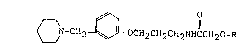Note: Descriptions are shown in the official language in which they were submitted.
2 ~ ? ~ ,'3
TITLE OF THE INVENTION
NOVEL ACETAMIDE DERIVATIVE AND APPLICATION THEREOF
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a novel compound,
2-substituted-N-{3-[3-(1-piperidinomethyl)phenoxy]-
propyl}acetamide, derivatives thereof, and
pharmaceutically acceptable salts thereof. The present
invention also relates to a drug composition, especially
to an antiulcer drug composition, comprising said
compound as an effective component.
Description of the Back~round Art
Ulcers, typified by stress ulcer, are diseases
characteristic to modern human being. Their increase in
the future is anticipated. For example, in the case of
stress ulcer, depression of gastrointestinal tract
movement, degradation of gastrointestinal tract vascular
flow, and the like are reported to be caused by the
action of the adrenocorticotropic hormones releasing
factor which is induced by hypothalamus irritation.
Simultaneous actions of parasympathetic nerves and
sympathetic nerves render the conditions even more
complicated. In the case of peptic ulcer, the ulcer is
considered to develop when a balance between aggressive
factors, such as pepsin, gastric acid, or the like, and
defensive factors, such as mucus, mucosal vascular flow,
or the like, is lost. Thus, an ulcer is caused by
various reasons, including abnormal secretion of gastric
acid, hormones, and the like; inhibition in the synthesis
of mucus; inhibition in the synthesis of prostaglandin;
and the like. Ulcers are thus considered to be formed
due to coincidence of various factors.
On the other hand, in any types of ulcers, reducing
gastric acid secretion, accelerating synthesis of muco
polysaccharide which consists of gastric mucus, or
increasing gastric mucosal vascular flow are believed to
alleviate deep pains caused by ulcers and to degenerate
the ulcers.
Nowadays, roughly classified, two types of antiulcer
drugs are on sale; one is Histamine H2-antagonist which
has an action to depress gastric acid secretion, and the
other is a gastric mucosa protective agent which exhibits
actions to protect gastric mucosa. Although histamine
H2-antagonist shows a superior action and exhibits its
effect rapidly, it has a problem that the rebound
phenomenon is caused by repeated administration. The
gastric mucosa protective agent, on the other hand,
exhibits only a weak action, and, depending on the
circumstances, it takes a long period of time for the
agent to exhibit the its effect.
In view of the above-mentioned drawbacks in
2~t~2
;
conventional antiulcer drugs, t:he present inventors have
synthesized a number of compounds in order to develop a
new antiulcer drug and were successful in obtaining a
novel compound possessing both the gastric acid secretion
inhibitive activity and the gastric mucosa protective
activity.
Accordingly, an object of the present invention is
to provide a novel compound which can exhibit both the
gastric acid secretion inhibitive activity and the
gastric mucosa protective activity, and which can be used
as an effective component for a new type of antiulcer
drug.
Another object of the present invention is to
provide a drug, particularly an antiulcer drug,
comprising said novel compound as an effective component.
Still another object of the present invention is to
provide an intermediate compound for preparing said novel
compound.
The present inventors have synthesized a number of
compounds for the purpose of obtaining a novel compound
possessing both of the above-mentioned activities, and
found that 2-substituted-N-{3-t3-(1-piperidinomethyl)-
phenoxy]propyl}acetamide exhibits both the gastric acid
secretion inhibitive activity and the gastric mucosa
protective activity.
SUMMARY OF THE INVENTION
The above object can be resolved according to the
present invention by the provision of novel compounds, 2-
substituted-N-{3-[3-(1-piperidinomethyl)phenoxy]-
propyl}acetamide, repxesented by the following foxmula,
~N-cH2¢~LocH2cH2cH2NHccH2o-R
wherein R is an aminomethylcyclohexane carbonyl group,
-co{3~ CH2NH2
or an N-carbobenzoxy-aminomethylhexane carbonyl group,
-CO{~CH2NHCOCH2~
derivatives thereof, and pharmaceutically acceptable
salts thereof.
Among the above compounds, acetamide compounds
having the first-mentioned group, i.e., aminomethyl-
cyclohexane carbonyl group, are compounds exhibiting both
of the aforementioned actions, and the compounds of the
last-mentioned group, i.e., N-carbobenzoxy-aminomethyl-
hexane carbonyl group, are intermediates for producing
the compounds having the first-mentioned group.
~ mong the compounds represented by the above
formula, the following compounds are particularly noted.
2-(aminomethylcyclohexanecarboxy)-N-{3-[3-(1-
piperidinomethyl)phenoxy]propyl}acetamide,
CH2-N
/ O O
- OCH2CH2CH2NHCCH20C ~ CH2NH2, and
2-(N-carbobenzoxy-trans-p-aminomethylhexanecarboxy)-N-{3-
[3-(1-piperidinomethyl)phenoxy]propyl}acetamide,
CH2-N >
~ / O O O
~ I - ocH2cH2cH2NHccH2oc{}cH2NHc-ocH2~
The above object the present invention is further
resolved by the provision of a drug composition,
particularly, an antiulcer drug composition, comprising a
compound possessing said groups as an effective
component. Derivatives of the present invention include
compounds with.the terminal amino group, piperidino
group, or the like substituted by other groups.
The compounds of the present invention may be
present as cys or trans steroisomers and may form a
2 ~
pharmaceutically acceptable salt with an acid such as
hydrochloric acid, citric acid, maleic acid, or the like.
Thus, the compounds of the present invention include such
steroisomers and pharmaceutically acceptable salts.
The above object is still further resolved according
to the present invention by the use of the compounds of
the last-mentioned group, i.e., N-carbobenzoxy-
aminomethylhexane carbonyl group, as an intermediate for
producing the compounds having the first-mentioned group,
i.e., aminomethylcyclohexane carbonyl group.
Other and further objects, features and advantages
of the present invention will appear more fully from the
following description.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an IR spectrum (2R) of 2-(trans-p-amino-
methylcyclohexanecarboxy)-N-{3-t3-(1-piperidinomethyl)-
phenoxy]propyl}acetamide, which is a compound of the
present invention.
Figure 2 is an NMR spectrum of the compound of
Figure 1.
Figure 3 is a mass spectrum of the compound of
Figure 1.
Figure 4 is an IR spectrum (2R) of 2-(N-carbo-
benzoxy-trans-p-aminomethylhexanecarboxy)-N-{3-[3-(1-
piperidinomethyl)phenoxy]propyl}acetamide, which is
another compound of the present invention.
Figure 5 is an NMR spectrum of the compound of
;Figure 4.
Figure 6 is a mass spectrum of the compound of
Figure 4.
DETAILED DESCRIPTION OF THE INVENTION
AND PREEERRED EMsoDIMENTs
There are various methods of preparing the compounds
of the present invention. The following methods are
preferred from the aspect of both the yield and the
easiness.
First, 3-piperidinomethylphenol is synthesized from
3-hydroxybenzaldehyde and piperidine. The 3-piperidino-
methylphenol is then reacted with 3-chloropropylamine to
produce N-[3-(3-aminopropoxy)benzyl]piperidine, which is
reacted with acetoxyacetyl chloride to obtain N-t3-(3-
piperidinomethylphenoxy)propyl]hydroxyacetamide.
5eparately, tranexamic acid and carbobenzoxy chloride are
reacted to produce N carbobenzoxyaminomethylhexanecarboxy
chloride. The two compounds thus obtained are then
reacted first to afford N-{3-[3-(1-piperidinomethyl)-
phenoxy]propyl}-2-(N-carbobenzoxy-aminomethylhexane-
carboxy)acetamide. This compound is then reduced into
2-(aminomethylcyclohexanecarboxy)-N-{3-[3-(piperidino-
methyl)phenoxy]propyl}acetamide.
Among the compounds thus prepared 2-(trans-p-
aminomethylcyclohexanecarboxy)-N-{3-[3-(1-piperidino-
methyl)phenoxy]propyl}acetamide (hereinafter referred toas Compound RT) and 2-(N-carbobenzoxy-trans-p-amino-
methylhexanecarboxy)-N-{3-[3-(1-piperidinomethyl)-
phenoxy]propyl}acetamide (hereinafter referred to as
Compound RTP) are preferable.
N-[3-(3-piperidinomethylphenoxy)propyl]hydroxy-
acetamide, which is an intermediate compound, is known as
roxatidine. N-carbobenzoxyaminomethylhexanecarboxy
chloride, which is another intermediate compound, is also
a known compound. Both compounds can be prepared by
known processes other than the above-described process.
The derivatives of the present invention can also be
obtained by introducing appropriate groups by
substitution at any appropriate stage in the synthesis of
the compound of the present invention.
The above intermediate compounds dissolved in an
organic solvent are stirred under ice cooling or at room
temperature in the presence or absence of a catalyst to
produce crystals, which is then collected by filtration.
Triethylamine or the like is used as a catalyst. 2-(N-
carbobenzoxy-trans-p-aminomethylhexanecarboxy)-N-{3-[3
(l-piperidinomethyl)phenoxy]propyl}acetamide of the
present invention can be obtained by collecting the
crystals. The crystals are dissolved into alcohol and
catalytically hydrogenated in hydrogen gas in the
2 ~
presence o~ a catalyst to give 2-(aminomethylcyclohexane-
carboxy)-N-{3-[3-(1-piperidinomethyl)phenoxy]propyl}
acetamide, which is another compound of the present
invention. The catalytic hydrogenation is carried out at
room temperature under atmospheric pressure using
palladium-on-carbon as a catalyst. Isolation and
purification of the target compound from the reaction
mixture can be performed by means of conventionally known
methods, such as separation by column chromatography,
crystallization by vacuum concentration, and the like.
Regarding physicochemical characteristics of the
compounds thus obtained, Compound RT is a light yellow or
colorless oil and its IR, NMR and mass spectra are as
shown in Figures 1-3; and Compound RTP is a light yellow
paste and its IR, NMR and mass spectra are as shown in
Figures 4-6.
Experiments on the suppression of ulcers induced by
the water dipping stress and ulcers induced by
hydrochloric acid-ethanol were carried out using rats to
investigate the pharmaceutical activities of Compound RT
and Compound RTP. The both compounds were found to
effectively suppress the ulcers, confirming that the
compounds of the present invention are useful as a drug,
especially as an antiulcer drug.
Although a dose to human varies depending on the
symptom, sex, age, and the like of the patients, all
s a ~
compounds of the present invention can cure ulcers
induced by indigestion or stress, or can suppress their
inducement, by administering them in an amount of 1-300
mg/day once a day or dividedly in several times a day.
The compounds may be administered either orally or
parenterally, even though oral administration is normally
more preferred. Parenteral administration includes
subcutaneous injection, intramuscular injection,
intravenous injection, and, in cases, arterial injection.
The compound of the present invention may be
formulated together with conventional carriers,
disintegrators, lubricants, and the like, and formed into
powders, granules, tablets, capsules, drinks, cataplasms,
suppositories, or the like. Alternatively, it can be
used as injection after dissolved into distilled water,
physiological saline, or the like, followed by
sterilization.
Other features of the invention will become apparent
in the course of the following description of the
exemplary embodiments which are given for illustration of
the invention and are not intended to be limiting
thereof.
EXAMPLES
Example 1 <Synthesis>
(1) Synthesis of 3-pyridinomethylphenol
CHO CH2-N
OH ~ ~ OH
(III) (IV) (V)
3-Hydroxybenzaldehyde (III) (0.246 mol, 30 g) was
dissolved into 150 ml of methanol. Piperidine (IV) (0.6
mol, 52 g) was added to the solution and the mixture was
stirred at room temperature to dissolution. To the
solution was added under ice cooling sodium hydrogen
borate (0.247 mol, 9.4 g) while stirring over 1 hour,
followed by continued stirring for 1 hour at room
temperature. The reaction mixture was concentrated ~nder
vacuum. The residue was dissolved in 200 ml of 3 N
hydrochloric acid and washed twice with 50 ml of ethyl
acetate. The water layer was alkalinized (pH 10) with
about 50 ml of concentrated aqueous ammonia to deposit
crystals. The crystals were collected by filtration,
washed with water, dried under vacuum, and recrystallized
from a mixed solvent of acetone and n-hexane, to obtain
40 g of 3-piperidinomethylphenol (V) (yield: 84.7%).
m.p. 135-138C.
(2) Synthesis of N-[3-(3-aminopropoxy)benzyl]piperidine
-N ~ CH2-N ~
~ ClCH2C112CH2NH2- E~Cl ~\
OH OCH2CH2cH2NH2
(V) (VI) (VII)
3-Chloropropylamine hydrochloride (VI) (0.2 mol, 39
g) was dissolved into 3 N sodium hydroxide solution
(containing 10% sodium chloride) and extracted with 300
ml of benzene. The benzene layer was dried over
anhydrous magnesium sulfate. Separately, 3-piperidino-
methylphenol (V) (0.2 mol, 38.2 g), sodium hydroxide
(0.25 mol, 10 g), 100 ml of dimethylsulfoxide, and 70 ml
of benzene were charged to a flask equipped with a water
quantitative measurement tube, and heated at 130C for 3
hours while stirring to remove water produced by the
tube, followed by continued stirring for 2 hours at 140-
150C. To the resulting reaction mixture was dropwise
added said benzene solution of 3-chloropropylamine at
150C while stirring over 4 hours. The reaction mixture
was allowed to stand still to cool to room temperature to
separate deposited sodium chloride by filtration. The
filtrate was concentrated under reduced pressure and
vacuum distilled to obtain 42.7 g of N-[3-(3-amino-
propoxy)benzyl]piperidine (VII) (yield: 86.0%). m.p.148-151C/0.25 mmHg.
(3) Synthesis of N-[3-(3-piperidinomethylphenoxy)propyl]-
hydroxyacetamide
2 N ~ CH2-N
CH3COOCH2COCl -->~ O o
OCH3cH2cH2NH2 OCH2CH2CH2NHCCH20CCH3
(VII) (VIII) (IX)
CH2-N ~
~D--'
OCH2CH2CH2NHCCH20H
(X)
N-[3-(3-aminopropoxy)benzyl]piperidine (VII) (0.15
mol, 37.2 g) was dissolved into 200 ml of anhydrous benzene.
To the solution was added triethylamine (0.18 mol, 18 g)
and the mixture was stirred under ice cooling, followed
by the dropwise addition of a solution of acetoxyacetyl
chloride (VIII) (0.18 mol, 24.5 g) in 50 ml of dry
benzene under ice cooling over l hour. After further
stirring for 1 hour at room temperature, the deposited
crystals of compound (IX) were separated by filtration.
The filtrate was subjected to evaporation under reduced
pressure, the residue was dissolved into lO0 ml of 2 N
sodium hydroxide, and the solution was stirred for 5
2 ~ 2
hours at 50-60C. The resultant reaction mixture was
extracted with dichloromethane, the extract was dried
over anhydrous magnesium sulfate, and dichloromethane was
evaporated under reduced pressure. The residue was
purified by silica gel column chromatography (solvent:
chloroform:methanol=lo:l) to obtain 43.6 g (yield: 95~)
of yellow oil of N-[3-(3-piperidinomethylphenoxy)propyl]-
hydroxyacetamide (X).
(4~ Synthesis of N-carbobenzoxy-trans-p-aminomethyl-
hexanecarboxy chloride
O O
NH2-CH2- ~ COOH + ~ CH2OCCl -> ~ CH2OCNHCH2 ~ COOH
(XI) (XII) (XIII)
CH2OCNHCH ~ COCl
(XIV)
Tranexamic acid (XI) (0.25 mol, 39.3 g) was
dissolved into 100 ml of 2 N sodium hydroxide and the
solution was stirred under ice cooling, following which
carbobenzoxy chloride (XII) (0.3 mol, 51 g) was added
dropwise over 1 hour. The mixture was allowed to become
room temperature, followed by the addition of 5 N
hydrochloric acid to adjust the pH to 3. The resultant
reaction mixture was ex~racted with 300 ml of
dichloromethane, the extract was dried over anhydrous
magnesium sulfate, and dichloromethane was evaporated
under reduced pressure. 65 g of crystals of compound
(XIII) obtained by recrystallization of the residue in
chloroform was dissolved in 100 ml of anhydrous
chloroform, and, after the addition of 50 ml of thionyl
chloride, refluxed for 1.5 hours. The reaction mixture
was distilled under reduced pressure and the residue was
recrystallized in chloroform to obtain 54.4 g of N-
carbobenzoxy-trans-p-aminomethylhexanecarboxy chloride
(XIV) (yield: 80%). m.p. 79C.
(5) Synthesis of 2-(N-carbobenzoxy-trans-p-aminomethyl-
hexanecarboxy)-N-{3-[3-(1-piperidinomethyl)phenoxy]-
propyl}acetamide (RTP)
-N ~ + ClCO ~ CH2NHl-OCH
OCH2cH2cH2NHc CH20H
(X) (XII)
CH2-N ~
--> ~ ` O O O
, . `OCH2CH2cH2NHccH20c{~cH2NHc_ocH2~
(II)
CH2-N
_~ ~ ~ o o
OcH2cH2cH2NHccH2oc{}cH2NH2
(I)
N-[3-(3-piperidinomethylphenoxy)propyl]hydroxy-
acetamide (X) (0.012 mol, 3.84 g) was dissolved into 100
ml of anhydrous benzene, and, aftei the addition of
triethylamine (0.024 mol, 2.1 g), the mixture was stirred
under ice cooling. 6.2 g (0.02 mol) of N-carbobenzoxy-
trans-p-aminomethylhexanecarboxy chloride (XIV) dissolved
in lOOml of anhydrous benzene was added dropwise over 1 hour.
The mixture was allowed to become room temperature and
stirred for 30 minutes. The deposited crystals were
collected by filtration to obtain 5.4 g (0.0094 mol) of
light yellow paste of 2-(N-carbobenzoxy-trans-p-
aminomethylhexanecarboxy)-N-{3-[3-(1-piperidinomethyl)-
phenoxy]propyl}acetamide (II). IR, NMR and mass spectra
of the compound thus obtained are as shown in Figures 4-
6.
(6) Synthesis of 2-(trans-p-aminomethylcyclohexane-
carboxy)-N-{3-[3-(1-piperidinomethyl)phenoxy]propyl}-
acetamide
2-(N-carbobenzoxy-trans-p-aminomethylhexanecarboxy)-
N-{3-[3-(1-piperidinomethyl)phenoxy]propyl}acetamide (II)
was dissolved into 10~ palladium/carbon solution in ethanol
16
Q ~
and catalytically hydrogenated by hydrogen gas at room
temperature under atmospheric pressure. Palladium/carbon
was removed by filtration and the filtrate was evaporated
under reduced pressure to obtain an oily product, which
was developed on a thin layer chromatography plate using
a chloroform-methanol (1:1) solvent as a developer. The
spotted portion was scraped and extracted with methanol.
The solvent was removed by evaporation under reduced
pressure to obtain 2.5 g (0.0056 mol) of 2-(trans-p-
aminomethylcyclohexanecarboxy)-N-{3-[3-(I-piperidin
methyl)phenoxy]propyl}acetamide as a light yellow or
colorless oily substance (yield: 60%). IR, NMR and mass
spectra of the compound thus obtained are as shown in
Figures 1-3.
Example 2 (Pharmacological activity)
Experiments were carried out to investigate the
pharmacological activity of Compound RT using ulcer
models in Wister male rats. Comparative tests were
performed using commercial antiulcer drugs; Cetraxate
(trade mark) and Roxatidine (trade mark), each having the
following chemical structure.
o
Cetraxate: HOOCCH2CH2 ~ OC ~ CH2NH2
Roxatidine: ~ N-CH2 ~ OCH2CH2CH2NHCCH2OH
3 ~
TABLE 1
ED50 values (mg/kg) in rat ulcer models
Cetraxate Roxatidine
Hydrochloric acid-ethanol60 40
induced ulcer
Water immersion restrained
stress induced model 200 25
Water immersion restrained stress induced model
Groups of Wister male rats weighing 250-270 g, each
group consisting of four rats, were fasted for 24 hours
and subjected to the experiment. Cetraxate and
Roxatidine, each suspended in 0.5% carboxymethyl
cellulose (CMC), were administered to the rats using a
per os probe, in an amount of 0.4 ml/100 g. 10 to 20
minutes after the administration, the animals were placed
in an stress cage (manufactured by Natsume Manufacturing
Co.) and dipped into water in a thermostat water bath at
21C to a depth of their processus xiphoideus to give a
stress. After 7 hours, rats were taken out from the cage
and sacrificed to cut out their stomach. 10 ml of a 2%
formalin solution was charged into each stomach and the
stomach was fixed in the solution for 10 minutes. After
fixing, the stomach was opened along the greater
curvature, and its mucosal surface was roughly washed
with water. The length of hemorrhagic mucosal damages
18
produced along the gastric glancl was measured. The total
of such length (mm) in each animal was taken as an index
for the ulcer production. The ulcer suppression rate (~)
was determined based on the index applying the following
formula.
[(Ulcer index for the control)-(Ulcer index for the
tested animal)]/(Ulcer index for the control)xlOO
The resulks are shown in Table 2.
TABLE 2
Antiulcer activity of Compound RT in water
immersion restrained stress induced model
Drug Dose (mg/kg) Ulcer index (mm) Suppression (%)
. _ . _ . . .
Control 10.3 + 4.5
Compound RT 36 0.7 + 0.5 93.2
Cetraxate 25 6.0 + 3.3 41.7
Roxatidine 25 2.5 + 1.6 75.7
_. _ . . _
(2) HYdrochloric acid-ethanol induced ulcer
Groups of Wister male rats weighing 200-230 g, each
group consisting of four rats, were fasted for 24 hours
and subjected to the experiment. Cetraxate and
Roxatidine, each suspended in 0.5% CMC, were administered
to the rats using a per os probe, in an amount of 0.4
ml/100 g. 30 minutes after the administration, 1 ml (per
rat) of 60% ethanol containing 150 mM HCl was
19
administered using a per os probe at the same intervals
as the test compound. After 1 hour, rats were sacrificed
to cut out their stomach. 10 ml of a 2~ formalin
solution was charged into each stomach and the stomach
was fixed in the solution for 10 minutes. After fixing,
the stomach was opened along the greater curvature, and
its mucosal surface was roughly washed with water. The
length of hemorrhagic mucosal damages produced along the
gastric gland was measured. The total of such length
(mm) in each animal was taken as an index for the ulcer
production. The ulcer suppression rate (%) was
determined in the same manner as above.
The results are shown in Table 3.
TABLE 3
Antiulcer activity of Compound RT in hydrochloric
acid-ethanol induced ulcer model
DrugDose (mg/kg) Ulcer index (mm) Suppression (%)
Control41.0 + 10.9
Compound RT 58 5.1 + 5.5 87.6
Cetraxate 40 35.9 + 14.7 12.5
Roxatidine 40 14.7 + 9.9 64.2
In the both ulcer models, the novel compound RT of
the present invention exhibited a lower ulcer index and a
higher rate of suppression than Cetraxate and Roxatidine,
indicating its high antiulcer activity.
(3) In order to more clearly identify the activity of
Compound RT, both Roxatidine and Cetraxate hydrochloride
were administered altogether. Since tranexamic acid is
hardly absorbed by gastrointestinal tracts due to its
abundant solubility in water, Cetraxate hydrochloride was
used in its place. The experiments were performed by
administering a dose corresponding to the ED50 value of
Roxatidine. The results are shown in Tables 4 and 5.
TABLE 4
Antiulcer activity of Compound RT in hydrochloric
acid-ethanol induced ulcer model
. _ . .
Drug Dose (mg/kg) Ulcer index (mm) Suppression (%)
Control 41.0 + 10.9
Compound RT 58 5.1 + 5.5 87.6
Cetraxate ~ 40 4.9 + 5.5 88.0
Roxatidine
. _ _ _ . _
TABLE S
Antiulcer~activity of Compound RT in water
immersion restrained stress induced model
_
Drug Dose (mg/kg) Ulcer index (mm) Suppression (%)
Control 10.3 + 4.5
Compound RT 36 0.7 + 0.5 93.2
Cetraxate + 25 6.8 + 2.5 66.5
Roxatidine
As can be seen from the Tables, the activity of
.3~ a~ ~
Compound RT against the hydrochloric acid-ethanol induced
ulcer was almost the same as the case where both
Roxatidine and Cetraxate hydrochloride were administered
altogether. This is presumed the activities of Cetraxate
hydrochloride and Roxatidine, having the ED50 value of 60
mg/kg and 40 mg/kg, respectively, were exhibited
synergistically to promote the same degree of effect as
Compound RT. On the other hand, Compound RT exhibited a
remarkably greater effect on the suppression of the acute
gastroduodenal ulcer than the combined use of the two
comparative drugs, demonstrating that its effect is not
due to simple synergism.
Example 3
10 g of Compound RT, 45 g of perfiller-101, 42 g of
carboxymethyl cellulose, and 3 g of magnesium stearate
were blended and made into granules according to a
conventional method. The granules are orally
administered in an amount of 1-3 g/day dividedly several
times a day.
Obviously, numerous modifications and variations of
the present invention are possible in light of the above
teachings. It is therefore to be understood that within
the scope of the appended claims, the invention may be
practiced otherwise than as specifically described
herein.