Note: Descriptions are shown in the official language in which they were submitted.
W~ /()X7-l~ PCT/US91/~7~X~
92290
~EACTOR WITH FOAM SHEARING MEANS FOR SOLUTION
POLYMERIZATION PROCESS
The present invention relates to a process and
reactor design for the polymerization of compounds in a
nonaqueous system. More particularly the present
invention relates to a reactor which is operated
partially full. That is, a portion of the volume of the
reactor is vapor space. The vapor space additionally
contains an agitator which aids in reducing the build up
of foam uncler certain reaction co~ditions. The reactor
is particularly suited for use in an anionic
polymerization process.
Processes for the polymerization of compound~,
especially for the anionic polymerization of monovinyl-
idene aromatic monomers and mixtures thereof with
conjugated dienes are well known in the art. Because of
the exothermic nature of most polymerizations and
anionic polymerizations in particular, it is generally
necessary to remove heat from the reaction mixture. If
the temperature becomes excessive, undesirable chain
transfer, premature termination, or side reactions may
- occur. At reduced temperatures, the time for each
polymerization step to occur increases significantly,
thereby lowering productivity. Therefore, lower and
:
~`
~ . .
.~ .
~. ` .
.',' ' '. . ' :
WO')2/~)X7~ PCr/US9l/0748~
~ 0 9'~ 2 ' ~
upper reaction temperature limits are normally chosen
for optimum results. Because of such temperature
constraints, it is generally necessary to provide a
control system to regulate the temperature of
polymerization mixtures.
In ~nited States Patents 3,231,635; 3,681,304;
and 3,801,555 there are disclosed reaction processes for
the anionic polymerization of monomers that employ
ebullient temperature control systems. In these
processes, a relatively low boiling solvent is employed
and the heat of vaporization of the reaction mixture is
used to remove heat from the reaction medium. A
condenser in operative communication with the reactor is
employed to cool and condense the vaporized components
and return the same to the reaction mixture. The latter
two U.S. Patents particularly note the problems
associated with foam generation. Boiling reactors may
also be utilized in other polymerizations such as in the
~0 free radical polymerization of monovinylidene aromatic
monomers and/or alkenyl nitriles, especially in the
preparation of styrene~acrylonitrile copolymers.
Ebullient cooling methods are highly efficient
so that only small amounts of solvent need be used.
Disadvantageously however, the previously known
ebullient cooling processes have been limited in their
ability to polymerize re~ction mixtures due to the
formation of a tenacious foam under certain reaction
3 conditions which occupies the available vapor space and
enters the condensation equipment, thereby fouling the
; heat exchanger and rendering the system inoperable. In
other solution polymerization systems, such as free
~ radical, ring opening, cationic, condensation or
`` coordination polymerizations, foaming of the
.
W(~ X7~ PCT/US91/0748~
_3- ~9~
polymerization mixture may be equally undesired even in
the absence of a condenser.
Accordingly, there remains a need to provide a
suitable reactor for use in solution polymerizations,
particularly those operating under ebullient cooling
conditions, that provides a means to control the level
of foam.
It would also be desirable if there were
provided a polymerization process, particularly a
process employing ebullient cooling conditions, that is
characterized by a means for the control of ~oam
production.
According to the present invention there is
provided a reactor suitable for use in the
polymerization of polymerizable compounds, said reactor
comprising a vessel fitted with an inlet, outlet,
agitation means immersed in the liquid reaction mixture,
~!0 and condenser means for the conde~nsation and return of
volatile reaction components to t,he reactor, said
reactor characterized by the presence of a mechanical
means for imparting shearing forces to foam accumulating
in the vapor space of the reactor.
Also provided according to the present
invention is a process for the polymerization of
polymerizable compounds conducted in a reactor equipped
with vapor condensation means and operating at least in
part under ebullient cooling conditions with the
gencration of foam, characterized in that shearing
agitation is imparted to the foam in the vapor space of
the reactor sufficient to prevent fouling of the reactor
condensation means.
W~92/n87~1~ PCT/VS9l/0748~
2092'~90 ~ ~
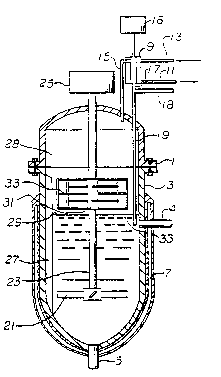
The invention is further illustrated by
reference to Figure 1 where there is illustrated a
vertically disposed reactor, comprising top, 1, and
bottom, 3, fitted with inlet, ~, outlet, 5. and a
heating jacket, 7; for circulation of a heat transfer
fluid for thermal control. Also fitted to the reactor
is a condenser, 9, supplied with circulating cooling
fluid through connections at 11 and 13. The condenser
is in operative communication with the reactor by means
of an inlet, 15, which allows vapors to enter the
condenser and discharge, 17, which allows condensate to
return to the reactor. It is understood that a pressure
regulating means, 16, may be employed to improve the
condensing system. Such pressure regulating means may
be a means to control the pressure of the reactor
contents such as a source af vacuum or venting,
?referably to a monomer scavenging and environmental
control system. Alternatively however, the pressure
regulating means may be a contro:L system to provide an
increased or reduced condensation rate by the condenser.
Additional monomer, initiator or other reaction
components may be added to the returning condensate
through line, 18, in operative communication with a drop
tube, 19, for discharging condensate, initiator, and
other reaction components beneath the surface of the
reaction mixture, 29, in order to obtain rapid dispersal
thereof.
The interior of the reactor is fitted with
agitation means, 21, turned by means of a shaft, 23,
connected to a source of rotational energy such as a
motor driven gear reduction unit, 25, to provide
sufficient mixing of the reaction mixture to ensure
substantial homogeneity thereo~. A foam shearing means,
.
.,, ~
.
. ~. -: , , , ~
~VO'J2/~l~7.~.~ PCT/US~1/07~8~
~5~ 2~2~0:
31, in ~he embodiment of the invention illustrated in
Figure 1, is also fitted to the shaft, 23, and moves in
the vapor space, 28, above the surface of the reaction
mixture to contact and cause shear degradation of foam
that forms in the vapor space. Baffles, 27, on the
inside surface of the reactor and in the vapor space
serve to improve mixing of the reactor contents and to
retard movement of foam in the vapor space to increase
the differential velocity between the foam which is
retarded by the baffles and the foam shearing means,
thereby increasing the shearing force imparted to the
foam. The foam shearing means (also referred to as a
foam breaker) comprises elongated members, 33, that are
spatially ordered and activated so as to provide
shearing forces to the foam that accumulates in the
vapor space. By disrupting the structure of foam formed
in the reaction, vapor is released from the accumulated
bubbles and may thereafter be drawn into the condenser,
and drainage of liquid back to the reaction mixture is
~ improved.
The elongated members o~ t,he foam breaker
assembly are desirably arranged to provide bracing and
rigidity, and the foam breaker is symmetrically balanced
with respect to the axis of rotation to reduce
vibrational forces when in use. The elongated members
preferably include bars of polygonal cross-section
(thereby providing edges for greater shear forces)
projecting radially from the same shaft used to activate
the agitator of the reactor as well as bars arranged
parallel to such shaft. The bars are joined together by
welding, me~hanical fastening or other means into a
unitary structure. The arrangement of the individual
elongated members is not critical to success, however,
~V(~ 7~ PCT/US91/0748~
2 n 9 '~ 6- ~
in a preferred arrangement for use in a substantially
cylindrical shaped reactor, the foam breaker preferably
includes bars that are positioned parallel to the axis
of rotation of the foam breaker and in close proximity
with the reactor wall or baffles. Preferably the
clearance between the foam breaker and the outer wall,
or baffles located on the outer wall, is from 1 to 50
mm, more preferably from 5 to 15 mm. The best shearing
effect on foam is obtained at clearances within this
range.
While the foam breaker may be located ln any
portion of the vapor space of the reactor, most
desirably it is located in the region closest to the
1~ surface o~ the reaction mixture without intervening
mechanical devices separating the foam breaker from the
surface of the reaction mixture. This arrangement has
been found to be particularly effective because
controlling foam generation in close proximity to the
surface of the reaction mixture allows monomers to
return rapidly to the liquid reaction mixture. Polymer
uniformity is improved by such rapid return of monomers
to the liquid reaction mixture. It should further be
understood that whereas the present invention has been
; 25 illustrated in a preferred embodiment by a common shaft
for activating both the agitator and foam breaker as
previously described, separate means for powering the
foam breaker and agitator may also be employed. It
should be noted, however, that the foam breaker of the
present invention should be sized appropriately to
generate a swept area substantially equal to the free
and unimpeded cross-sectional area of the reactor at the
sur~ace level of the reaction mixture. By the term
"unimpeded" is meant the area available for rotation of
:~`
WO')2/~)~7~ PCTtUS91/~74X3
~7~ 2~22~0
the foam breaker that is unimpecled by drop tubes and
baffels located near the reactor wall. Preferably, such
swept area is from 50 to 99 percent, most preferably 80
to 99 percent of such unimpeded cross-sectional area.
The reactor and associated equipment, including
the foam breaker are constructed from steel, stainless
steel, glass lined steel, or similar materials of
construction. The foam breaker is rotated generally
from 10 rpm to 300 rpm, preferably from 20 to 200 rpm,
so as to provide effective reduction in foam buildup
within the reactor due to shear induced foam collapse.
Suitably, the foam breaker is rotated to provide a
tangental velocity of the foam breaker of from 0.5 to 10
M/sec.
Pr-ocess conditions for polymerizations,
including free radioal, cationic, anionic, condensation
and coordination polymerizations are well known in the
art. Solvents particularly useful for the practice oP
ebullient cooling are inert hydrocarbons or mixtures of
hydrocarbons having a boiling point at or near the
desired temperature range for th~ polymerization.
PrePerred solvents are butane, pentane, isopentane,
cyclopentane, hexane, cyclohexane, toluene, and mixtures
thereof. The polymerization may be conducted at a wide
range of temperatures. Preferred temperatures are ~rom
30C to 110C, most prePerably from 45C to 100C. The
temperature may be adjusted by controlling the pressure
3 of the reactor to induce boiling of the reactor
contents. Once the reactor is at equilibrium, minor
adjustments to the reactor pressure as small as 0.1 psi
~700 Pa) are generally sufPicient to regulate the
; boiling behavior. Additional heating and cooling can be
~ incorporated into the reactor design, if desired. For
~ ' -
, - . ,
.. ~ .
W~ iJ2/~l~7 l~ 2 n ~ o PCT/US91/074X~
--8--
example a jacket enclosing a circulating heat transfer
fluid may be employed for partial control of
temperature, or for use when ebullient cooling is not
desired.
Preferred polymerization processes for which
the present invention is especially suited are anionic
polymerizations, especially such polymerizations
utilized to prepare block copolymers of vinylaromatic-
and conjugated diene monomers. In the preparation of
such block copolymers any of three well known anionic
polymerization techniques: use of multifunctional
initiators, sequential polymerization or coupling of
living polymer anions, may be used. All of the monomer
may be added to the reactor before initiation of
polymerization or monomer may be added continuously or
incrementally during all or some of the polymerization.
The anionic initiator employed in the anionic
process is not critical. Lithium alkyl compounds having
from 2 to 6 carbons in the alkyl group, especially sec-
butyl lithium and hydrocarbon soluble, difunctional
lithium initiators are preferred. Suitable
difunctional lithium initiators are well known and have
been previously disclosed in the following U.S. Patents:
4,169,115; 4,172,100; 4,172,190; 4,427,837; 4,196,154;
and 4,205,016, the teachings of which are herein
incorporated by reference.
Particularly desirable difunctional lithium
containing &ompounds are selected from the group
consisting of compounds corresponding to the formula:
~' .
. .
,
:` ~
W~')2/0~74~ PCT/US91/0748~
_g_
20~9~
~ - C - (~2) - C - ~
R1 CH2 lH2 Rl
R1
R3 R3
wherein
Rl is independently each occurrence hydrogen or
an inert radical having from 0 to 16 carbon atoms;
R2 is a divalent organic radical having at
least 6 carbon atoms, R2 having at least one aromatic
ring and the aromatic ring being directly attached to a
carbon which is attached to an aromatic ring of the
above formula.
R3 is independently each occurrence selected
from the group consisting of alkyl, cycloalkyl,
aromatic, mixed alkyl/aromatic, and mixed cyclo-
alkyl/aromatic radicals containing from l to 20 carbonatoms. Especially preferred are initiating compounds of
the formula:
; Rl Rl
Li Li
Rl CH2 CH
R1 l l
R3 R3 . .
wherein Rl and R3 are as previously defined.
By the term "inert" as used in this context is
meant substituents that do not interfere with the
, ,,
, .
. . . .
W(~J2~7-l~ PCT/US91/07483
~0/~2'~`~0 -lO- ``
desired anionic polymerization. In a most preferred
embodiment, R1 is selected from the group consisting of
hydrogen, alkyl~ cycloalkyl, alkoxy, aryl and mixtures
thereof. Specific examples of difunctional initiators
(DFIs) corresponding to the above formula are
1,3-phenylene bis(3-methyl-1-phenylpentylidene)bis-
(lithium), 1,3-phenylene bis(3-methyl-1-(4-methyl-
phenyl)pentylidene) bis(lithium), 1,3-phenylene bis(3-
methyl-1-(4-ethylphenyl)-pentylidene) bis(lithium), 1,3-
phenylene bis(3-methyl-1-(4-(1,l-dimethyl-
ethyl)phenyl)pentylidene) bis(lithium), and 1,4-
phenylene bis(3-methyl-1-(4-dodecylphenyl)-pentylidene)
bis(lithium).
Diene monomers suitable for use in the practice
of the present invention inalude conjugated dienes,
preferably 1,3-butadiene, isoprene and mixtures thereof.
In addition to diene monomers, one or more olefin
comonomers are additionally suitably employed. Any
copolymerizable olefin comonomer may be employed.
; Preferred olefin comonomers are alkenyl aromatic
monomers. By the term alkenyl aromatic monomer is meant
a monomer of the formula:
` 25
R5
(R4)n ~ C=CH2
where n is an integer from 0 to 3, R4 is an alkyl
radical containing up to 5 carbon atoms and R5 is
hydrogen or methyl. Preferred alkenyl aromatic monomers
W(~)z/~7.l~ PCT/US~l/0748~
2 2 ~ 0
are styrene, vinyl toluene (all isomers, alone or in
admixture), n-methylstyrene, and mixtures thereof.
Particularly preferred alkenyl aromatic monomers are
styrene and mixtures of styrene and Q-methylstyrene.
Monomer and sol~ent purities are carefully
monitored. Purification by contacting with molecular
sieves, distillation, degassing, etc. may be employed to
remove water, oxygen, and other contaminants. Prior to
addition of initiator, reactive impurities may be
removed by "blanking", that is, by addition of a small
amount of lithium initiator to react with and remove the
contaminant, but not enough to begin polymerization.
The polymerization is conducted for time
periods suitable to achieve the desired product
properties and conversions. Suitable reaction times are
from 10 minutes to 3 hours, preferably from 20 minutes
to 2 hours.
Having described the invention, the following
examples are provided as further illustrative and are
not to be construed as limiting. Unless stated to the
contrary, parts and percentages are based on weight.
ExamPle 1
Preparation of Styrene/Isoprene/Styrene Triblock
Copolymer
A 30 gallon (0.1 m3) reactor substantially
according to Figure 1 is loaded with 50.2 kg o~ solvent
comprising o~ a blend of 65 weight percent cyclohexane
and 35 weight percent isopentane. To the solvent was
added 8.15 kg isoprene. (All solvents and monomers were
purified for the remoYal o~ polar impurities such as
W()~2/OR743 PCr/VS91/07~8;~
-12-
water and degassed to remove oxygen.) After 20.18
millimoles pentamethyldiethylenetriamine were added, the
solution was blanked using dilute l,3-phenylene-bis(3-
methyl-l,-[4-methylphenyl]pentylidene)bis(lithium) in
order to remove any residual impurities that would
consume any initiator. Polymerization was then
initiated at 48C and 16.2 psia (111 kPa) with 910 g of
a solution of 1,3-phenylene-bis(3-methyl-1,-[4-
methylphenyl]pentylidene)bis (lithium) (concentration
was 0.073 mmole/g in cyclohexane).
The agitator/foam breaker speed was set at 150
rpm. The polymerization temperature and boiling were
controlled by venting as needed to induce boiling and
heat removal. Foam levels were limited to a few
centimeters Prom the solution surface and no condenser
fouling occurred. The temperature did not exceed 70C
during the exotherm period. After the isoprene
polymerization was completed, about 65 minutes into the
run, 1.44 kg oP styrene monomer was added in order to
prepare the pure triblock polymer. During the second
polymerization the reaction was conducted adiabatically,
that is, no boiling oP the reaction mixture took place.
The reaction mixture was cooled and isopropanol
added to terminate the active polymer anion. Polymer
was recovered by devolatilization. Number average
molecular weight was determined by gel permeation
chromatography according to the technique of Runyon
3 et al., J. Appl. Poly~. Sci., 13, 2359 (1969) to be
146,000.
W(~ '.)2/()~7'1;~ PCTtUS91/07~183
. -13- 2 0 ~2'~ ~0
Comparative
In experiments similar to that of Example 1 but
conducted in reactors without a mechanical foam breaker,
the foam formed during the isoprene polymerizations was
uncontrollable and fouled the condensers.
Example 2
Preparation of Styrene/Butadiene/Styrene Tapered
Triblock Copolymer
The procedure of Example 1 was substantially
repeated using 50.09 kg of solvent comprising a blend of
65 weight percent cyclohexane and 35 weight percent
isopentane. 6.28 Kg butadiene, 3.09 kg styrene and
4.96 millimoles of pentamethyldiethylenetriamine were
added to the reactor. Polymerization of the mixture of
both monomers was initiated at 54C and 26.4 psia (181
kPa) with 1421 g of a solution of 1,3-phenylene-bis(3-
methyl-1,-[4-methylphenyl]pentylidene)bis(lithium)
(aoncentration was 0.075~ mmole/g in cyclohexane).
The agitator/~oam breaker speed was set at 150
rpm. The polymerization temperature and boiling were
controlled by controlling pressure through a vent. The
foam level was kept within control specifications and
the temperature did not exceed 75C during the period of
butadiene polymerization. After the butadiene
polymerization was completed tabout 65 minutes) the
3 boiling phase was terminated by increasing the pressure.
Styrene homopolymerization commenced and the reaction
mixture was allowed to increase in temperature. The
reaction was terminated by addition of isopropanol and
the polymer recovered by devolatilization. The
w~3 ~vn~7~3 PCr/VSgl/0748.~
`2 ~ J~ 14-
resulting product was a tapered triblock copolymer
styrene-butadiene-styrene having Mn of 87,200.
Example 3
Preparation of Styrene/Isoprene/Styrene Triblock
Copolymer
The same reactor as was employed in previous
Examples was loaded with 49.81 kg of solvent composed of
a blend of 85 weight percent cyclohexane and 15 weight
percent isopentane. (All solvent and monomers were
purified for the removal of polar impurities such as
water and degassed to remove oxygen.) The solution was
then blanked in order to remove any residual impurities
that would consume any initiator. The reactor was
heated to 62C under 21.5 psia (147 kPa) pressure.
45.5 ml of a solution of sec-butyllithium (1.4835
normality in cyclohexane) was added and the
agitator/foam breaker speed was slet at 150 rpm.
Polymeri7ation initiated immediat~ly upon addition of
0.714 k~ of styrene. The styrene polymerization was
allowed to oontinue for 36 minutes. At a solution
temperature of 64C, 4.12 kg of iC~oprene was added.
Ebullient cooling w~s utilized to control the reaction
temperature. Foam generated during the polymerization
was controlled by the foam breaker throughout the
polymerization so that foam did not enter the condenser.
After 16 minutes of polymerization, the temperature
reached 68C. The agitator speed was increased to 200
rpm, and 2.04 kg more isoprene were added. After
polymerizing for an additional 25 minutes, the
temperature reached 69C and an additional quantity
(1.82 kg) o~ isoprene was added. After 27 more minutes,
the temperature reached 72C. The pressure was
W~)2/OR743 PC~/US91/07~83
-15-
2~32290
increased so that no further boiling occurred. A second
quantity of styrene (0.710 kg) was then added and
allowed to polymerize to form a triblock copolymer.
: Recovery was according to the techniques of Example 1.
The polymer's number average molecular weight (Mn) was
168,000.
:; 25
: 30
.
.
\
~. .
;, . , ~
:
'~