxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butyloxanilide, 2-ethoxy-2'-ethyl-
oxanilide, N,N'-bis(3-dimethylaminopropyl)oxalamide,
2-ethoxy-S-tert-butyl-2'-ethyloxanilide and its mixture with
2-ethoxy-2'-ethyl-5,4'-di-tert-butyloxanilide, and mixtures of o- and p-methoxy- and also
of o- and p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-~ydroxvphenYI)-1.3.5-triazines, for example 2,4,6-tris(2-hydroxy-4-octyloxy-
phenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-
1 ,3,5-triazine, 2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl~- 1 ,3,5-triazine,
2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl~-4,6-bis(4-methylphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1,3 ,S -triazine,
2-[2-hydroxy-4-(2-hydroxy-3-butyloxypropyl-
oxy)phenyl]-4,6-bis(2,~-dimethylphenyl)-1,3,5-triazine, 2-[2-hydroxy-4-
(2-hydroxy-3-octyloxypropyloxy)phenyl~-4,6-bis(2,4-dimethylphenyl)- 1 ,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxalic acid diamide, N-salicylal-N'-
salicyloylhydrazine, N,N'-bis(salicyloyl)hydrazine, N,N'-bis(3,5-di-tert-butyl-4-hydroxy-
phenylpropionyl)hydrazine, 3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalic acid
dihydrazide, oxanilide, isophthalic acid dihydrazide, sebacic acid bis-phenylhydrazide,
2al90082
- 28 -
N,N'-diacetyladipic acid dillydrazide, N,N'-bis-salicyloyloxalic acid dihydrazide, and
N,N'-bis-salicyloylthioyropionic acid dihydrazi(le.
4. F~urthe ~sphites ~Ind phosl)honites, for example triphenyl phosphite, diphenyl alkyl
phosphites, phenyl dialkyl phosphites, tris~nonylphenyl) phosphite, trilautyl phosphite,
trioctadecyl phosphite, distearylpentaerythritol diphosphite, ~ris(2,4-di-tert-butylphenyl)
phosphite, diisodecylpentaerythritol diphosphite,
bis(2,4-di-tert-butylphenyl)pentaerythritol diphosphite,
bis(~,6-di-tert-butyl-4-methylphenyl)pentaerythlitol diphosphite, bis-isodecyloxypenta-
erythritol diphosphite, bis(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol diphosphite,
bis(2,4,6-tri-tert-butylphenyl)pentaerythritol diphosphite, tristearylsorbitol triphosphite,
tetrakis(2,4-di-tert-butylphenyl)4,4'-biphenylenediphosphonite, 6-isooctyloxy-2,4,8,10-
tetra-tert-butyl-12~-dibenz[d,g]-1,3,2-dioxaphospnocin and 6-fluoro-2,4,8,10-tetra-tert-
butyl- 12-methyldibenz[d,g~- 1 ,3,2-dioxaphosphocin, bis(2,4-di-tert-butyl-6-methylyhenyl)
methyl phosphite and bis(2,4-di-tert-butyl-6-methylphenyl) ethyl phosphite.
5. Peroxide-destrovin~ compounds, for example esters of ,t3-thiodipropionic acid, for
example the lauryl, stearyl, myristyl or tridecyl ester, mercaptobenzimidazole, the zinc salt
of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide and
pentaerythritol tetrakis(,B-dodecylmercapto)propionate.
6. PolYamide stabilisers, for example cupper salts in combination with iodides and/or
phosphorus compounds and salts of divalent manganese.
7. Basic costabilisers, for example melamine, polyvinylpyrrolidone, dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids, for
example calcium stearate, zinc stearate, magnesium behenate, magnesium stearate,sodium ricinoleate, potassium palmitate, antimony pyrocatechinate or tin pyrocatechinate.
8. Nucleatin~ ag~nts, for example 4-tert-butylbenzoic acid, adipic acid and diphenyl acetic
acld.
9. Fillers and reinforcin~ algents, ~or example ~alcium carbonate, silicates, glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and metal hydroxides, carbon
black and graphite.
2090082
- 2'~ -
__~ciitivcs, for example plasticisers, lubrica-lts, emulsifiers, pigments, fluorescent
wilitenillg a~ents, flallleproofillg ngellts, nntistatic agents all(l propellants.
11. 1~5~n(iol _lles, as clescribed, tor exnmple, hl US-A-4 325 863 or
US ~-4 338 244.
The conventional additives are added, for example, in concentrations of from 0.01 to 10
%, with respect to the total weight of the material to be stabilised.
The incorporation of the compound of the formula I and, if desired, further additives in the
organic material is effected by known methods. Incorporation into the materials can, for
example, be effected by mixing in or applying the compounds of the formula I and, if
desired, further additives using the methods customary in the art. 1~ the materials are
polymers, in particular synthetic polymers, the incorporation can be effected before or
during shaping, or by applying the dissolved or dispersed compounds to the polymer, with
subsequent evaporation of the solvent where appropriate. In the case of elastomers, these
can also be stabilised AS latexes. A further possibility for incorporatin~ the compounds of
the formula I in polymers comprises adding said compounds before, during or
immediately following the polymerisation of the corresponding monomers or prior to
crosslinking. The compounds of the formula I can be added as such, but also in
encapsulated form (for example in waxes, oils or polymers). In the case of addition before
or during the polymerisation, the compounds of the formula I can also act as regulators for
the chain length of the polymers (chain stoppers).
The compounds of the formula I can also be added to tho materials to be stabilised in the
forrn of a masterbatch which contains said compounds, for example in a concentration of
from 2.5 to 25 % by weight.
The materials stabilised in this way can be used in very diverse forms, for example in the
form of films, fibres, tapes, m'dulding compositions or sections or as binders for coating
compositions, adhesives or putties.
The following examples further illustrate the invelttion. Unless indicated otherwise, parts
and percentage data in these examples are by weight. In the formulae the prefix n
designates a straigllt-chain alkyl radical and the prefix i a mixture of isomers. "Melting
209~082
- 30 -
point" is abbreviated ns "m.p." in thc tables.
Ex~: Prepara~ion of 4-~3-ter~-hutyl-5-methyl-4-llydroxyphellyl)butimol.SO g of potassillm hy(lro~ide, 316 g of 2-tert-butyl-6-Metllylphenol and 866 g of
1,4-butnnediol are initially inLroduced into an autoclave. Af~er introdllcing nitrogen gas,
the mltoclave is close(l nnd the mixture is heated at 235C t4 bar) for 6 hours, with
stirring; n further rise in prcssure as a consequence of the reac~ion is prevented by blowing
of gas. When the reaction is complete, the reaction mix~ure is cooled to 80C and the
autoclave is emptied. Excess butanediol is rcmoved by distillation. The distillation residue
is poured into 1 1 of water and S00 ml of toluene. The organic phase is then washed with
water until neutral and the toluene is stripped ot`f by applying a vacuum. The remaining
residue (310 g) contains 68-70 % of the title product of the formula
OH
(CH3)3C~,CH3
CH2 CH2~ ~OH
Further purification is effected by f~actional distillation under vacuum: the pure product
boils at 115C and 35 Pa (= 0.35 mbar). It has a refractive index n20D of 1.5322.
Example lb: Preparation of 6-(3-tert-butyl-5-methyl-4-hydroxyphenyl)hexanol.
Example la is repeated except that in place of 1 j4-butanediol an equivalent amount of
1,6-hexanediol is used. The resulting product of the formula
OH
(CH3)3C~CH3
has a boiling point at 35 Pa (= 0.35 mbar) of 135C and
(CH2)6--OH
melts at 45C.
Example lc: A solution of 25.5 g (84 mmol) of stearoyl chloride in 10 ml of toluene is
added dropwise at 10C, under nitrogen, to a solutlon of 18.9 g (80 mmol) of
4-(3-tert-butyl-5-methyl-4-hydroxyphenyl)butanol (product from Example la) and 9.5 g
2~90~82
(120 mmol) of pyridine in 90 ml of toluene. The white suspension is then allowed to warm
to 20-25C mld filtered and thc filtrate is poured into approximately 1 mok~r aqueous HCI
and extracted wi~l ethyl acetate. Af~er drying an(l concentrating the organic phase, the
resulting crude product is purit`ied by chromntography (siO2; hexane:ethyl acetate = 19:1).
0~1
(CH3~3C ~,CH3
37.2 g (93 %) of the product of the formula
(C~12)4 0--C - n cl7l~35
(compound No. 1) are obtained in the form of a colourless liquid. Characterisation (IR)
and analysis are given in Table 1.
Examples 2-16: In order to prepare compounds Nos. 2 to t6, corresponding
t~-(3-tert-butyl-5-methyl-4-hydroxyphenyl)alkanols or
co-(3,5-di-tert-butyl-4-hydroxyphenyl)alkanols are first synthesised by the method
described in Example la. The starting compounds (compounds of the formula IV) thus
obtained are then reacted using corresponding acid chlorides in accordance with the
methotl descri~ed in Exarnple lc to give the said end praducts (compounds of the formula
I). Data on the purity and characterisation of the compounds are summarised in Tables 1
and 2. Where no melting point is indicated, the compounds are liqùid at room temperature.
0 8 2
- 32 -
Tclb. 1: Analytical and IR data Ior compounds Nos. 1-8
OH
(CH3)3C~,~,CH3
CompoIlnd t.ype: ~
(CH2)~r 0--C ~--A
__ _----~ ANALYSIS: IR~
Compouud. n A ~O C: calc. foul~d % H: C~lc. found C=O [cm 13
_~
1 4 -n-Cl7~3s 78.8 78.7 11.6 l l .4 1738
2 4 -n-c7H1s 76.2 76.1 10.6 I0.~ 1719
3 4 -i-c7H1s 76.2 76.2 10.6 11.0 1717
4 4 -i-Gl2~C22H 77.7 77.7 11.2 11.1 1717
4 - CH2 CH- CH~ C2Hs 76.2 76.0 10.6 10.4 1715
6 6 - CH2 - CH- cH2 - C2Hs 76.9 76.9 10.8 10.6 1715
7 6 -n-C11H23 78.0 77.9 11.3 11.5 1732
8 6 -n-Cl7H3s _ 79.2 79.1 11.8 12.2 1720
2 ~ 2
Tab. 2: Melting points, analytical ancl IR data ~o~ compollnds Nos. 9- l 6
OH
(CH3)3C~,~ C(C~3)3
Compoun(ltype: ~
~CH2)rr 0 ~ A
_ _ ~NALYSIS: IR: 1/ A
CmNPound n C2Hs m.p ['CI % C: c~c. found % H: cnlc. found C=0 ¦om'~¦
9 4 - CH~ CH- CH~ C2Hs 77.2 77.0 11.0 10.8 1732
4 -1)~G7H1 ~ - 77.2 77.2 11.0 11.4 1736
11 4 -i'C12H25 . 78.4 78.3 11.5 11.5 1735
12 4 'n C17HSS 30-33 79,4 79,5 11.8 12.1 1738
13 5 ~n'C17HH5 31-32 79.5 79.6 11.9 11.9 1738
14 6 - cH~cH-cH~c2H6 77,7 77.7 11.2 1 I.5 1738
6 -n-C11H23 7~,6 78.5 11.6 11.9 1737
16 6 -n-C17H35 35 79,7 79.6 12.0 12.2 1738
Exarnple 17: 13.6 g (0.074 mol) of adipic acid dichloride are added dropwise at 10C
under nitrogen to a colourless solution of 33 g (0.14 mol) of the product from Exarnple la
and 16.6 g (0.21 mol) of pyridine in 200 ml of toluene in a 350 ml sulfonation flask. The
mixture is then allowed to warm to room temperature9 while continuing to stir. After 2 h
the reaction mixture is poured into aqueous hydrochloric acid and extracted with ethyl
acetate. After drying and concentrating the organic phase, the resulting crude product is
purified by chromatography (siO2; hexane:ethyl acetate = 19:1). 36 g (88 %) of the
product (compound No. 17) of the formula
- 2090~82
- 34 -
OH OH
(CH3)3C~,c~l3 C~3 ~ C(CH3~3
~l o o l~J . whicll has a meîting point
tCH2)4--o--c--(CH2)4--C O -(CH2)4
of 50-52C are obtained Charnc~elisation (IR) an(l analysis are given in Table 3.
_xamples 18-22 and 24-34: In order to prepare compounds Nvs. 18 to 22 and 24 to 34
corresponding ~-(3-tert-butyl-5-methyl-4-hydroxyphenyl)alkanols or
~o-(3 5-di-tert-butyl-4-hydroxyphenyl)alkanols are first synthesised by the method
described in Example la. The starting compounds (compounds of the formula IV) thus
obtained are then reacted using conesponding dicarboxylic acid dichlorides in accordance
with the method described in Example lc to give the said end products (compounds of the
formula I). Data on the purity and characterisation of the resulting compounds are
summarised in Tables 3 4 and 5.
Example 23: 4.98 g (30 mmol) of isophthalic acid 14.4 g (70 mmol - 2.3 equivalents) of
dicyclohexylcarbodiimide and 150 ml of dichloromethane are mixed at 20-25C under
nitrogen in a 750 ml sulfonation flask. A solution of 15.9 g (60 mmol = 2 equivalents) of
6-(3-tert-butyl-5-methyl-4-hydroxyphenyl)hexanol (product from Example lb) in 80 ml of
dichloromethane and a solution of 0.73 g (6 mmol) of dimethylaminopyridine in S ml of
dichloromethane are added to the resulting white suspension. The mixture is refluxed for 4
hours. It is then cooled to 20-2~C and filtered and the filtrate is evaporated. The resulting
crude product (viscous oil) is purified by chromatography (SiO2; hexane/ethyl acetate =
1~/1). 11.3 g (57 %) of compound No. 23 are obtained in the form of a colourless oil. Data
on the purity and characterisation are given in Table 3.
209~2
- 35 -
Tab. 3: Melting points, analytical and IR dati1 for compollnds Nos. 17-23
OH OH
(C~13)3c~cH3 C~3 ~C(CH3)3
Compoun(l ~ypc: 1~1 ~1
(CH2)1~ 0 - C--A ~ C ~ O -(CH2)n
. _ _ ANALYSIS: îR: 1/ A
Com~ound n . ID p.¦ Cl % C: cslc. fouod % H: calc. found C=O ¦cm'~
17 4-(CH2)4- 50-52 74.2 73.8 09.3 09.5 1718
18 4-(CH2)g- ~ 75.2 74.7 09.8 09.9 1717
19 6 . 74.2 74.1 09.3 09.7 1738
6-(CH2)2' 90-103 74.7 73.9 09.6 09.8 1713
21 6-(CH2)4- 91 -95 75.2 75.0 09.8 09.7 1718
22 6-(CH2)g- 76.0 75.5 10.2 10.2 1718
23 6 ~ - 76.6 76.1 08.9 09.1 1723
2~9~82
- ~6 -
Tab. 4: Meltin~ pC)illtS, analyliclal allcl IR (I,ata tor compounds NC)S. 24-3()
0~1 01-1
(CH3)3C~ C(CH3)3 (CH3)3c~,c(cH3)3
Compouncl lype:
(CH2)jr 0--C--A--C--O -(CH2)n
_~ ANALYSIS: IR~
No. n A m.p.[~c] % C: calc. found % H: calc. found C=O [cm'1]
. _ _
24 4 -(CH2)4- 86-88 75.6 75.5 10.0 09.8 1734
89-90 75.2 75.1 09.8 os.s 1743
26 5 -(CH2)4- 92-95 76.0 75.6 10.2 10.0 1735
27 6 76.6 75.7 10.0 10.1 1743
28 6 -(CH2)2- 70 76.0 75.9 10.2 10.4 1736
29 6 -~CH2)4- 54~57 76.4 76.5 10.3 10.6 1735
6 -~CH~ 77.1 77.1 10.6 10.6 1735
2 0 ~ 2
Tab. 5: Analy~ical and IR data for compounds Nos. 31 to 34
Compound type:
OH OH
(C1~3)3C~,R1 R~ c(CH3)3
(CH2),r 0--C--(CH2)r S--(CH2)r C--O ~(CH2)n
_ _ AN~LYSIS:IR. 1/ A
No. n R1 i yO C: c~lc. found % H: c~lc. found % S: c~lc. found C=O [cm 1]
31 4 CH3 2 70.3 70.5 8.9 9.05.2 5.4 1731
32 6 CH3 2 71.6 71.5 9.3 9.54.~ 4.9 1732
33 6 C(CH3)3 2 72.7 72.6 9.7 9.7 4.4 4.7 1736
C(CH3)3 1 72.1 72.0 9.5 9.6 4.6 4.7 1734
Example 35: Example 23 is ,repeated except that in place of the product from Example lb
6-(3,5-di-tert-butyl-4-hydroxyphenyl)hexanol is used as the starting material. The product
(compound No. 35) of the formula
OH OH
(CH3)3C~ C(CHg)3 (CH3)3C~¢~C(CH3)3
(C~2)6--O--C ~ C--O -(CH2)6
has an IR absorption of the carbonyl band at 1723 cm~l.
Analysis: calculated 77.6 % C 9.5 % H, found 77.1 % C 9.5 % H.
xample 36: 7.57 g (30 mmol) of methyl trimellitate, 33 g (117 mmol) of the product from
Example lb and 0.45 g (1.8 mmol) of dibutyltin oxide are mixed in a round-bottomed
~90082
flask fitted with a fracliollating column. Thc mixture is heale(l ~o I X0C, metlmnol being
distilled off. After 2 hours the pressure abovc ll-c rnixlllrc is r~dllce(l to 533 hPa (= 400
mmHg) and the temper~lllrc is then kcp~ ~It 180C t`or a t`~lrlhcr ~ hollrs. The excess
6-(3-tert-butyl-S-methyl-~-hydroxyphenyl)llexnnol is lhell removed by dislillation (150C,
4 Pa) antl the resulting cru(le product is purit`ied by chromatograplly (SiO2; first hexane,
ttlell hexnne/ethyl ncetate = 19/1). 24.7 g (87 %) ot` a prodllct of the formula
OH OH
(CH3)3C~3,CH3 CH3 ~,C(CH3)3
(C~l2)6- 0--C ~ C--O (C~I2)6
C--O -(Cl2)6
CH3 J~C(CH3)3
01~
(compound No. 36) are obtained; IR absorption of the carbonyl band at 1722 cm-l.Analysis: calculated 75.9 % C 8.9 % H, found 75.3 % C 9.3 % H.
xarnple 37: 16.5 g (70 mmol) of 4-~3-tert-butyl-S-methyl-4-hydroxyphenyl)butanol,
18.05 g (91 mmol) of methyl 10-undecenoate and 80 mg (1.4 mmol) of dibutyltin oxide
are introduced into a round-bottomed flask fitted with a fractionating column.
The mixture is heated to 180C, methanol being distilled off. After 2 hours, the pressure
above the mixture is reduced to 400 mmHg and the temperature is then kept at 1 80C for a
further 2 hours. The excess methyl 10-undecenoate is then removed by distillatiorl and the
resulting crude product (brown oil) is purified by chromatography (SiO2; hexane/ethyl
acetate 9:1).
23.15 g (82 %) of a product of the formula
~90082
- 39 -
H3C ~ C ~ 3 OH
Ch3 ~C~3
(C~2)4
o ", (CH~)b C~
ll CH2
are obtained.
Anfllytical and IR data: see Table 6.
Examples 38-42: Compounds 38-42 are prepared analogously to lhe method described in
Example 1. Analytical and IR data of cornpounds 38-40 are given in Table 6 and the
corresponding data for compounds 40 and 41 are given in Table 7.
Tab. 6: Analytical and IR data for cormpounds Nos. 37-40; cornpound type:
OH
(CH3)3C~ CH3
O
(CH2)~--0--C--A
_ __ _ . _ -: _
ANALYSIS: IR~
No n A % C: c~c. found % H: c~c. found C=O [cm
_ __ ~ _ _ _
37 4 -(CH2)8-CH=CH2 77.56 77.59 10.52 10.58 1719i1735
38 4 -C(CH3)~CH2 74.96 74.95 9.27 9.48 1705
39 6 -CH=CH2 75.43 75.20 9.50 9.53 1712l1725 .
6 -C(CH3)=CH2 75.86 75.83 9.70 10.16 1705/1718
~0082
- ~o -
Tab. 7: Ana1y~ical and IR ~ata for compollnds Nos. 41 and 42
OH
(CH~)3C~,~C(c~13)3
Compoulld ~yp~
(CH2)~r 0--C A
_ _ . _ _
ANALYSIS: IR: 1/ A
No. n . _ _ % C: calc. fo~ d % H: calc. found C=O ¦Gm ~¦
41 6 -C~H=CH2 76.61 76.44 10.06 10.08 1725
42 6 -C(CH~)=CH2 76.96 76.85 10.23 10.16 1719
Exam~es 43-4S: Compound No. 43 is prepared by the method described in Example l;compound No. 44 is prepared by the method described in Example 37; compound No. 45
is prepared by the method described in Example 23. Analytical and IR data for compounds
43-45 are given in Table 8.
Tab. 8: Analysis and IR data for compounds Nos. 43-~5
OH OH
(CH3)3C ~,CH3 CH3 ~C(CH3)3
Compound type: l~ 0 l~)
(CH2~n C--A--C--~(CH2)n
.
_ ANALYSIS: IR~
No. n A % C: calc. found % H: calc. found C=O ¦cm~1]
_ _ _ ~
43 4 -CH2-CH2- 73.61 73.14 9.08 9.21 1722
44 4 -(CH2)2-O-(CH2)2- 72.21 71.93 9 09 9.20 1728
6 ~ 72 .25 72.41 8.49 8.53 1717
_ _ _ %S: calc.4.82 found 4.69 _
2090~82
- 41 -
Example 46: ~? Esterit`ic~tion ol` the dicarboxylic acid mixtule with methanol
22 ml/0.4 l mol of concentl llted sulfuric acid are added dropwise at 10C~ to a solution of
52 g ~0.4 mol) ot` dicarboxylic aci(l mixtu~e (mnmlfacturer B~SF; comprising 25-35 % of
SUCCitliC acid, 37-47 '~, of glutaric ncid and 25-30 % ot' adipic acid) in 450 ml of methanol.
Thc reslllting solution is kept at the reflux temperatl1re for 3 hours. After cooling to
20-25C, the mix~llre is neutralised Wittl SS g of potassium carbonate, poured into water
and extracted with ethyl acetate. Evapor~tion of the solvent yields 23 g (36 %) of the
desired ester mixture in the form of a pale yellow oil.
Gas chromatographic analysis of the compound mixture of the formula
H3C-OCO-(CH2)~-COO-CH3:
x = 2 23 mol-%
x-3 44mol-%
x = 4 33 mol-%
b) Preparation of compound 46
30.7 g (130 mmol) of 4-(3-tert-butyl-5-methyl-4-hydroxyphenyl)butanol, 8.01 g (50
mmol) of the diester mixture described under (a) and 490 mg (2 mmol) of dibutyltin oxide
are introduced into a round-bottomed flask provided with a fractionating column.
The mixture is heated to 180C, methanol being dis~lled off. The mixture is kept at 180C
for a further 15 hours. After cooling to 20-25C, the crude mixture is purified by
chromatography (siO2; hexane/ethyl acetate 40:1~19:1~ 9:1).
17.1 g (60 %) of compound No. 46 are obtained in the form of a yellow oil.
Analytical and IR data: see Table 9.
Examples 47-Sl: The preparation of compounds 47-48 is carried owt using corresponding
phenolic alcohols in accordance with the method described in Example 46.
In order to prepare compounds 49, 50 and 51, the corresponding methyl ester is first
synthesised from Poly-THF-Dipropionic acid~ 350 (manufacturer: Bayer A.G.,
Leverkusen) by the method described in F,xample 46. The high molecular weight
~090~82
- 42 -
dicarboxylic aci~l methyl estel thus obtained is then re~ctecl using currespon~ting phenolic
alcohols in accordanc~ wilh the method c!escribed in Example 46 to give the said end
pro(lucts.
Annlytical and IR datn for compollnds 46-51 ar~ given in Tnble 9,
Table 9: Analytical ~m~l IR (lata tor compoun(ls 46-51
Compound type (diester mixtures and oligomers)
R1
~CI ~2) n (Cl H2) n
O ll A 11 - O
O O
_ _ ~ _ _
Com- ANALYSIS: IR~
~und R 1 n A C %: H %: C-O [cm 1]
No. _ _ calc. foundcalc. found
46 Me 4 ~ where X = 2 20-25% 73.91~ 73.79 9.22 9.37 1719
47 t-Bu 4 J - (CH2)X x - 3 40 43% 75.43~ 75.05 9.88~ 9.98 1735
48 Me 6 21 % x=2; 42% x=3; 37% x=4; 74.96~ 74.91 9.68b 9.81 1720
m.p.79-93 C
49 Me 4 70.26 69.92 9.61 9.78 1735
t-Bu 4 - (CH2~2 O ~ (CH2)4- O ~y (CH2)2 - 71.87 71.92 9.95 10.21 1737
51 Me 6 y - 2.6 71.70 71.18 9.96 9.97 1736
Me = methyl; t-Bu ~ tert-butyl
~ Analysis calculated for x = 3
~9~82
- 43 -
le 52: u) Prcpar.~ ?n Or me~hYl n_rilolriaccl~
7~.8 n~l (137.7 ~; 1.4 ~lol) of collcen~ra~e(l sulrllric aci(l nre a(lde(l dropwise to a
sllspensioIl of l()0 ~ (0.52 mol) of nitrilolrincelic aci(l in ')00 ml of methnllol nt about
lOC.
The mixturc is kept at the reflux temperature for 22 hours. After cooling to room
temperature, the mixture is neuLralised by careful addition of 150 g of potassium
carbonate, poured in~o water and extracted with ethyl acetate.
Evaporation of the solvent and drying of the residue under vacuum yields 57 g (47 %) of
methyl nitrilotriacetate in the form of a colourless oil.
IR (film on KBr crystal): carbonyl absorption at 1751 cm-
Analysis: calc. 46.35 % C, 6.4~ % H, 6.0 ~O N
found 4~S.47 % C, 6.53 % H, G. 14 % N
b) Preparation of compouncl 52
1.76 g (7.5 mmol~ of methyl nitxilotriacetate, 7 g (29.4 mmol) of
4-(3-tert-butyl-5-methyl-4-hydroxyphenyl)butanol and 25 mg (0.45 mmol) of dibutyltin
oxide are introduced ~nto a round-bottorned flask provided with a fractionating column.
The mixture is heated to 180C, methanol being distilled of After 3 hours, the pressure is
reduced to 400 mmHg and the mixture is kept at 180C for a further 2 hours.
The excess 4-(3-tert-butyl-5-methyl-4-hyd~oxyphenyl)butanol is then removed by
distillation (150C/0.4 Pa) and the resuldng crude product is purified by chromatography
(siO2; hexane-ethyl acetate 9:1_3:1).
5.6 g (88 %) of a product of the formula
~9~
- 4~ -
1-1 C CH3
c~C~13
(CH2)4
o~CH2----N
are obtained.
Analytical and IR data: see Table 10.
Examples 53-SS: Compounds 53-55 are prepared by the method desclibed in Example 52.
Analytical and IR data of the compounds are summar~sed in Table 10.
'CCH~ R 1
Table 10: Compound ~ype
(C, H2)n
O~rCH2~--N
Com- _ -- ~ ANALYSIS: IR~
pound Rl n m.p. C%: H%: N%: ~ C_O [cm-1]
No. calc. found calc. found calc. found
52 Me 472.39 72.39 8.93 8.83 1.66 1.48 1738
53 t-Bu 474.11 73.42 9.64 9.68 1.44 1.23 1747
54 Me 673.59 72.98 9.43 g.67 1.51 1.31 1739
t-Bu 675.03 74.18 10.02 10.28 1.33 1.33 1748 _
. _ ~ ~
Examples 56-60: Compounds 56-60 are prepared by the method described in Exarnple 36.
Analytical and IR data of the eompounds are summssed in Table l l.
2~90~82
- 45 -
T~lble~ Compo~ l ty~ R~
(C~l2)6
~--A
_ _ ~ _ ~ ANALYSIS: IR 1/ A
pOoum~d R1 A z tn.p.-Cc%: H%: C=O [cm-~]
No. _ _ c~lo. found c~lc found
56 t-Bu ~ 3 77~05 77.28 9.56 9.53 1727
57 Me ~ 3 62-6575.91 75.95 8.92 8.92 1728
58 ~ I ~ 1 ~95 91 77.~6 76.09 9.5 9.67 1 7~9
59 Me ~ 4 75.57 74.95 8.94 9.65 1725
60 t Bu 4 ~ 76.77 71.14 9.69 6.91 1730
Example 61: Preparation of 4-(3.5-dicyclohexYl-4-hYdroxyphenYl)hexanol
258 g (1 mol) of 2,6-di.cyclohexylphenol and 591 g (5 mol) of 1,6-hexanediol are melted
in a sulfonation flask and heated with 36 g (0.64 mol) of potassium hydroxide for 10 hours
at 238C. The mixture is then slightly acidified at 80C using a 2 molar aqueous HCl
solution. The phases are separated, the inorganic phase is washed with toluene and the
combined organic phases are concentrated in a rotary evaporator. Excess 1,6-hexanediol
and unreacted 2,6-dicyclohexylphenol are removed by distillation.
2nsoos2
- 46 -
Crystallisation of the resi(llle from special boiling point ben~.ine (80- l l0C) yields 163 g
(45 %) of a prodllct of the formukl
(f H2,6
OH
m.p.: 96C
Analysis: calc. 80.39 % C, 10.68 % H
found78.62%C, 10.62%H
Example 62: 32.3 g (90 mmol) of 4-(3,5-dicyclohexyl-4-hydroxyphenyl)hexanol are
reacted using 9.2 g (50 mmol) of adipic acid dichloride, in accordance with the method
described in Example lc, to give 18.7 g (47 %) of a product of the formula
C,~,C C~
(CH2)6 (ci~2)6
l I :
O C (CH2)4 11 -
O O
The compolmd is obtained in the form of a viscous oil.
IR (film on KBr crystal~: 1736, 1717 cm-l (C = Oj
Analysis: calc.77.76 % C, 10.29 % H
found 77.62 % C, 10.05 % H
20~0082
- 47 -
Vse ,examples
,Exam~,: _a_s tl of polypropYlenc
100 parts of polypropylelle powder, con~ailling 0.1 % ot`calcium slearate, are mixed with
0.3 % of distearyl thiotlipropion.lte ~DS rDp) ~u~(l o~ l % of the stabiliser according to the
invention indicate(l in Tablc 12 antl the mixlure is ~hen kneaded for 1() minllteg at 200C
in a Brabender pklstogr.lph.
The composition thus obtained is pressed in a press which has a surf.lce temperature of
260C to give l mm thick plates, from which strips l cm wide and 10 crn long arepunched. For comparison purposes, a further sample is prepared without stabilisers.
Several such strips from each plate are suspended in a circulating air oven heated to 135C
or 149C and observed at regular intervals. The oxidative decomposition of these strips
can be recognised by an incipient circular yellow discoloration. The time in days until
decomposition takes place is a criterion for the stability of the sample.
2090082
Table 12: Time (in days) belore d~composilion of lhe samples containing the stabiliser
combina~ioll û.3 % DSTDP ~ (). I % compoun(l according to ~he invention an(l containing
no stabiliser takcx phlce
Number of days oven ageing before
Stabilisers dccomposition tnkes place
at 135C at 149C
none 1 d
DSTDP+compoundNo. 8 126 50
DSmP-~compoundNo. 13 115 40
DSTDP + compoun:l No. 17 156 65
DSTDP+ compound No. 20 162 60
DSTDP+ compound No. 21 175 38
DSTDP~ compound No. 22 247 57
DSTDP ~ compound No. 23 245 85
DSTDP+ compound No. 24 140 43
DS'~DP + compolmd No. 25 121 24
DSTDP+ compound No. 26 151 33
DSTDP~compoundNo. 28 147 47
DSTDP+ compound No. 29 145 49
DSTDP+ compound No. 30 194 50
DSTDP~ compound No. 35 148 50
DSTDP ~ compound No. 36 190 71
DSTDP ~ compound No.52 158 55
DSTDP+ compound No. 53 158 48
DSTDP~ compound No. 54 161 55
DSTDP+ compound No. 55 150 43
DSTDP~ compound No. 56 170 52
I:)STDP + compound No 57 193 70
DSTDP-~ compoundNo 58 173 52
DSTDP + compound No 59 163 63
DSTDP + compound No 60 159 4~
Example 63: Stabilisation of acrYlonitrile-butadiene-styrene terpolymer (ABS3
The stabilisers indicated in Table 13 are dissolved in 40 ml of a mixture of hexane and
isopropanol. The solution is added ~o a dispersion of 100 g of ABS in 600 ml of water,
with vigorous stirring, after which the solution is completely absorbed by the ABS within
about one minute. The polymer powder containing the stabilisers is then filtered off and
dried for 40 hours at 40C under vacuum.
For comparison purposes, the subsequent processing steps are also carried out using~ a
sample without stabilisers.
2 % of titanium dioxide, as pigment, and 1 % of ethylene-bis-stearic acid amide, as
~090082
- 4') -
lubricant, are added to thc dry l)owdcr. The rni~ r~ is lhen compollnde(l within 4 minutes
on a 2-roll mill al l X0C.
A plat~ ().8 mm thick is i r~s~d t`rom th~ rollc(l ~ cl al 175C and 45 x 17 mm2 les~
pieces are pllnchc(l lrolll this plat~. The te~t to determin~ th~ etfectiv~ncss of the added
stabilisers is carr;e(l olll by heal al~in~ in a circul~ltill~ air oven at lX~)C. Thc criterion is
the colour development ~ter a test tirne of 45 minules. The colour intensity is determined
in accordance with ~STM D 1')25-70 (Yellowness Index) Tlle test results are summarised
in Table 13. Higher figures indicate more intensive yellowing. The tests show that
yellowing is ef~ectively suppressed by ~he compounds according to the invention.
able 13: Yellowness Index after 45 minute oven ageing of samples containing no
stabilisers, 0.5 % of dilauryl thiodipropionate ~DLTDP) and also 0.5 %
DLTDP+0.25%of the indicated compound nccording to the inventio
Stabilisers Yellowness Index after 45 Minutes at L80C
none 78
DLTDP 75
DLTDP+compoundNo.l 26
DLTDP+ compound No. 7 25
DLTDP+ compound No. 8 26
DLTDP+ compound No. 12 34
DLTDP+ compound No. 13 39
DLTDP+ compound No. 15 33
DLTDP+compoundNo.16 34
DLTDP+ compound No. 17 26
DLTDPt compound No. 19 31
DLTDP+ compound No. 20 26
DLTDP+ compound No. 21 33
DLTDP+ compound No. 22 21
DLTDP+ compound No. 23 29
DLTDP+ compound No. 24 32
DLTDP+ compound No. 25 41
DLTDP + compound No. 26 28
DLTDP+ compound No. 28 30
DLTDP+ compound No. 29 32
DLTDP+ compound No. 31 29
DLTDP+compound No. 32 27
DLTDP+ compound No. 33 32
DLTDP+ compound No. 34 30
DLTDP+compound No. 39 36
DLTDP+ compound No. 40 26
DLTDP+ compound No. 42 32
209~82
- s() -
l~xample 64: _abi!is.lti_rl of X-S~R l~l x c~oxylated SBR latex)
In ench case 0.25 parts by weight of ~he compoun(ls accor(lillg lo the inventiotl lisîed ;n
Tablc l4 are dissolve(l in a little methanol and stirred into lO() par~s by weigllt of X-SBR
latex (styrene-butadienc copolymer). A precisely clefined nmount of latex is then filled
into Petli dislles an(l dried in an oven at 80C. Transparetlt films havillg n layer thickness
of about 0.2 mm are obtained. For comparison purposes, a sample without stabilisers is
prepared.
The test to determine the elfectiveness of the added stabilisers is carried out by heat
ageing in a circulating air oven at 135C. The discoloration ot the samples is deterrnined
in accordance w;th ASTM D 1925-70 (Yellowness Index) after the intervals indicated in
Table 14. The test results are surrlmarised in Table 14. Higher tlgures indicate more
intensive yellowing. The tests show that yellowing is efFectively suppressed by the
compounds according to the invention.
Table 14: Yellowness Index after the indicated ageing time at 135C
Yellowness Index after ageing time (in hours)
Stabiliser 4 h 12 h 24 h 40 h
none 92 * ~ *
0.25 % compound No. 31 46 52 64 75
0.25 % compoundNo. 32 40 46 58 69
*: not measurable (sample black)