Note: Descriptions are shown in the official language in which they were submitted.
' W092/05189 1 PCT/SE91/~628
2~0~:~9 t
ANALOGUES OF CYCLOLINOPEPTIDE A AND THE USE TH~QE
The present invention relates to new analogues of Cyclolino-
peptide A, in particular analogues having a cyclic formula
derived from the sequence of Cyclolinopeptide A, which se-
quence is extended between any two amino acid residues with a
defined cystine derivative residue. Additionally, the inven-
tion relates to the analogues of the invention for use a6 a
medicament, in particular for use a9 an immunosuppressive
agent. Furthermore, the invention relates to the use of the
analogues of the invention for the preparation of a medica~
ment for immunosuppressive treatment, to pharmaceutical pre-
parations comprising an analogue of the invention, and a
method of treating a mammAl, including man, in need of
immunosuppressiVe treatment.
BACKGROUND
Cyclolinopeptide A w~s first ~solated from linseed cake by
Kaufmann H.P. and Tobschirbel A., in 1959 ~Chem. Ber., 92,
2805 ~1959)) and ~t8 chemclal Ltructure was suggested ln 1966
by Prox,A. and Weygand, F. t~1967) in "Peptides: Proceedings
of the 8th European P-ptide Symposium", Beyerman, H.C., van
de Linde, A. and van den Brink, W.M. Ed~., North Holland,
Am~t-rdam, pp. 158-172~. The chemlcal structure of Cyclol~no-
p-ptid- A i~ :~
Pro-Phe-Phe-L ~
¦ le
Pro-Val-Leu-11e ~
K---ler, H., et al ~An9. Chemie Int. Ed. 25, 997-999 ~1986))
how-d that natural Cyclolinopeptide A inhibits the uptake of
cholate into hepatocyte-, and stated that, to thelr know-
ledge, this was the first biological activity that have been
found for this natural product.
SUBSTITUTE SHEET
W092/05~89 2 PCT/SE91/~K~
t~
2~8~9
However, it wa~ surprisingly found that Cyclolinopeptlde A
can be used a~ an immunosuppressive agent, and this has been
dificlosed in our previou~ Swedlsh patent application number
ô904640-4 filed December 23, 1988 (corresponding PCT
application PCT/SE89/00732)
Immunosuppressive treatment of a patient in need thereof re-
quires large amounts of the immunosuppressive agent used
When Cyclolinopeptide A is synthesized according to the
method used in our prevlou~ patent application, 60-70X of
the linear peptide 18 lost durlng the cyclization I~ 18
evident that large scale production of Cyclolinopeptide A is
very expensive, and lndustrial production of Cyclolinopeptlde
A is thus deferred
Surprisingly, we found that the immunosuppressive actlvity of
Cyclolinopeptide A is retained when the sequence thereof is
extended by a cystlne derlvat~ve residue The new analogue6
of Cyclolinopeptide A enables lar~e scale production thereof,
slnce ln the cycllzatlon ~tep practically no linear pept~de
is lost
DESC~IPTION OF THE INVENIION
In one aspect ot the lnventlon there is provided new
analogu-s of Cyclolinopeptide A havln~ a cyclic formula
d-riv-d from the eguence of Cyclolinopeptide A
Val-Pro-Pro-Phe
he
eu-lle-lle-Leu
.
... . . . , .. ~....... .
' ' ~
WO92/05189 ~ PCT/SE91/00628
,~. 2~9V535~
which sequance i~ extended between any two amino acid
residues with the followlng cy~tine derivative residues
C=O N~
I
X-C-H H-C-Y
I
; CH2 CH2
~0
S S -, :
in which X is ~elected from -H and -OH and
Y is selected from -C=O and -H
NH2
wherein the amino acid residues are of L- or D-configuration.
.~
In a preferred embodlment of thls aspect of the inventlon the
above cystine derivative residue is located between the amlno
ac~d residues Leu and Phe ~n the formula of Cyclollnopept de
A. In fact the locatLon of said cystine derivative residue ir.
the sequence Or Cyclol~nopeptlde A was randomly chosen. Thus
lt can bo expocted that the immunosuppressive activity of
~yclolinop-ptido A ~ r-ta~ned ~rrespect~ve of between whlch
two amino acid residue~ in the sequence thereof the cyst-ne
derivatlve ree~due ~ ntroduced.
In an additional aspect of the invention there is provided an
analogue of the ~nvent~on for u~e a~ a medicament.
In another aspect of the invention there is provided the use
of an analogue of the ~nvent~on for the preparation of a me- :
dicament for immunosuppressive treatment.
SUE~STITUTE SHEET
... , . . . ~ . . : ~ ,
`~: . , : ` , : . : :. -
. ~ . . .
WO92/05189 4 PCT/SE91/00628
8 ~ 9
In still another aspect of the invention there i~ provided a
method of treating a mammal, including man, in need of
immunosuppressive treatment, comprislng administering to sald
mammal a pharmacologically effective amount of an analogue of
the invention
In yet another aspect of the invention there i8 provided a
pharmaceutical preparation comprising an analogue of the in-
vention together w~th phnrmaceutically acceptable carrier(s),
excipient(s) and/or diluent( 8 ) . h
The amount of an analo~ue of the ~nvention to ~e administered
to a mammal in need of immunosuppressive treatment has to be
decided by a phys~cian who 18 experlenced in immunosuppres-
~ive therepy
The pharmaceutlcal preparation compri~ing an analogue of the
invention can be formulated into oral preparations or prepa-
rations for infuslon using pharmaceutically acceptable
carrier(s), excipient~s) and/or diluent(s) suitable for such
preparations, but k--pin~ in mlnd that said analogue i8 ln-
oluble in water The pharmaceutical pr-paration can e g
compri-e an analogue of the lnventlon ln a pharmacolo~lcally
tt-ctiv- amount urp-nd-d in an olive oil
The pr-~en- ~nvent~on r-v-al- hat analogues of the lnventlon
ha~- immuno-upPre-sive activity, which i8 comparable to that
of Ciclosporin ~al-o call-d Cyclo-porin A) and Cyclollno-
p-ptid- A Accordin~ly, the analo~ues o~ the inv-ntion are to
be u~-d in medlc-m-nt- for immuno-uppre--ive treatment of
mammal-, including man, for all indications where immuno-
suppre--ive treatment 1~ warrant-d Examples of when lmmuno-
uppr-~-ive treatment i- warranted is for the prevention of
allogratt reJectlon aft-r or9an transplantation and bone
marrow transplantation, prophylactic or therepeutic treatment
ot graft-versus-ho~t-dlsease ~GVHD), and autoimmune dlsease6,
~. . -: : . -
.. ~ .
W092/05189 5 PCT/SE91/00628
~, ,?;
2~8~9
such as systemic lupus erythematosus (SLE), rheumatoid
arthritis (RA) and diabetes mellitus.
SHO~ DES~R~'rION OF THE DRAWINGS
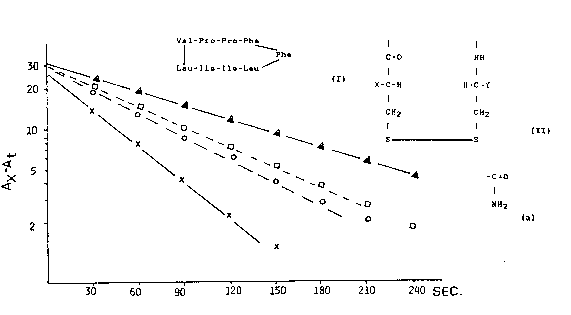
Fig. 1 shows a graph of the inhibition of PPIase actl~ity by
Cyclosporin A.
Fig. 2 shows a graph of the inhibition of PPlase actlvity by
CLA, LA, and 1-Mpa-LA-Cys-NH2.
SYNTHESIS OF CLA ANALOGUES OF THE INVENTION
The CLA analogues`of the invention can be produced by any
known method in the art of peptlde chemistry, whereby a
linear peptide i8 formed, which is then subjected to an
oxidatlon step ln order to cycllze two cysteine related
residues, whereby ~ sulphur bridge is formed between said
residues, thus formlng a cystlne derivative residue.
The linear linopeptide ~LA) analogues according to the inven-
tlon can thus be synth-s~z-d ln conventional manner e.g. by
~tep wi~e coupling of one amino acid residue to the next in
liquid pha~e, e.g. accord1n~ to the method of Law, H.B. 8 Du
Vigneaud, V.:J. Am. Chem. Soc. 82 ~1960) 4579-4581; Zhuze,
A.L., Jo~t, K. Kasafir-k, E. ~ Rudin~er, J.: Coll. Czecho-
lovak Chem Commun. 29 ~1964) 264B-2662, modified by Larsson
r .-E., Lindeberg, G., Melln, P. ~ Pliska, V.: J. Med. Ch-m.
21 ~1978) 352-356. The coupling of amino acid residues to one
another, whereby ~o call-d peptide bonds are produced may
also be performed starting with a solid phase ~usually a
re~in) to which the C-terminal of the first amino acid ~8
coupled, whereupon the C-terminal of the next amino acid is
coupled to the N-terminal of the flrst amino acid, etc.,
finally releasing the built-up peptide from the solid phase.
In the example6 below thls 80 called rolid phase techn~que
SU13STITUTE SHEET
~ . :
~ 5~ 6 PCT/SE91/~628
has been utilized in accordance with the method of
Merrifield, R.~.; J. Am. Chem. Soc. 05 ~1963) 2149;
Merrifield, R.B.: Biochem. 3 (1964) 1335 and Konig, W. 8
Geiger, R.: Chem. Ber. 103 ~1970) 758.
GENER~L DESCRIPTION OF SYNTHESIS
All the peptides in the examples below were synthesized on an
Applied Bio~ystems 430A Pept~de Synthesizer using a double
coupling program with termination step after the second
coupling; The resln used was of 4-methyl-benzhydrylamine type
with a theoretical loading of 0.66 meq/g ~Peptides Interna-
tional, Louisville, KY, USA). The flnal product of the syn-
thesis was dried in vacuo over night. The peptide was then
cleaved from the re~in by treatment with liquid hydrogen
fluoride in the presence o anisole and ethyl-methyl-sulfide
as scavangers ~HF:anisole:EMS - 10:05:05). After removal of
hydrogen fluoride by evaporation the residue wa6 ~uspended ln
ethyl acetate ~100 ml) and flltered. The solid was washed on
~ilter with addltional ethyl acetate ~3 x 100 ml) and the
cleaved pept~de extracted with acet~c acid ~100 ml). The
extract was promptly diluted to a volume of 2 cm3 with 20X
acetic acid ln methanol and trested w~th 0.1 M solut~on of
iodine ln methanol until the fa~nt brown colour persisted.
Th-n the Dow-x 1 x 8 ~on exchanger ~n acetate form was added
~3 ~) ~3io-Rad, Richmond, CA, USA~ and the mixture was
filter-d. She filtrate was evapora-ed and the re-idue freeze-
-dri-d from 1~ acetic acid in water. The product was then
purifi-d by rever~-d pha-e liqu~d chromatography on a column
~ d with Vydac 20-25 ~ tSeparation Group, CA, USA~ in a
~uitable system containinp acetoni-r~le in O.lX trifluoro-
acetic acid water olution. The samples collected from the
column were analyzed by analytical HPLC ~Varian 5500, Sunny-
vale, CA, USA) equipped with a ~ondapak C1g column
~Millipore, Milford, Ma~s., VSA~. Fractions containing pure
~ubstance were pooled, the ~olvent was evaporated and the
SUBSTITUTE SHEET
.. . .
. . .
- .
,. . ` , ~ . ..
WO92/05189 7 PCT/SE9l/00628 7
} ~ ! .
product freeze-drled from lX acetic acid in water. The final
HPLC analysi~ was performed on ready product and the struc-
ture of the peptlde in question was confirmed by amlno acid
~nalysis and FAB-MS ~Fast atom bombardment spectrometry). The
FA~-MS analyses were performed by M-Scan Ltd. Sunnln~hill,
Ascot, Berkshire, England.
All amino acids used during the synthesi~ were protected with
tert-butoxycarbonyl group at -amino function. The thiol
function of Mpa, Hmp and Cys wa~ protected with 4-methoxy-
bensyl group. The amino acid derivative~ were dellvered by
Bachem AG, Switzerland.
~ .
In the Examples below the abbreviation LA is used for the
linear peptide Leu-lle-lle-Leu-Val-Pro-Pro-Phe-Phe
EXAMPLE I
1-Mpa-LA-Cy6-NH2
1 1
The peptide was prepared accordlng to the gener~l de~crlptlon
of synthesi6. 3-Mercaptopropionic acid tS-~p-methoxy-)benzyl~ -
was used for po~ltion 1.
2S
Pur~ty ~HPLC):99. ax
The sructure was conf~rmed by am~no acid analy~is and by FAB-
MS. tM~H~ = 1246.
EXAMe~E 11
1-Mpa-LA-D-Cys-NH2
SUBSTITUTE SHEET
W092/05189 8 PCT/SE91/00628
2~9~38~9
The peptide wa~ prepared according to the general de~cript~on
of ~ynthesis. 3-Mercaptopropionic acid ~S-~p-methoxy-)benzyl]
was used for positlon I and Boc-D-cysteine
~-(p-mothoxy-)-benzyl] for po6ition 2.
s
Purity ~HPLC):95.7X
The structure was confirmed by amlno acid analy6i6 and by
FA~-MS. CM+H]+ = 1246.
EXAMPLE III
l-Hmp-LA-Cys-NH2
The peptide was prepared accord~ng to the general descrlptlon
ot ~ynthesis. 2-Hydroxy-3-mercaptopropionic acid was used for
position I.
Purity ~HPLC):97.5X
The structure wa6 conflrmed by amino acid analysls and by
FAL-MS. ~M~H~+ = 1262.
EXAMPLE 1V
l-Mpa-D-LA-D-cys-NH2
The p-ptide was pr-par-d accordlns to the goneral descript~on
Or synthesis using amino acids of D-configuration.
3-Mercaptopropionic acld tS-~p-methoxy-)bezyl~ was used for
postion 1.
Purity ~HPLC~:96.0X
The structure wa6 conflrmed by amlno acid analysi~ and by
FAL-MS: tM~H~ = 1246.
~U~STITUTE SltEET
. , . ~ ., ~
. ,. . .. ~.. . ` .
.
- ..
WO92/05189 9 PCT/SE91/00628
2~08~9 ~
LIST OF COMPOUNDS USED AS ENZYME INHIBITORS AND AS IMM~NO-
SUPPRESSIVE AGENT
CS-A = Cyclo~porln A ~Referenc8)
CLA = cyclolinopeptide A (Reference)
1-Mpa-LA-Cy~-NH2 Leu-Val-Pro-Pro-Ph ~
¦ ¦ = Phe
/ .: ,'
Ile-Ile-Leu-Mpa ~ s-NH2
~5~Compound of Example 1)
1-Mpa-LA-D-Cys-NH2 Leu-Val-Pro-Pro-Phe
I ¦ = Phe . -
I: Le-Ile-Leu-Mpa D-Cys-NH2
~Compound o~ Ex~mple 2~
1-Hmp-LA-Cys-NH2 L-u-Val-Pro-Pro-Phe
¦ ¦ _ Phe
1. .e-lle-Leu-Hmp Cys-NH2
~Compound of Example 3~
SUBSTITUTE SHEET
W092/05189 10 PCT/SE91/00628
2~9~8~9~-~
l-Mpa-D-LA-D-Cys-NH2 D-Lju-D-Val-D-Pro-D-Pro-D-Phi . ,
D-Ile-D-Ile-D-Leu-Mpa D-Cy~-NH ~ -
(Compound of Example 4)
wherein Mpa i~ 3-mercapropropionyl residue (-S-CH2-CH2-CO-)
and Hmp is 2-hydroxy-3-mercaptopropionyl residue
OH
I
(-5-CH2-CH-CO-)
INH181TION OF THE ENZYME PPlase
It i~ known that the peptidyl-prolyl cis-tran~ isomerase
~PPIase~ catalyses the cls-trans ~somerization of prollne
imidic peptide bonds in oligopeptides and Nobuhiro Takahashi,
et al (Nature vol. 337, Letters to Nature, pp 473-475, 1989)
suggested that the peptidyl-prolyl cis-trans isomeriz~ng ac-
t~v~ty of PPIa~e may be ~nvolved ln events, such a~ those
occuring oarly in T-cell activation, that are suppressed by
Cyclorpor~n A. Gun--r F~scher, et al ~Nature Vol. 337,
L-tters to Nature, pp 476-478, 1989) reported that PPIase ~8
probably identical to cyclophilln, a recently dlscoverod
mammalian protoin which binds tightly to Cyclosporin A. We
have purified PPS-~- and tosted analogues of the ~nvent~on a8
inhibitor~ of PPIase.
PURIFICATION OF PPla~e
The pig kidney cortox was homogenized in equal volume of
3accharose (0.2 M) and centrifuged 60 min. at 10,000 x 9. The
SUBSTITUTE SHEET
. ~ ~
. . ..
.. . ~ , . ` ,
,. .~ ,' .
W092~05189 11 PCT/SE91/~628 ~,
~ 2 ~ 9
supernatant wa~ ad~ueted to pH 5.5 by addition of ~ M acetate
buffer, pH 4Ø The precipitate was discarded and the ~uper-
natant wa~ brought to pH 7Ø
The extract was brought to 40X saturation of ammonium sul-
phate. To the supernatant obtained after 15 min. centrifuga-
tion at 20,000 x g solid sodium sulphate was added to get a
~inal ~aturation of 60X. The precipitate obtained by centri-
fugation was dissolved in a small volume of 10 mM Tri~
buffer, pH 7.6 and dialysed 24 h. again6t the same buffer.
The dialysed solution was loaded onto a DEAE-Sephadex A-50
column, that had been prevlously eguilibrated with the same
buffer and eluted without changing the buf~er. The eluate
rich in PP~a~e act~lty pa~ed through the column ln one
lS active peak.
The active fract~ons were appl~ed on a CM-Sephadex C-50
column and eluted with a linear gradient of KCl (0-1.2 M) as
one actlve peak.
The enzyme was etored as euspens~on in 60X saturated ammon~um
sulphate. After 5 month8 the enzyme shows full enzymatic
~ct~ity.
DETERMINAT~ON OF PPlase ACSIVITY
The ci~-trans iromerlzation of the Ala-Pro peptide bond of
the peptide N-succinyl-Ala-Ala-Pro-Phe-p-nitroanlllde waa
m-a~ur-d in a coupled assay with chymotryp~in. Substrate
~50 ~M~, prev~ously dic~olved in DMSO, wae equilibrated at
10C w~th the appropr~ate amount of PPlaee in the thermostat-
ed ~pectrophotometer cell. The reaction wa8 started by adding
chymotryp~in (f~nal concentration 21 ~M). The trans-peptide
was cleaved within the d-adtime. The rate of cis-trans isome-
rizat~on was followed by the decrease in tran~m~ttance at
390 nm. The ~pectrophotometer Specord UV-Vic ~Jena, GDR~ :~
eguipped with a cell stirrer was used.
;, . . ,. ;. . , . ' ....................... , . ,. :, . :.
- ... . i ., . : . ~ .. .. . .
:, ~. , : '
W092/05189 S2 PCT/SE91/~628
'~ 0 ~
The effect of the lnhibitors on PPIase was examined by mixing
the studied sub~tance with PPIase for 1 min. before incubat-
ing the ~ubstrate The substances tested were dissolved in
dioxane containing 0.5 - lX of Triton X-100 or in DMSO. There
was no difference ob~erved in the effect on the PPIase acti-
vity depending on the kind of solvent.
The result~ are given in Fig. 1 and Fig. 2, where the log of
the difference between transmittance at ~teady state Ax and
transmittance at a given time At was plotted against time.
The first order rate constant k ( 5-l ) was calculated from the
resulting straight lines.
IMMUNOSUPPRESSIVE ACTIVlTY OF CLA, CS-A AND THE ANALOGUES
OF THE ~ NVENTION.
Material~ and methods
Animals: CBA/Iiw mice ~ - 10 wee~ old
Antlgen: SR8C ~sheep red blood cells)
Reagents: 1-Mpa-LA-Cys-NH2, 1-Mpa~ -LA-D-CT8-NH2~
1-Mpa-LA-D-Cyr-NH2, and CLA were prepared by
FERRING AB, Sweden, and Cyclosporin A ~CS-A), Sand~mmun
Sandoz, Ba-el, Switzerland
Solv-nts: Mixture of Cr-mophor EL ~SIGMA) and 94X ethanol
~6.5 : 3,5~, eth~nol 96X, olive oil, intralipid
and PBS ~pho~phate buffer ~olution~
T~QatmQnt çf mice with reaoent intraPeritoneallv ~i~.) or
ntravenouslv ~iv.):
The reagents dissolved ~n one of solvents were diluted to
desired concentration in PBS or in intralipid, and 0.2 ml
SUBSTITUTE SHEET
~. ~
t
' , ' ' ' '; ' ': '
.: ",'. ' ~ .' ~' `'
W092/05189 13 PCT/SE91/~628
~r
~39~ 9
thereof wa~ introduced lp in two dose~ First 3 hr~ before
the antigen, second 24 hrs later The activity of analogues
studied wa~ compared to the activity of CLA and CS-A
S Treatment of mice with ~ea~ents Der 09 (PO.). directlv into
the ~tom~ch
The reagents dis~olved in one of the solvents diluted to
desired concentration in olive oil, PBS or in intralipid was
introduced in the volume of 0 2 ml in to the animal First
dose 3 hrs before the antigen, second dose 24 or 4~ hr~ ~ -
later
The activity of the derivatives studied was compared to the
activity of CLA and CS-A
.:
Effect of CLA analoaues on humoral immune resDonse in mlce
immunized with SRBC
Mice were injected lntraperitoneally wlth 0 2 ml of lOX sus-
pension of SRBC in PBS, 3 hrs before the antigen the first
dose of the reagen- WaB introduced, ip , po or iv After 4
days the number of plaque forming cells (PFC) in the spleen
was detsrmined accordlng to the Mishell-Dutton <Mishell R
1 , Dutton R W J Exp Med , 1967, 126, 423) The magnl-
tude of th- humoral lmmune resPOnSe wa8 expressed as the num-
b-r ot PFC p-r 106 ~plenocytes The results obtained are al-
ven in table~ A, B and C
Ett-ct of CLA analoau-s on humoral immune re~Donse to SRBC ln
vitro
Priming ot mice and l-olat~on of spleen cell~ Mice were
primed intrav-nously with 0 2 ml of lX 8uspension of SRBC in
PBS Four days later the an~mals were killed and thelr
spleens were minced, pres~ed through a pla8tic screen into
0-~3X NH4Cl buffered with 0 017 M Tris buffer to remove
SU~STITUTE SHEET
. ., ., . .. . . - .. ,; . .
, `. . . .~ . . .
.
.. . . . .
,. , . .. - , :
.. ; . ~ .:
- , ~ . .
` . , - . . . ~ ,,
WO92/05189 14 PCT/SE91/00628
2'~3~9
erythrocytes. Then the cell~ were washed three time wlth PBS
and finally resu6pended in RPMI medium supplemented with lOX
of fetal calf serum.
Culture conditions and determ~nation of PFC num~er:
To one milliliter of spleen cell suspension (S x 106
cells/ml) 0.1 ml of the reagent was added followed by addl-
tion of 0.1 ml of 0.005X suspension of SRBC in the cell cul-
ture medium, and incubated for 4 days at 37C, S~ of C02 and
lOOX of humidity. The number of PFC was measured using the
procedure of Mlshell-Dutton. As a control PBS or approprlate
concentration of the 601vent was u6ed. The experiments were
performed using NUNC 24-well Tissue Culture Plate.
The result6 are 6hown in Tables D and E.
Effect of CLA analoaues on delaved tY~e hvDersensitivitv
(DTH)
The re~ults and de~criptlon of the test are given ~n Table F.
ImmunosuDDresslve actlvitY of CLA analoaues in
Graft-versus-host reaction (GvH)
Anlmals: Hybride Fl (C3H~I~w x 86/Iiw~ and female 86 mlce,
~-10 weeks old.
Pr-para~lon of parental cells:
F-male B6 mice were killed, their popliteal nodes were
pre--ed through a pla~tic ~creen lnto PBS. Then the cell~
were wa-hed three times with PDS ~nd re~u~pended in RPMI
medium.
SUBSTITUTE SHEET
. .
.. ~ . ~ - -.
.. . . . ~ . . i
. . ...
. . . .
W092/05189 15 PCT/SE9~628
f
i............................................................................. .
GvH test:
GvH reaction was perormed according to Twist and Barnes
~Twist V.S., Barnes R.D.: Transplantation, 197~, 15, l9B2).
Hybride mlce, F1 ~C3H x B6) were injected subcutaneously in
the left hind footpad with 5 x 106 parental lymphoid cells
tB). After 7 days, drainlng lymph node6 were isolated and
weighed. As a control, the weight of popliteal node isolated
from the right leg was measured.
The intensity of GvH reaction was expressed as a ratio of
weights of popliteal lymph nodes isolated from the left and
right legs ~index of GvH reactlon). The results are given ln
Table G.
SUBSTITUTE SHEET
_ ............ . . .. . . .
.~ - . , . -.`--; ` `.`,, ., ~ :
. - . ` ~ . ~ . . ..
!, : . .
'
- WO92/05189 16 PCT/SE91/00628
(~
085
Table A
The number of PFC in the spleen cell of mice treated intra-
peritoneally with two doses ~-3 hrs and + 24 after SR~C) of
1-Mpa-LA-D Cys-NH2, 1-Mpa-LA-Cys-NH2 and CLA dissolved in
Intralipid ~ tint.) ~A fatty emul~ion for intravenous
nutritlon from Kabi, Stockholm, Sweden)
~5
Reagent P
~s/mouse PFC~106 I SE Student test
Control int. 3047 35~ -
l-Mpa-LA-D-Cys-NH2 1.0 1139 162 0.01
I I 10.0 5~6 77 0.001
1-Mpa-LA-Cys-NH21.0860 64 0.001
~ _ I10.0 798 32 0.001
CLA 1.0 1649 339 0.05
10.0 864 74 0.001
Preparation and ~ntroduction of the substance, see explana-
tion after Table C.
The results are expressed as a mean ~ SE of 6 mice.
SUBSTITUTE SHEET
~ . - .. ... . ..
, WO92/05189 17 PCT/SE91/00628
~ I ' .
- .
~00~9
Table B
The number of PFC in the ~pleen of mice treated with two
doses ~-3 hr and 24 hr after SRBC~ of CLA, CS-A and analogue6
ot the in~ention dl~solved in olive oil, introduced per o~
~po. )
Reagent p
~g/mouse PFC/106 + SE StudPnt test
15Control olive oil 21~3 166
CLA 10 1016 125 0.05
100 314 73 0.001
20 CS-A 10 1092 209 0.01
100 753 51 0.001
1-Mpa-D-LA-D-Cys-NH2 10 602 112 0.01
I I 100 680 86 O.OOl
l-Mpa-LA-D-Cy~-NH2 lO 764 60 0.01
I I ~00 477 45 0.001
l-Mpa-LA-Cy~-NH2 10 687 136 0.01
30 1 î 1OO 501 109 0.001
The r--ults ~re expressed aY a mean ~ SE of 6 mice.
SUBSTITUTE SHEET
.-~ . . .. ~ . : . . : . .
. . . . ~ . . . . . . ' . . . . . .
.- . . . . . . . . . ..
. . . .. . ... . ..
- . . .
. , . . , . . - . ..
. ... ..... .. .. . . . ~ . .. .. . . .
WO92/05189 18 PCT/SE9l/00628
2~8~9
Table C
T~e number of PFC in the spleen of mi~e treated intravenou~ly
tiv.) with two doses o~ 1-Mpa-LA-Cy~-NH2, CLA and CS-A dis-
~olved in intralipid ~int.) I
Reagent p
~g/mouse PFC/106 + SE Stud~nt test
Control P~S 19O5 B2
Control int. 2326 67 NS
1-Mpa-LA-Cys-NH2 0.01 1649 126 0.05
1 1 0.11364 43 0.01
1.0 903 39 0.001
CLA 0.011943 77 NS
0.11475 97 0.02
1.01203 85 0.01
CS-A 0.011042 122 NS
0.11633 lel o . 05
1.0115e 50 0.01
i
Th- pr-parat-s in the volume of 0.2 ml were lntroduced twice,
rirst dose 3 hrs befor- sens~tizatlon with antigen second 24
hrs later. Preparation o CLA and 1-Mpa-LA-Cys-NH2: 2 mg of
th- substance was di-solv-d ~n 0.15 ml of hot ethanol and
0.35 ml of intralipid, then diluted to desired concentration
in intral~pid.
CS-A was diluted directly ~n intralipid to desired concentra-
~5 tion. In the control 0.2 ml of PBS or intralipid was intro-
duced.
The re~ults are expres~ed a~ a mean + SE of 6 mic~.
.- : , : , ' .:: . .::': . . :. :, ' ,, : . ' : : ' : :
WO~2/05189 ~9 PCT/SE91/00628
2 1Sa ~
Table D
The number of PFC ln the spleen cell cultures treated with
CLA, CS-A ~nd analogues of the invention dissolved in
intralipid ~int.)
.. . . . .. _
Reagent P
~g/well PFC/106 I SE Student test
Control PBS 4212 404
Control int. 4495 26B NS
CLA 0.012879 236 NS
lS 0.~865 27 0.001 :
1.0537 ~0 0.001
CS-A 0.013020 184 NS
0.1892 31 0.001
1.0569 84 0.001
1-Mpa-D-LA-D-Cys-NH2 0.01 3047 370 NS
I 1 0.1217B 306 0.05
1.0841 B9 0.001
ZS
I-Mpa-LA-D Cys-NH2 0.01 29B5 272 NS
I 1 0.13100 279 NS
~.0119 ~5 0.001
1-Mpa-CLA-Cys-NH2 0.0123BB 226 0.05
0.122~6 ~01 0.05
1.0~36 70 0.001
~9 - 65 17
The results are expressed as a mean + SE of 6 wells.
Bg - background
SU13STITUTE SHEET
. . .
.- .. .. ..
W092/05189 20 R~T~SE91~00628
Table E
2 ~ 9
Imunosuppressive activity of CS-A and analogues of the
invention mea~ured by the number of PFC. ~n in vivo studie~,
mice were tre~ted with two doses of the preparations
dissolved in cremophor or in olive oil. First dose ~ hours
before immunization, ~econd 24 hour~ later.
Reagent Route of P
~g/mouse introduc- PFC/106 + SE
tion
Control "C" i.p. 1330 lD9
1-Mpa-LA-Cys-NH2 1 ~g l.p. 1263 124 NS
1 1 10 ~3 i.p. 700 90 0.01
1-Hmp-LA-Cys-NH2 1 ~g i.p. 1178 111 NS
I I 10 ~9 i.p. 831 58 0.05
20 Control PBS i.p. 1186 109 - i
CS-A 1 ~9 ~.p. 1350 120 NS
10 ~9 i.p. 839 105 0.05
___________________________________________________________
Control "C" i.p. 1122 144
1-Mpa-LA-Cys-NH2 10 ~9 ~.p. 238 23 0.001
30 Control PBS i.p. 1056 95
CS-A 10 ~9 i.p. 124 36 0.001
___________________________________________________________ ~
Control "C" - cremophor in Appropriate concentr~tion need-d
to prepare 10 ~9 of the pept~de.
CS-A - dissolved in PBS.
SUBSTITUTE SHEET
. . . ~..... . , .. :, ~ . . ; :-: . :
WO92/05189 21 PCT/SE91/~628
~'~7.
Continued
_________________________________________________________
Control "Ol" p.o.2428 329
1-M~a-LA-Cys-NH2 10 ~9 p.o.1214 91 0.02
I I100 ~9 p.o. 406 73 0.001
CS-A 10 ~g p.o.1888 272 NS
100 ~9 p.o. 834 142 0.01
Control 'Ol p.o.1693 133
1-Mpa-LA-Cys-NH2 100 ~g p.o. 444 66 0.001
1-Hmp-LA-Cys-NH2 100 ~9 p.o.672 74 0.001
Control PBS p.o.1788 219
CS-A 100 ~9 p.o.561 .88 0.001
_____________________________________________________________
Control "C" ln v~tro 3690 321
1-Mp~-LA-Cys-NH2 1 ~g/ml in vitro 65 11 0.001
CS-A 1~g/ml in vitro 20 12 0.001
_____________________________________________________________
Control "Ol' - olive oil
SU~STITUTE SHEET
.. ~ . .:
- ~ . - ., . : ~ .
. .. - . ~ . .. . : . .- .
~ . ..
. : . .
W O 92/05189 22 PCT/SE91tO0628
~ !
~ ,.,i '
2 a ~ ~ Table F
The effect of CLA, CS-A and analogues of the invention dis-
solved in olive oil on delayed type hypersensitivity (DTH) in
mice Preparates were lntroduced per 08 ~po ) twice, first
dose 3 hours before sensitization, second 48 hours later
Reagent P
~g/mouse Vnits I SE Student test
Control olive oil 22 50 1 60
CLA 10 15 20 1 26 0 05
100 10 90 0 62 0 001
t
lS CS-A 10 13 5 0 86 0 01
100 10 2 0 95 0 001
1-Mpa-D-LA-D-Cys-NH2 10 12 6 0 70 0 01
I 1 100 10 00 0 71 0 001
1-Mpa-LA-D-Cy6-NH2 10 13 70 0 94 0 02
I 1 100 11 20 0 65 0 00l
1-Mpa-LA-Cys-NH2 10 11 7 0 50 0 01
l 1 100 8 50 0 71 0 001
On- unit = 0 1 mm
DTH was induced ln m~c- accordin~ to Lagrange et al ~Lagrange
P H , Mackaness G E , Miller T E , Pardon P J Immunol ,
1975, 114, 447) Mice were sensitized intra~enously with 105
SRBC. After 4 d~ys the react~on was elicited by an intra-
d-rmal introductlon of lo8 SRBC into left, hind foot pad The
ma~nitude of the reaction was mea8ured as the increa~e of the
foot pad thickness at 24 hrs following administratlon of the
challenging dose
The results are expressed as a mean ~ SE of 10 mice
SUBSTITUTE SHEET
:~.
W092/05189 23 PCT/SE9t/~628
f.~,,,,., .
~03~!~
Table G
Immunosuppressive activity of 1-Mpa-LA-Cys-NH2 and
1-Hmp-LA-Cy~-NH2 in GvH reaction (cellular immunity). `,
Preparations dissolved in cremophor were given i.p. -4 and 48
hrs before and after introduction of parental cells,
respectively.
,'
Reagent Route of GvH ~ SE P
~g~mouse introduc- (index)
tion
Control "C" i.p. 3.17 0.36
1-Mp~-LA-Cys-NH2 10 ~g i.p. 1.96 0.27 0.05
l-Hmp-LA-Cys-NH2 10 ~g i.p. 1.54 0.22 0.01
,
Control PBS ~.p. 3.09 0.42
2S
CS-A 10 ~g ~.p. 1.41 0.16 0.001
Control PBS - phorphat- buffer ~olution
Control "C" - cremophor at a conc-ntration needed to
di~olve 10 ~g of the peptide~ or 1 ~9
~in in vitro)
Control "Ol' - olive oil
SUt~STlTUTE SHEET
.
.
.
. . .. - . .: . , . ;~
.
W 0 92/05189 PCI/SE91/00628
2~9V~ 24 ~ I
Description of the drawings:
Fig.1 Inhibition of PPIase activity by Cyclosporin A.
Catalysis of proline isomerization by PPIase:
- X -X - without CS-Ak=26 X 10 3s 1
- O -O - 0.25 ~g CS-A 12.9 , i
o -O - 0.50 ~g CS-A 10.9
-A - 1.5 ~g CS-A 7.0
~ without PPIase 7.0
Fig.2 Inhibition of PPIase activity
Catalysis of proline isomerization by PPIase:
- X - X - without inhibitor k=52 x 10 3s
- O - O - with 1-M ~ ys-NH2 2~g 41
- - - LA - inactive 10~g 35
_ O - O - with CLA 20~g 24.1
without PPIase 7.5
SUBSTITUTE SHEET
, "
. .
.. . . . ~ . . ~ .. . -