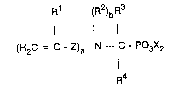Note: Descriptions are shown in the official language in which they were submitted.
r~
HOECHST AKTIENGESELLSCHAFT HOE 92/F 079 Dr.Kl/rh
Alkenylaminoalkylenephosphonic esters and process for the
preparation o~ copolymers comprising alkenylamino-
alkylenephosphonates and ethylenically unsaturated
compounds
Copolymers of unsaturated phosphonic acids and unsatur-
ated monocarboxylic and dicarboxylic acids are proposed
in US-A-5 126 418 as alkaline earth metal and heavy metal
complexing agents, as antiscalants in the petroleum
industry, and as builders, cobuilder and peroxide
stabilizers and also granulation auxiliaries for bleach-
ing activators in detergents. Such copol~mers combine the
properties o~ polycarboxylates and phosphonates.
:
Phosphonomethylated polyvinylamines are known from
DE-A-3 926 059, which are prepared by phosphono~
methylation of polymers containing N-vinylamide groups or
vinylamine groups and are used as additives in detergents
and as water treatment agents (~equestering agents).
.~
JP-A2-54/135 724 describes a procsss for preparing
aminomethylenephosphonic esters and also their acid
hydrolysis, for example the Yynthesis of tetrethyl
N-diallylaminomethylenediphosphonate and of the corres-
;~ ponding acid.
The homopolymerization and copolymerization of diallyl-
aminomethylenephosphonic acids with ethylenicallyunsaturated monomer~ to form high molecular weight
(co)polymers is described in JP-A2-50/72 987. In the
polymerization of diallylammonium salts, polymers that
, ~ are onIy slightly branched and that contain piperidinium
groups are obtained (Lancaster et al., Polym. Lett. 1976,
`~ 14, 54~).
:
: .
(Co)polymerizable phosphonic acid derivatives can be
obtained for ~xample by the Mannich reaction of
k ~
- 2 ~
(di)allylamine with aldehyde~ and phosphorous acid in
concentrated mineral acid solution (K. Noedritzer, R.R.
Irani; J. Org. Chem. 1966, 31, 1603). The amine is
reacted with formaldehyde and phosphorous acid in the
presence of at least equimolar amounts of a mineral acid.
In the subsequent neutralization of the reaction solution
at least equimolar amounts of salts are corre~pondingly
formed, which are separated by comp:Lex operations for
specific applications, for example prevention of scale in
cooling waters and also for modern detergents, and
finally have to be disposed of. If hydrochloric acid is
used the formation of carcinogenic halogenated dimethyl
ethers, which occur as by-products, is moreover a serious
disadvantage.
The need therefore exi.sted to provide a proce~s for
preparing ~ao)polymerizable alkenyl~minoalkylene-
phosphonic acid derivatives which produces little~ if
any, inorganic salt and in which the formation of car-
cinogenic halogenated dimethyl ethers i5 avoided.
We have surprisingly found that the esters of alkenyl-
aminoalkylenephosphonic acids can be prepared without the
aforementioned disadvantages occurring. These esters are
suitably copol~merizable with ethylenically unsaturated
compounds.
The present invention relates to alkenylaminoalkylene-
phosphonic esters of the formula 1
R1 ~R2)bR3
(H2C = C ~ Z)a - N --- C - PO3X2
I
R4
,;
: :
i
2 ~
-- 3 --
where
R' is hydrogen or methyl,
R2 is hydrogen, C1-C22-alkyl, preferably Cl-C~-alkyl,
C3-C6-alkenyl, preferably propenyl, aryl, preferably
phenyl or a group of the formulae -C(R3)(R4)-Po3X2
and -C(O)Rs,
R3 is hydrogen, C~C22-alkyl J preferably Cl-C6~alkyl, or
aryl, preferably phenyl,
R4 is hydrogen, C1-C22-alkyl, preferably Cl-C6-alkyl,
aryl, preferably phenyl, or a group of the formula
: -PO3X2 ~
Rs is C1-C22-alkyl, preferably Cl-C4 alkyl, or aryl,
: preferably phenyl,
:~ Z is Cl-C3-alkyl, ;.
a is 1 or 2, b i8 0 or 1, a + b is 2, and
X is Cl-C4-alkyl, or aryl, preferably phenyl,
:~. with the exception of the compound~ of the formulae
~ (H2C-CH-C~2)2N-CH[PO3 ~ C2~5 ) 2 ] 2 a~d (H2C-C~-C~2)2~-CHR-~PO3x2-
s~ '
~he present invention also relates to a proce~s for
preparing copolymer~ ~omprising
; from 0.1 to 99.9% by weight, preferably from 1 to 50% by
`~ weight, of at least one monomer unit of the formula 2
R1 (R )bR
( 2 )
(H2C = C ~ Z)a ~ N --- C - PO3MY
: R7
~ where
:~; 25 Rl is hydrogen or methyl,
R6 is hydrogen, Cl-C22-alkyl, preferably C1-~6-alkyl,
C3-C6-alkenyl, preferably propenyl, aryl, preferably
phenyl, or a group of the formulae -C~R3)(R4)-Po3MY
-~; and -C(O)Rs,
30 R3 is hydrogen, C1-C22-alkyl, pre~erably Cl-C6-alkyl, or
.~ aryl, preferably phenyl,
: ~ R7 is hydrogen, C,-C22-alkyl, preferably C~-C6-alkyl, ~:
2 ~
-- 4 --
aryl, preferably phenyl, or a group of the formula
-PO3MY,
Rs is C~-C2~-alkyl, preferably C,-C4-alkyl, or aryl,
preferably phenyl,
Z is Cl-C3-alkyl,
a is 1 or 2, b is 0 or 1, a + b is 2,
Y is hydrogen, C,-C~-alkyl, or aryl, preferably phenyl,
and
M is, independently in each instAnce, hydrogen or a
cation, preferably sodium, potas~ium, or ammonium
and
from 99.9 to Ool~ by weight, preferably from 99 to 50~ by
weight, of at least one monomer unit from the group
comprising ethylenically unsaturated carboxylic acids,
sulfonic acids and their derivativeq, and also other
ethylenically unsaturated compounds, wherein alkenyl-
aminoalkylenephosphonic esters of ~he formula 1 and
monomers from the group comprising ethylenically unsatu-
rated carboxylic acids, sulfonic acids and their deriva-
~: 20 tives and also other ethylenically unsaturated compounds
are polymerized in aqueous medium or organic medium at a
: pH of less than 5, and if necessary the remaining
: phosphonic ester groups of the copolymers obtained are
~- hydrolyzed.
The preparation of the alkenylaminoalkylenephosphonic
esters of the formula l according to the invention is
. described hereinafter.
If the compounds of the formula 1 are monophosphonic
esters (R4 different from PO3X2), these esters are
obtained by reacting an aldehyde or ketone of the formula
R3-C(o)-R4, preferably formaldehyde, with a diester of
phosphorous acid and an amine of the formula 3
R1 (R2)b
I
;~ (H2C = C-Z~a-N-H (3)
;..
,,,,, .. ~ . . . . . - - , - . - -
- , . -
-- 5 --
where R1, R2, z, a and b are as defined above. Suitable
diesters of phosphorous acid ar~ for example dialkyl
esters with Cl-Cs-alkyl, such as the dimethyl ester and
the diethyl ester, diphenyl ester and alkyl aryl esters.
In the preparation of the monophosphonic esters of the
formula 1 (R4 different from PO3X2) the diester of phos-
phorous acid and the aldehyde or ketone of the formula
R3-C[o)-R4 are normally placed in thle reaction ve~sel
first and the corresponding amine is then added so that
the exothermic reaction proceeds in a controlled manner.
Alternativsly, however, the diester of phosphorous acid
and the amine may be placed in the reaction vessel first,
following which the aldehyde or ketone of the formula
R3-C(o)-R4 is slowly added.
It has been found that from 0.5 to 1.5 mol, preferably
from 0.3 to 1.2 mol, of aldehyde or ketone of the formula
R3-C~o)-R4 and from 0.5 to 1.5 mol, preferably from 0.8 to
1.2 mol, of diester of phosphorous acid per mole of the
secondary amine axe reacted with one another. It is
part.icularly preferred to react the individual components
with one another in equimolar amounts.
With primary amines~ double the amounts of aldehyde or
ketone of the formula R3-C(o)-R4 and of the diester of
phosphorou~ acid are correspondingly used. ~he reaction
temperatures are in the range from 20 to 200C, prefer-
ably from 50 to 150C.
If the compounds of the formula 1 are l,l-diphosphonic
esters (R4 the same as -PO3X2), these esters are obtained
by reacting diesters of phosphorous acid with an alkyl
ester of orthoformic acid and an amine of the formula 3.
Suitable alkyl estexs of orthoformic acid are Cl-C4-alkyl
esters, for example ethyl orthoformate.
In the preparation of these l,l-diphosphonic acid esters
advantageously from 0.5 to 2 mol, preferably from 1.0 to
1.3 mol, of alkyl ester of orthoformic acid and from
.l 1.5 to 3.0 mol, preferably 2.0 to 2.5 mol, of diester of
phosphorous acid are reacted per mole of the rslevant
,'.
. ~ . ., .. . ~ -
. .
-
~: :
~ 3
- 6 -
primary or secondary amine at temperatures in the range
from 50 to 150C, the alcohol formed being distilled off,
and the addition of the amine to the mixture of diester
and alkyl ester of orthoformic acid normally being
5 effected in a controlled manner. It is particularly
preferred to react the individual components in the molar
ratio 1:2:1.
The aforementioned processes for preparing compounds of
the formula 1 have the advantage that no inorganic salts,
such as sodium chloride, occur, and the ~ormation of
carcinogenic halogenated dimethyl ethers is avoidPdl
As compounds of the formula 1 there may be mentioned in
particular allylaminobis~methylenephosphonic) acid
tetraethyl ester, methallylaminobis(methylenepho~phonic)
acid tetraethyl ester, diallylaminomethylen2phosphonic
acid dimethyl ester, diallylaminomethylenephosphonic acid
diethyl ester, diallylaminomethane~ diphosphonic acid
: ~ tetraethyl ester, diallylaminomethane-1,1-diphoæphonic
: acid tetramethyl ester, diallylaminomethane-l,l-diphos-
phonic acid tetrapropyl ester, and N-methylallylamino-
methane~ diphosphonic acid tetraethyl ester.
Th~ salt-~ree compounds o~ the formula 1 may be used
without further purification or drying in the preparation
of the aforementioned copolymers comprising monomers of
the formula 2 and monomers of the group comprising
ethylenically unsaturated carboxylic acids, ~ulfonic
acids and their derivatives, and also other ethylenically
~: unsaturated compounds.
If, however, drying i~ necessary, the water present can
be removed by suitable methods, such as distillation or
;. the addition of drying agents.
, . . .
¦ Both novel and known alkenylaminoalkylenephosphonic
esters may be used as starting compounds for the prepa
ration of copolymers by the process according to the
.~
. :
.i ~. - - . . ~
- 7 ~
invention. The known compounds include diallylamino-
methane~ diphosphonic acid tetraethyl ester and the
diallylaminomethanephosphonic acid dies~ers named in the
still unpublished German patent application with the file
No. P 4100760.3.
Suitable comonomers are ethylenically unsaturated
carboxylic acids and their derivative~, for example
acrylic acid, methacrylic acidr crotonic acid, maleic
acid, maleic anhydride, fumaric acicl, itaconic acid,
itaconic anhydride, methyl acrylate, ethyl acrylate,
methyl methacrylate, butyl methacrylate~ dimethyl-
aminoethyl acrylate, dimethylaminoethyl methacrylate,
monoethyl maleate, diethyl maleate, hydroxyethyl
acrylate, hydroxypropyl acrylate, hydroxyethyl meth-
acrylate, hydroxypropyl methacrylate, methacrylamido-
propyldimethylammonium chloride, dimethylaminopropyl
acrylamide~ acrylonitrile and methacrylonitrile.
~; Also suitable are ethylenically unsaturated sulfonic
acids such as vinyl~ulfonic aaid, allylsulfonic acid,
methallylsulfonic acid, styrenesulfonic acid, acrylic
acid (3-sulfopropyl)ester, methacrylic acid (3-sulfo-
propyl) ester, and 2-acrylamido-2-methylpropane~ulfonic
~ acld.
- Other suitable compounds include neutral unsaturated
compounds such as N-vinylacetamide, N-vinylpyrrolidone,
N-vinylcaprolactam, N-vinylimidazole, vinyl acetate,
vinyl propionate, vinyl butyrate, styrene, olefins having
2 to 10 carbon atoms, such as ethylene, propylene,
isobutylene, hexene, diisobutene, and vinyl alkyl ethers,
such as methyl vinyl ether, ethyl vinyl ether, n-butyl
vinyl ether, isobutyl vinyl ether, hexyl vinyl ether and
octyl vinyl ether.
The aforementioned starting substances may be used as
~` individual substance6 or in the form of mixtures.
The copolymers may be prepared by bulk, solution, preci-
pitation, suspension or (inverse)emulsion polymerization.
~he preferred preparation process is solution
.~
.:
;, ;, : ,
~ ~ ~ 2 ~ ~ ~
polymerization, further details of which will be
discussed hereinafter.
The copolymerization normally takes place in the presence
of initiators that form free radicals under the poly-
merization conditions, for example in the presence ofperoxides, hydroperoxides, psrsulfates, azo compounds ox
redox catalysts.
Suitable solvents are aqueous media and organic media.
The aqueous media comprise mixtures of water and water-
miscible organic solvents, such as alcohols, cyclicesters or, preferably, only water. The organic media
comprise water-miscible or wa~er-immiscible organic
solvents, which also include aromatic hydrocarbons such
as toluene and xylene and paraffins. Solution poly-
merization is carried out at a total monomer concen-
tration of from 1 to 80% by weight/ preferably from 10 to
60% by weight, The temperatures are from 0 to 120C,
preferably from 10 to 100C.
The starting materials may be added to the solvent
separately or together. The addition of the free radlcal
chain initiator, i~ necessary dissolved in a ~uitable
solvent, may take place at the same time as, or after the
addition of the starting materials. In order to improve
the solubility in water of the alkenylaminoalkylene-
phosphonic esters employed, it i~ advisable to add short-
chain aliphatic alcohols such as ethanol or isopropanol.
In addition it may be advantageous, in order to improve
the solubility of the compound of the formula 1 and
furthermore to prevent oxidation, to add acids, prefer-
ably in equimolar amounts, to the aqueous medium beforethe actual polymerization, in which connection the pH of
the aqueous medium should be less than 5, and preferably
less than 3. Suitable acids are mineral acids, for
example hydxochloric acid, sulfuric acid, phospboric
acid, and also organic acids, such as alkanecarboxylic
acids, for example formic acid and acetic acid, and
aromatic carboxylic and ~ulfonic acid~, for example
benzoic and p-toluenesulfonic acid.
; ;
~: :
,
.
'~; ' ' ' ~ : '
9- ~$~7~
If copolymers with free phosphonic acid groups of the
formula -PO3H2 are to be obtained, t~len the copolymeriza-
tion may be carried out in strongly acidic solution with
the addition of the aforementioned acids, or the
phosphonic ester groups of the copolymers obtained by the
process according to the invention are hydrolyzed by
heating in strongly acidic solution, at a p~ of less than
3. In a special variant of the process the hydrolysis of
the phosphonic ester groups may be effected by treating
the copolymers with copolymers containing free phosphoni~
acid groups, with the addition of water, and distilling
off the alcohol that is formed. In addition to the
aforementioned acids the ethylenically unsaturated
carboxylic acids or sulf onic acids used as starting
materials may also act as proton donor~, so that if
desired the addition of acids can be omitted. In this
connection, it has been found that, under the acid
reaction conditions, hydrolysis of the alkenylamino-
alkylenephosphonic esters occurs in parallel to the
polymerization, and the formation of ethyl chloride can
be excluded. Pref erred monomers in this reaction are
acrylic acid, methacrylic acid, maleic acid and 2-acryl-
amido-2-methyl-propanesulfonic acid.
.,
In the copolymerization of phosphonic e~ters of the
formula 1 with comonomers carrying acidic groups in
aqueous solution, the ester groups are hydrolyzed during
the polymerization, as can be shown by 3lP-NMR spectros-
copy. For example, the 3lP-NMR sp~ctrum of a copolymer of
acrylic acid and diallylaminomethane-l,1-diphosphonic
~ 30 acid tetraethyl ester (Example 8) exhibits a broad signal
`` between 8 and 9 ppm, whereas the diallylaminomethane-1,1-
- diphosphonic acid tetraethyl ester that is used exhibits
a substantially sharper signal at 19.8 ppm.
; The 31P-NMR spectrum of the pyrrolidine-1,1-methanedi-
phosphonic acid prepared for the purposes of comparison
(Comparative Example 2)~ which constitutes a structural
element of the polymer according to Example 4, also
~`
''"
.; .: ,1
.::
.~
.,,;, ~ ~ .
lo~ 7~
exhibits a signal at 8.25 ppm.
According to the process of the invention, copolymer can
be prepared by reacting oil~soluble alkenylamino-
alkylenephosphonic esters with oleophilic ethylenically
un~aturated comonomers in organic solvents in the
presence of free-radical chain i.nitiator~ ~uch as AIBN or
organic peroxides, followed by hydrolysis of the copoly-
mer~ thereby obtained by adding minera.1 acids at a pH of
less than 3.
The molecular weight of the copol~mers prepared depends
on the intended use, but in principle is not subject to
any restrictions. Pre~erably, copolymers are prepared
having low and mean molecular weights in the range ~rom
1000 to 500,000. Thi~ can also be achieved by adding from
: 15 0.001 to 30~ by weight of regulators such as thiogly~_olic
acid, ethanethiol, dodecanethiol, phosphorous acid, hypo-
phosphorous acid, sodium hydrogen sulite~ or water~
soluble salts of transition metals such as copper, iron,
manganese and nickel to the reaction mixture before or
during the addition of the catalyst. The preferred
intrinsic viscosity K (determined according to Ubbelohde)
of the polymers i9, for example if the polymers are used
as antiscalants, from 10 to 100, in particular from 10 to
50.
The copolymers prepared by the process according to the
invention have a broad range of application and can be
used in many sectors, with utilization of their advan-
tageous properties. The copolymers are preferably used as
anti~calants, for example in the cleaning of machinery,
bottle cleaning, steam generation, cooling water treat-
ment and in oil conveyance, as complexing and/or
sequestering agents, for example in water treatment, in
the production of leather and in textile and paper
blèaching, and as builders and cobuilders in detergents.
: . .
. . . ;
, . . . ;
~: : . , . , -
. . . .. ..
7~ j
Examples
The percentage figures are, unless otherwise speci~ied,
by weight. The water used in the examples is deionized.
The intrinsic viscosity values K were determined accord-
ing to Ubbelohde at 25C in water at a polymer concentra-
tion of 5~ ~y weight. 3lP-NMR spectra were recorded with
a 121-MHz spectrometer in D2O with
3-(trimethylsilyl)propionic acid d4-sodium salt and in
CDCl3 with tetramethyl silane as internal standard.
~he polymerizations were carried out in 1 1 5 necked
flasks with plane-ground cover~. The 1asks are each
equipped with an anchor stirrer, thermometer, reflux
condenser, gas inlet tube and dropping funnel. The
solutions added to the flasks for the polymeriæation were
flushed with nitrogen.
Example 1
Preparation of allylaminobis(methylenephosphonic) acid
; tetraethyl ester
14.3 g (0.25 mol) of allylamine were added at 80C during
; 20 the course of hal~ an hour to a mixture of 15 g (0.5 mol)
of paraformaldehyde and 69 g (0.5 mol) of diethyl
phosphite. ~he reaction wa~ complete after stirring for
half an hour at 85C. The crude product, which contained
9% of water, was dried by adding Na2SO4. It was used
without further purification for the polymerization.
H-NMR (DMS~-d6)~ ~ = 1.23 (t, 12H); 3.05 (d, 4H); 3.38
(d, 2H); 4.0 (m, 8~); 5.2 ~m, 4H); 5.58-5.g5 ppm (m, lH).
31P-NMR (D2O): ~ = 27-55 ppm-
Example 2
,,
Preparation of diallylaminomethylenephosphonic acid
diethyl ester
30 g (1 mol) of paraformaldehyde were slowly added at
60C to a well-stirred mixture of 138.1 g (1 mol) of
diethyl phosphite and 97.2 g (1 mol) of diallylamine. The
reaction was complete after 30 minutes' stirring at 85C.
The crude~ product ~(comprieing approximately 90% of
:.
:., ~ - .
g~
- 12 -
diallylaminomethylenephosphonic acid diethyl ester and 6~
of water) can be purified by distillation (bp35 ~r ~
139-143C).
1H-NMR (DMSO-d6): ~ = 1.23 (t, 12H)i 2078 (d, 2H); 3.18
(d, 4~); 4.0 (m, 4H); 5.2 (m, 4H); 5.58-5.95 ppm (m, 2EI).
3'P-NMR ~CDCl3): ~ = 25.9 ppm.
Example 3
Preparation of diallylaminomethane-1,1-diphosphonic acid
tetraethyl ester
4.85 g (O.05 mol) of diallylamine, 8.9 g tO.06 mol) o~
; ethyl orthoformate, and 13.8 g (0.1 mol) of diethyl
phosphite were heated to 150C with the addition of
O.2 ml of boron trifluoride etherate. The ethanol fonmed
(8.2 ml) wa9 distilled off over about 2 hours~
The crude product thus obtained was taken up in toluene,
dried over sodium sulfate, and the toluene was removed
under reduced pressure. The product was then purified by
fractional vacuum distillation. 8.1 g (42% of theory) of
N,N-diallylaminomethane~ diphosphonic aaid tetraethy~
ester having a boiling point of 118-121C (0.04 mbar)
were obtained.
31P-NMR (D2O~: ~ = 19.8 ppm-
Example 4
Copolymer of acrylic acid and 15% of allylamino-
bis(methylenephosphonic) acid tetraethyl ester
7.5 g (0.021 mol) of allylaminomethyl~ne-bis(phosphonic)
acid diethyl ester according to Example 1 were dissolved
in a mixture of 80 g of water and 40 g of isopropanol and
heated to 75C while passing a stream of nitrogen through
the mixture. A catalyst solution comprising 42.5 g
(0.6 mol) of acrylic acid and 1.5 g of (~H4)2S2O~ in 30 g
of water was added dropwise ~ynchronously from 2 dropping
funnels at thi~ temperature. After the end of the exo-
; thermic reaction phase the reaction mixture was stirred
for 4 hours at 80C. The resulting polymer had an
intrinsic viscosity K of 22.
. . ~ . . . ~
, ~ , . -
.
~ ~ 9 2 7 r~ ~
- 13 -
Example 5
Copolymer of acrylic acid and diallylaminomethylene-
phosphonic acid diethyl ester
7.5 g (0.030 mol~ of the distilled diallylaminomethylene
phosphonic acid diethyl ester according to Example 2 were
copolymerized as described in Example 4 with 42.5 g
(0.6 mol) of acrylic acid. The resulting pol~mer had an
intrinsic viscosity K of 20.
Example 6
Copolymer of acrylic acid and diallylaminomethylenephos-
phonic acid diethyl ester in the presence of ~Cl
22.5 g (0.091 mol) of diallylamino methylenephosphonic
acid diethyl ester (crude product) according to Example
2 were dissolved in a mixture of 240 g of water and 120 g
of isopropanol with the addition of 40 g (0.364 mol) of
33% hydrochloric acid and heated to 75C while passing a
stream of nitrogen through the mixture. A catalyst
solution aomprising 4.5 y of (N~4)~S2O8 in 90 g of water,
and 120 g (1.8 mol) o~ acrylic acid was added dropwi~e
synchronously from 2 dropping funnel~ at this tempera-
ture. After the end of the exothermic reaction phase the
reaction mixture was stirred for 2 hours at 80C. ~he
.
~; resulting polymer had an intrinsic viscosity K of 23.
After distilling off isopropanol the reaction mixtur~ was
refluxed ~or 1 hour to hydrolyze the phosphonate esters.
Example 7
Copolymer of acrylic acid and diallylaminomethane~
diphosphonic acid tetraethyl ester
10.0 g (0.026 mol) of diallylaminomethane~ diphos-
phonic acid tetraethyl ester are copolymerized, as
described in Example 4, with 40.0 g tO.56 mol) of acrylic
acid.
Example 8
Copolymer of acrylic acid and diallylamillomethane-1,1-
diphosphonic acid tetraethyl ester in the presence of ~Cl
; 10 g tO.026 mol) of diallylaminomethane~ diphosphonic
.
~` ~ ~ :'. ' ,
.. ~ :
- 14 -
acid tetraethyl ester are copolymerized with 40 g
(O.56 mol) of acrylic acid as described in Example 6,
with the addition of 2.89 g (0.026 mol) of 33% hydro-
chloric acid.
After distilling off the isopropanol the reaction mixture
was refluxed for 1 hour to hydrolyze the phosphonate
esters.
Example 9
Copolymer of 2-acrylamido-2-methylpropanesulfonic acid
and diallylaminomethylenephosphonic acid diethyl ester
A third of a monomer solution comprising 7.5 g (0O03 mol)
of diallylaminomethylenephosphonic acid diethyl e~ter and
42.5 g (0.2 mol) of 2-acrylamido-2-methylpropanesulfonic
acid in 120 g of water was placed in the reaction flask
and heated to 80C whil~ pa~sing a stream o~ nit;rogen
through the reaction mixture. The remainder of the
monomer solution and also a catalyst solution comprising
1.5 g of (NH4)2S2O8 in 30 g of water were added dropwise~
synchronously within 2 hours at this temperature~ The
; 20 reaction mixture was refluxed for 2 hours. The resulting
polymer had a X value of 47.
Comparative Example 1
Preparation of diallylaminomethane~ dipho~phonic acid
~similar to JP-A2 54/135724)
The diallylaminomethane-l,1-diphosphonic acid was
prepared by hydrolysis of the corresponding distilled
tetraethyl ester with concentrated HCl.
Comparative Example 2
' Preparation of pyrrolidine-1,1-methanediphosphonic acid
71.1 g (1 mol) of pyrrolidine, 170.4 g (1.2 mol) of ethyl
orthoformate, and 289.8 g (2.1 mol) of diethyl phosphite
were heated for 4 hours at 150C and the ethanol formed
was distilled off. The unreacted substances were then
removed by distillation under a high vacuum. 286 g of the
biphosphonate obtained were refluxed for 6 hours with 1 1
of concentrated hydrochloric acid. The excess
~ .
..
~,
. .
- 15 - 2~2~
hydrochloric acid was then removed under reduced pressur~.
142 g (58~ of theory) of pyrrolidine~ methanedi-
phosphonic acid were obtained as a colorles~ powder.
3lP-NMR ~D2O): ~ = 8.25 ppm
Comparative Example 3
Copolymer of acrylic acid and diallyl~ninomethylena-
phosphonic acid
Diallyl~ninomethylenephosphonic acid was prepared by
reacting diallylamine with formaldehyde and phosphorous
acid in equimolar amount6 (K. ~oedritzer, I.I. Irani
J. Org. Chem. 1966, 31, 1603-1607). Sulfuric acid w~s
used instead of hy~rochloric acid as mineral acid and,
a~ter the reaction, was neutralized with sodium
hydroxide. The phosphonic acid was separated from the
salt by extracting the reaction product, concentrated by
heating to dryness, with ethanol. 10.0 g (0.05 mol) of
diallylaminomethylenephosphonic acid were copolymerized
with 40.0 g (0.56 mol) of acrylic acid as described in
Example 4.
20 3lP-NMR spectroscopic investigations on diallylamino-
monophosphonate
~:;
Example 2 25.9 ppm
~; Example 6 17.2 ppm, 10.5 ppm, 9.2 ppm;
rel. intensity 3:1:0.1
' after 1 hour's
boi}ing 17.3 ppm, 10.7 ppm, 9.2 ppm;
rel. intensity 3:1:0.3
Comp. Ex. 3 8.6 ppm
3~P-NMR spectroscopic investigations on diallylamino-
l,l-diphosphonate
Example 3 19.8 ppm
Example 7 20.1-~0.3 ppm, 14.2-15.5 ppm;
rel. intensity 3:1
~' ~
~::
.
2~2~ l ~J
16 -
Example 8 16.9-17.1 ppm, 16.1-16.3 ppm,
12.9-13.4 ppm, rel. intensity
2:1:2
" after 1 hour's
boiling 8.8-9.0 ppm
Comp. Ex. 1 7.7 ppm
Comp. Ex. 2 8.25 ppm
: These measurements point to a continuing hydrolysis of
the phosphonate esters (Example~ 6, 7 and 8) during the
10 polymerization and in the thermal post-treatment.
.; ~ :
.,
' ,
' ' .
:
: ,
~ . :