Note: Descriptions are shown in the official language in which they were submitted.
20931 ~8
New 9-fllloro-7-o~o-7H=pvrido r 1,2 3-del r 1.4lbenzoxazine
6~carboxylic acids and esters
The present invention relates to new 9-fluoro-7-oxo-
7H-pyr.ido[1,2,3-de][1,4]benzoxazine-6-carboxylic acids
and esters, process for their preparation and -their use
as medicaments, in particular as antiviral medicaments.
1,8-bridged 4-quinolonecarboxylic acids having an anti-
bacterial action are already known ~rom EP-A-253,235.
The present invention relates to new g-fluoro-7-oxo-7H-
pyrido~l,2,3-de~[1,4]benzoxazine-6-carboxylic acids and
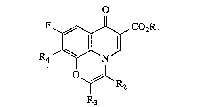
esters o~ the general formula (I)
o
, CO2RI
N ~ (I),
~R2
in which R3
R1 represents hydrogen or straight-chain or branched
alkyl having up ~o ~ carbon atoms,
R2 represents hydrogen, methyl, ormyl, benzyl, p-Cl-
benzyl, carboxyl or the -CONH2 group, or
represents straight-chain or branched alkenyl or
Le A 28 861 - 1 -
20931~8
alkoxycarbonyl each having up -to 6 carbon a-torns, or
represents straigh-t-chain or branched a].kyl having
up to 6 carbon atomsl which is substikuted by
carboxyl, ~enæyloxy, imidazolyl or by s-traight-chain
or branched alkoxycarbony:L having up to 4 carbon
atoms, or
represents cycloalkyl having 3 to 8 carbon atom~
R3 represents hydrogen,
represents carboxyl or
represents cycloalkyl having 3 to 8 carbon atoms, or
represents straight-chain or branched alkoxycarbonyl
having up to 8 carbon atoms, or
represents straight-chain or branched alkyl having
up to lO carbon atoms, which is substituted by
carboxyl, phenoxy or by straight-chain or branched
alkoxycarbonyl having up to 6 carbon atoms, or
represents biphenyl or a radical o~ the formula
~ ~RsR8 ~(CH~)n
in which
A denotes a nitrogen atom or the -CH group,
R5 and R5 are identical or different and denote
Le A 28 861 - 2 -
~i
2~31~8
hydrogen or straight-c~hain or branched alkyl or
~cyl eaoh having up to 6 carbon atoms,
R7 deno-tes hydrogen, halogen, hydroxyl or
straight-chain or branched alkoxy or alkyl each
5having up to 6 carbon atoms,
n denotes a number 1,2 or 3,
and
B denotes straight-chain or branched alkyl having
up to 6 carbon atom~ or hydrogen,
0 R4 repre~ents imidazolyl or 1,4-diazacycloheptanyl
bonded via N, or a radical o~ the formula
(H2C)p
~ ~ ` ~J
Ra
R.
or ~
/--\ HN N--
R~o--N :-- y
Rl2
Le A 28 861 - 3 -
,
.. . .
~. . . .
, : . : - ~
'. : . : '" ' ,, ':
.
2093~
in which
o denotes a number 2 or 3,
p denotes a number 1 or 2,
D denotes the -C~2-group or an oxygen atom,
RB and R9 are identical or different and denote
hydrogen, methyl or et:hyl,
Rl denotes cycloalkyl having 3 to 8 carbon atoms
or straight-chain or branched perfluoroalkyl
hav.ing up to 6 carbon atoms, or
denotes phenyl, pyridyl or pyrimidyl, each o~
which is optionally substituted by halogen or
straight-chain or branched alkyl or alkoxy each
having up to 6 carbon atoms,
R11 denotes hydrogen or methyl,
R12 denote straight-chain or branched alkyl having
up to 6 carbon atoms, which is substituted by
hydroxyl, benzyloxy, phenoxy or morpholino,
and in the case in which R3 does not denote hydrogen if R2
represents hydrogen or methyl,
~0 R4 additionally represents morpholino or a radical of
the formula
Le A 28 861 - 4 -
,: ~.. . ,, . . ; - : ~
. . " ,., ", "
. , . ,,, , . ~ ,
~ 3 1 ~ 8
R"--N N--
in which
Rl3, R14 and Rl5 are ide~tical or different and denote
hydrogen, hydroxyethyl, methyl or ethyl
and their hydrates and ~alts, if appropriate in an
isomeric form.
Physiologically acceptable salts of the compounds
according to the invention can be salts of the sukstances
according to the invention with mineral acids, carboxylic
acids or sulphonic acids. Particularly preferred salt~,
for example, are those with hydrochloric acid, hydro-
bromic acid, sulphuric acid, phosphoric acid, methane-
sulphonic acid, ethanesulphonic acid, toluenesu1phonic
acid, benzenesulphonic acid, naphthalenedisulphonic acid,
acetic acid, pxopionic acid, lactic acid, tartaric acid,
citric acid, fumaric acid, maleic acid or benæoic acid.
Physiologically acceptable salts can also be alkali
metal, alkaline earth metal, silver and guanidinium salts
of the compounds according to the invention.
Preferred compounds of the general formula (I) are those
Le A 28 86 - 5 -
,
.
:. . .
.
.
. : . . .
~3~
in which
Rl represents hydrogen, or
represents straight-chain or branched alkyl having
up to 6 carbon atoms,
R2 represents hydrogen, formy:l, methyl, benzyl, p-C1-
benzyl, carboxyl or the -CON~2 group, or
represents straight-chain or branched alkenyl or
alkoxycarbonyl each having up to 4 carbon atoms, or
represents straight-chain or branched alkyl having
up to 4 carbon atoms, which i~ substituted by
carboxyl, benzyloxy, imidazolyl, methoxycarbonyl,
ethoxycarbonyl or propoxycarbonyl, or
represent~ cyclopropyl, cyclobutyl, cyclopentyl or
cyclohexyl,
R3 represents hydrogen~
represents carboxyl, or
represents cyclopropyl, cyclobutyl, cyclopentyl or
cyclohexyl, or
represents straight-chain or branched alkoxycarbonyl
having up to 6 carbon atom~, or
represents straight-chain or branched alkyl having
up to 8 carbon atoms, which is substituted by
carboxyl, phenoxy or by straight-chain or branched
alkoxycarbonyl having up to 4 carbon atoms, or
represents biphenyl or a radical of the formula
Le A 28 861 - 6 -
~ , ; , , , : :
, ., . ~ .
2~3~
~ NFlsFI~ C~-(2)rl
in which
A denotes a nitrogen atom or the -CH group,
R5 and R6 are identical or different and denote
hydrogen or straight-chain or br a n c h e d a 1 k y 1 o r
acyl each having up to 4 carbon atomst
R7 denotes hydrogen, fluorine, chlorine, hydroxyl
or straight-chain or branched alkoxy or alkyl
each having up to 4 carbon atoms,
n denotes a number 1 or 2,
and
B denotes straight-chain or branched alkyl having
up to 4 carbon atoms or hydrogen,
R4 represents imidazolyl or 1,4-diazacycloheptanyl
bonded via N, or
represents a radical of the formula
Le A 28 861 - 7 -
~ :'
. .
. .
2~93~
N~J ~ N/
Rlo--N N-- or H: N--
in which
o denotes ~ number 2 or 3,
p denotes a number 1 or 2,
D denotes the -C~2 group or an oxygen atom,
R8 and R9 are identical or different and denote
hydrogen, methyl or ethyl,
Rl denotes cyclopropyl, cyclopentyl or
cyclohexyl, or
denotes straight~chain or branched perflu-
oroalkyl having up to 4 carbon atoms, or
denotes phenyl, pyridyl or pyrimidyl, each
of which is optionally substituted by
fluorine, chlorins or straight-chain or
branched alkyl or alkoxy each having up to
Le A 28 861 - 8 -
.
2~31~8
4 carbon atoms,
Rll denotes hydrogen or methyl,
Rl2 denotes straight-chain or branched alkyl
having up to 4 carbon atoms, which is
substituted hy hydroxyl, benzyloxy,
phenoxy or morpholino,
and in the case in which R3 does not denote hydroyen i~ R2
represents hydrogen or methyl,
R'' additionally represents morpholino or a radical of
the formula
R~" N ~--
in which
R13, Rl4 and R15 are identical or different and denote
hydrogen, hydroxyethyl, methyl or ethyl
and their hydrates and salts, if appropriate in an
isomeric form.
Particularly preferred compounds of the general formula
Le A 28 861 - 9 -
,,
:
.
20~3~
( I ) are -those
in which
Rl represents hydrogen or straight-chain or branched
alkyl having up to 4 carbon atoms,
S R2 represents hydrogen, formyl, methyl, benzyl,
p-Cl-benzyl, carboxyl or the -CONH2 group,
represents vinyl, allyl, methoxycarbonyl or ethoxy-
carbonyl,
represents straight-chain or branched alkyl having
up to 3 carbon atoms, which is sub~tituted by
carboxyl, benzyloxy, imidazolyl, methoxycarbonyl or
ethoxycarbonyl, or
represents cyclopropyl or cyclopentyl,
R3 represents hydrogen, or
represents carboxyl, or
represents cyclopxopyl or cyclopentyl, or
represents straight-chain or branched alkoxycarbonyl
having up to 4 carbon atom~, or
represents straight--chain or branched alkyl ha~ing
up to 6 carbon atoms, which iR substituted by
carboxyl, phenoxy or by straight-chain or branched
alkoxycarbonyl having up to 3 carbon atoms, or
represents a radical of the formula
Le A 28 861 - 10 -
-- ..
" ~ , :.
,
. :
2093t ~8
~ NRsFIo
in which
A denotes a nitrogen atom or the -CH group,
R5 and R6 are identical or diffe.rent and denote
hydrogen, methyl, ethyl or acetyl,
R7 denotes hydrogen, fluorine, chlorine, methyl,
ethyl, methoxy or ethoxy,
R4 represents imidazolyl or 1,4 diazacycloheptanyl
bonded via N, or
represents a radical o~ the formula
~N ~ ~ ~N~
~ or ~ \
R~o--N N- HN N--
~-2
Le A 28 861 - 11 -
,: : . . ,~ .
' ` ';,
. . . . .
2~931~
in whlch
o denotes the number 2,
p denotes a number 1 or 2,
D denotes the -CHz group or an oxygen atom,
R3 and R9 are identical or different and denote
hydrogen, methyl or ethyl,
R10 denotes cyclopropyl or straight-chain or
branched perfluoroalkyl having up to 3 carbon
atoms or phenyl, pyridyl or pyrimidyl, each of
which is optionally subst.ituted by fluorine,
chlorine or straight-chain or branched alkyl or
alkoxy each having up to 3 carbon atoms,
Rll denotes hydrogen or methyl,
Rl2 denotes stxaight~chain or branched alkyl having
up to 3 carbo~ atoms, which is substituted by
hydroxyl, benzyloxy, phenoxy or morpholine,
and in the case in which R3 does not denote hydrogen if R2
represents hydrogen or methyl,
R4 additionally represents morpholino or a radical of
the formula
Le A 28 861 - 12 -
.: . ,, . : :
: , . : , ,
2093~
R15
in which
Rl3, R1~ and Rl5 are identical or different and denotehydrogen, hydroxyethyl, methyl or ethyl
and their hydrates and salts, i~ appropriate in an
isomeric form.
Processes for the preparation of the compounds of the
general formula (I) according to the invention have
additionally been found, characterised in that
[A] compounds o the general formula (II)
O
F ~ C02RI
T ~ N (II),
~R2
R3
in which
R2 and R3 have the abovementioned meaning,
Le A 28 861 - 13 -
~ . . . . . .
, .: :
2~9~08
Rl has the abovementioned meaning, but preferably
represents hydrogen,
and
T represents halogen, preferably fluori.ne,
are reacted with compounds of the general formula (III)
R4-H (III),
in which
R4 has the aboveme~tioned meaning,
in i.nert solvents, in the presence of a base, under a
protective gas atmospherer
or
aldehydes of the general formula (IV)
F ~ CO2RI (IV),
F CH2-CHO
in which
Le A 28 861 - 14 -
, -, , : . ~ . . .
. .
! .: !~
2~93~
R1 has the ~bovementloned meaning,
are first reac-ted with compounds of the general formula
(III) or (IIIa), preferably wit:h (IIIa)
R4-H (III), W-H (IIIa),
in which
R4 has the abovementioned meaning,
W has the abovementioned meaning of R4, one of the
cyclic amine functions being protected by a protec-
t.ive group, preferably tert-butoxycarbonyl or
ethoxycarbonyl,
in inert solverlts, in the presence of a base, with
simultaneous cyclisation, and the protective group i8
removed according to a customary method
or in the case in which R2, as defined above, represents
substituted alkyl,
[B] compounds of the general forrnula (V~
F ~ CO2RI (V),
F ~ CH2
I
o
Le A 28 861 ~ 15 -
~. i
2~3.~g
in which
Rl has the abovementioned meanir~g,
are reacted with compounds of the general formula (VI)
Rl7-~ (VI),
in which
Rl7 represents the substituted alkyl mentioned under R2,
which is shortened by a carbon atom,
in one of the abovementioned solvents, if appropriate in
the presence of a base, with simultaneous cyclisation,
and in a last step the substituent R4 is introduced as
described above,
and in the case in which R3~H, the product is derivatised,
and then, depending on the desired meaning of Rl, either
an esterification or a hydrolysis is carried out by a
customary method.
The process according to the invention can be illustrated
by way of example by the following reaction scheme:
Le A 28 861 - 16 -
[~] ~93~3~
o ~ HN N ~3 DMSO
CH3
F ~, CO2H
¢~ J ~CH3
[B]
F ~CO2C2Hs N
F ~ H
~C~12 ~ ,
oo
F ~ ~ N
0~ CH2--N~=¦
F `r5~, C02H
F ~f N N --
~ C~2--N~ -
O
O N--~X~/ .
0~ CH2--N~=¦
Le A 28 861 - 17 -
- : , . :: :
~' ~ ; . , :, :,, . :
2~9~
Pro-tective groups in the contex-t of the invention are the
groups known from pepticle chemistry such a~, for example,
benzyloxycarbonyl, methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, isopropoxycarbonyl, hutoxycarbonyl or
isobutoxycarbonyl. Methoxy- and ethoxycarbonyl are
preferred.
Suitable solvents for all the process steps are the
customary inert solvents which do not change under the
reaction conditions. These preferably include organic
solvents such as ethers, for example diethyl ether,
glycol monomethyl ether or glycol dimethyl ether, dioxane
or tetrahydrofuran, or hydrocarbons such as benzene,
toluene, xylene, cyclohexane or mineral oil fraction~, or
halogenohydrocar~ons such as methylene chloride,
chloroform, carbon tetrachloride, or diimethyl sulphoxide,
N,N-dimethylformamide, hexamethylphosphoramide,
sulpholane, ethyl acetate, pyridine, triethylamine,
N-methylpyrrolidine, anisole or picoline. It is also
possible to use mixtures of the solvents mentioned.
Dimethyl sulphoxide and N,N-dimethylformamide are
preferred.
Suitable bases are the customary basic compounds. These
includie, for example, alkali metal or alkaline earth
metal hydroxides, alkali metal or alkaline earth metal
carbonates, pyridine, triethylamine, diisopropylethyl-
amine or N-methylpiperidine, or bicyclic amidines such as
1,5-diazabicyclo[3.4.0]-non-5-ene (DBN), 1,5-diaza-
bicyclo[3.4.0]undec-5 ene (DBU) or DABC0. Triethylamine,
Le A 28 861 - 18 -
. , .
` ~ ; !
,,
'
. ' ` ~,
'
2~9~
diisopropylethylamine, DABCO and potassium carbonate are
pre~erred.
The bases are in general employed in an amount from 1 to
3 mol, preferably from 1 to 1.5 mol, relative to 1 mol of
the corresponding carboxylic acid.
The process is in general carried out in a kemperature
range from ~0C to +150C, preferably from +0C to
+120C.
In general, the process is carried out at normal
pressure. However, it is also possible to carry out the
process at reduced pressure or at elevated pressure (for
example in a range from 0.5 to 5 bar).
Suitable solvents for the hydrolysis are water or water
in combination with a customary organic solvent. These
preferably include alcohols such as methanol, ethanol,
propanol, isopropanol or butanol, or ethers such as
tetrahydrofuran or dioxane, or dimethylformamide or
dimethyl sulphoxide~ Alcohols such as methanol t ethanol,
propanol or isopropanol are particularly pre~erably used.
The hydrolysis is carried out using acids such as, for
example, acetic acid, hydrochloric acid, hydrobromic
acid, methanesulphonic acid, sulphuric acid or perchloric
acid.
Le A 28 861 - 19 -
' ' : ' ` ~ ' ~ :'; : ,
.' ' '.'
;
2~931~g
The hydrolysis i~ in general carried ou-t in a temperature
range from 0c to -~130C, preferably from +20c to
-~llO~C .
In general, the hydrolysis is carried out at normal
pressure. However, it is also possible to work at reduced
pressure or at elevated pressure (Eor example from 0.5 to
5 bar).
When carrying out the hydrolysis, the acid is in general
employed in an amount from 1 to 3 mol, preferably from 1
to 1.5 mol, relative to 1 mol of the ester. Molar amounts
of the reactants are particularly pre~erably used.
The hydrolysis of tert-butyl esters is in general carried
out using acids, such a~, for example, hydrochloric acid
or trifluoroacetic acid, in the presence of one of the
abovementioned solvents and/or water or mixtures thereof,
preferably with dioxane or tetrahydrofuran.
The esterification of the acids is carried out according
to a customary method, by reacting the acids with the
corresponding alcohols, i~ appropriate in one of the
abovementioned solvents in the presence of a catalyst.
The corresponding alcohol is preferably then also
employed as a solvent.
Catalysts which can be employed are inorganic acids, such
as, for example, sulphuric acid, or inorganic acid
chlorides, such as, for example, thionyl chloride.
Le A 28 861 - 20 -
.
2~93~
In yeneral, 0.01 to 1 mol, preferably 0.05 to 0.5 mol, of
catalyst are employed relative to 1 mol of reactan-t.
The aminoprotective groups are removed by methods known
from the literature [cf. Houben-Weyl, "Methoden der
organischen Chemie" (~ethods of Organic Chemistry),
Synthese von Peptiden (Peptide synthesis) II, 4th
Edition, Vol. 15/1, 15/2, Georg Thieme Verlag Stuttgart],
preFerably using -trifluoroaceti.c acid in anisole.
The compounds of the general formula (II) are known as
actual substance representatives in some cases [cf. in
this connection EP 253,235 Al] or are new and can be
prepaxed, for example, by reacting compounds of the
general formula (VII)
o
F ~ C2~l6
(YII)
F H
in which
Rl6 represents a Cl-Cb-alkyl radical,
with an ~-halogenocarbonyl compound of the general
formula (VIII)
R3 C~ CH~--R2
¦ (VIII)
Le A 28 861 - 21 -
:
` :
~93~
in which
R2 and R3 have -the abovementioned meaning
and
X represents halogen, preferably bromine,
in one of the abovementioned solvents and bases,
preferably dimethyl~ormamide ancl potassium carbonate,
or
by reactlng compounds o~ the general formula (IX)
F ~ C ~ CO~Rj6 (IX)
F Rl7
in which
R16 has the abovementioned meaning and
Le A 28 861 - 22 -
.
21~3~8
R17 represents Cl-C"-alkoxy,
with compounds of the general formula (X)
NH2
1R (X)
in which ll 2
CHR,8
R2 has the abovementioned meaning
and
R1~ represents hydrogen or C1-C4-alkyl,
in one of the abovementioned solvents, preferably
ethanol, and if appropriate in the presence of an amine
base also mentioned there, and then converting with
dimethoxyethane and sodium hydride into the compounds of
the general formula (XI)
O
F ~ C2RI6 (XI~
F
R2
CHRl8
in which
Le A 28 861 - 23 -
.
~09~108
R2, Rl6 and Rl8 have the abovementioned meanings,
then cle~ving the double bond, by ozonolysis, to give the
carbonyl function according to customary methods,
and in a last step carrying out the cyclisation as
described above, and in the case of the other substi-
tuents mentioned above under R2 and R3, varying these
according to customary methods.
The reaction with the compounds of the general formula
(X) is carried out in a temperature range from -20C to
+30C, preferably from 0C to room temperature, and at
normal pressure.
The ozonolysis is in general carried out at -78C in a
solvent mixture of methanol~methylene chloride and under
a protective gas atmosphere.
The compounds of the general formulae (IX) and (X) are
known in some cases or can bé prepared according to
customary methods.
The compounds of the general formula (XI) are mainly new
and can be prepared according to the process described
above.
The compounds of the general formulae (VII) and (VIII)
are known per se [cf. in this connection EP-A~253,23S] or
can be prepared according to a customary method.
Le A 28 861 - 24 -
. .
.
.
2093~8
The compounds of -the general ~orm-llae (III) and (IIIa)
are also known, in some cases commercially available or
c~n be prepared according to customary methods.
The aldehydes of the general formula (IV) are known per
se or can be prepared according to published methods [cf.
EP-A-253,235].
The compounds of the general formula (V) are new and can
be prepared by converting compounds o~ the general
formula (XII)
~ ~ CO CO2RI
F ~ F ~ (XII),
F OC2H5
in which
Rl has the abovementioned meaning,
by a 2-step reaction with 3-amino-4-benzyloxy-1-butene of
the formula (XIII~
NH2
~ CH2-O - H2C- ~ (XIII)
Le A 28 861 - 25 -
.
.~
20931~
in one of the abovementioned solvents, preferably
ethanol, and then with sodium hydride in anhydrous
dimethoxyethane into the compound of the formula (XIV)
o
F ~ ~ ~ (XIV),
~H2
in which
RL has the abovementioned meaning,
and then carrying out an ozonolysis.
The ozonoly~is is in general carried out at -78C in a
solvent mixture of methanol/methylene chloride and under
a protective gas atmosphere.
The compound of the formula (XIV) is new and can be
prepared by the abovementioned process.
The compounds of the formulae (XII) and (XIII) are known.
The compounds of the formula tI) according to the inven-
tion show an additional unforeseeable, useful spectrum of
pharmacological action. Surprisingly, they are suitable
Le A 28 861 - 26 -
.
~ ' , ' ~ ' ' : ,
"' ~ ' .
2093~
for the treatment of virus-induced diseases.
The intermediates of the general formula (II) can also
exhibit this spectrum of action.
Detection of activlty was carried out in hepatoma cells
(HEP G 2.2.15) transfected with hepatitis s virus DNA.
The results of the examples listed below were determined
for the HBV test system described in the following
literature reference [Sells, M.A.; Chen. M.L., Acs, G.,
Proc. Natl. Acad. Sci. US~ ppO 1005 - 1009, Vol. 84
(1987)]:
Transfected hepatoma ce~ls (HEP G2.2.15) were incubated
with various concentrations of the respective compound.
By treatment of the transfected hepatoma cell line HEP
G2.2.15 with the compounds according to the invention,
the reduction of virus-specific HBV-DNA in the super-
natant as well as a reduction in the H~Ag level could be
~hown. It was furthermore found that the compounds
according to the invention lead to a reduction o~ the
replicative intermediates of hepatitis viruses. The
content of HBV-DNA was determined in the supernatant of
the cell cultures after PEG precipitation. This was
carried out using a nonradioactively-labelled HBV genomic
DNA sample [Pauly, P., 1982, Ph.D. Thesis, University of
Gottingen, F.R.G.; Kochel et al., 1990, EMBL, Accession
No. X 51790]. At the same time, the effect on HB~Ag
formation was determined by means of a commercially
available ELISA test. The IC50 value~ indicated relate to
Le A 28 861 - 27 -
.. ~ ... .
. .
' '1 , '~, " '
; ,: ~ :
;:
~07~3~ ~3
the substra-te concentra-t.ions which cause a 50% inhi~ition
of the HBV-DNA concentration in the s~lpernatant under the
abovementioned test conditions.
Table A
Ex. No. IC50~M) SI
I 0.5 ~ 100
VIII 0.5 > 100
9 0.1 ~ 20
At the same time, any cytotoxic effect of the compounds
according to the invention that might be present was
determined by means of staining with Crystal Violet or
via the incorporati.on of radioactively labelled thymidine
into the cellular DNA.
The action of the compounds according to the invention
on other cells was tested by incubation of HEL and MEF
cells. No effect on the vitality of these cell5 was seen
up to a concentration of 250 ~M.
As areas o~ indication for the compounds which can be
used according to the invention, there can be mentioned,
for example:
The treatment of acute and chronic virus infections which
can lead to hepatitis, for example infections with
hepatitis viruses; also virus .infections with herpes
viruses Types 1 and 2, cytomegaloviruses, varicella
Le A 28 861 - 28 -
.. .
:.
''; ' ,, ~
20~3~
231~9-74
zos-ter viruses and IIIV.
The treatment of chronic hepatitis B virus infections
and the treatment of acute hepatitis B virus infection is
particularly preferred.
The present invention includes pharmaceutical prepara-
tions which, besides non-toxic, inert pharmaceutically suitable
excipients, contain one or more compounds of the formula (r) or
(II) or which consists of one or more active compounds of the
formula (I) or (II), and processes for the production of these
preparations.
The active compounds of the formulae (I) and (II)
should be present in the abovemen-tioned pharmaceutical prepara-
tions in a concentration of about 0.1 to 99.5% by weight,
preferably of about 0.5 to 95~ by weight, of the total mixture.
The abovementioned pharmaceutical preparations can
also contain further pharmaceutical active compounds in addition
to the compounds of the formulae (I~ and (II).
The abovementioned pharmaceutical preparations are
prepared in a customary manner by known methods, for example by
mixing the active compound or compounds with the excipient or
excipients.
The invention also extends to a commercial package
containing a compound of the invention, together with instructions
for its use for combating viruses.
In general, it has proven advantageous both in human
and in veterinary medicine to administer the active compound
- 29 -
.. ...
- . ~ .
:. : . -:
~:,, ::
, `' ' . ,
~93~8
or compounds according to the invention in total amounts
from a~out O.S to about 500 mg/kg, preferahly 1 to lOO
mg/kg, of body weight eve~y 24 ho~lr~, if appropriate in
the form o~ several individual doses, to achieve the
desired results. An individual dose contains the active
compound or compounds preferably in amounts from about 1
to about 80 mglkg, in particular 1 to 30 mg/kg, of body
weight. However, it may be necessary to depart from the
doses mentioned, namely depending on the nature and the
body weight of the subject to be treated, the nature and
the severity of the disease, the method of preparation
and o-f administration of the medicament as well as the
period or interval within which administration takes
place.
Startinq Compounds
Example I
Ethyl 9,10-difluoro-2-ethoxycarbonyl-7-oxo-7H-
pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylate
o
F ~COzC~H5
0~
co2C2HS
Le A 28 861 - 30 -
.
. .
,
~9~11D8
Method [Al
The compound i~ prepared either by the process published
in EP-A-253,235 or via the intermediates a) and b)
mentioned in the following.
Method [B]
a) Ethyl 6,7,8-trifluoro-1 (2~ethoxycarbonyl-2-propen-
yl)-4-oxo-1,4-dihydroquinollne-3-carboxylate
O O
F
EtO O
7 g (25.9 n~ol) of ethyl 6~7,8-trifluoro-4-hydroxy-
3-quinolinecarboxylate and 4.3 g (31.3 mmol~ of KzCO3
in 60 ml of DMF are heated at 80C for 30 minutes
under argon. The heating bath is rPmoved and 5 ~
(25.9 mmol) of ethyl 2-(bromomethyl)-acrylate in
20 ml of DMF are added. The mixture is then stirred
at ~0C for 4 h. After cooling, the solid is fil-
tered off and the solution is concentrated. The
residue is rendered acidic using lN HCl, the mixture
is extractPd several times with CH~C12, and the
extracts are dried over MgSO~ and concentrated. This
Le A 28 861 - 31 -
,
. . :..... :
.
: ,: , -, ~ , :
` ~ : ~ ' : ': , :
;. :'
~0~311~
crude product fs combined with the solid ~iltered
of-f and purifled by chromatography orl ~ilica gel
(CH2Cl2:MeOH:NH3~aq~ = 100:5:2). After recrystallisa-
tion from ethyl acetate, 5.54 g (56 % of theory) of
the title compound are obt:ained.
Melting point: 188C.
b) Ethyl 6,7,8-trifluoro-1-(2-ethoxycarbonyl-2-oxo-
ethyl)-4-oxo-1,4-dihydroqllinoline-3-carboxyl~te
O O
F ~N
F J
0~
EtO ~ O
1 q (2.61 mmol) of the compound ~rom a) are dis-
solved in 150 ml of a mixture (1:1) of C~2Cl2 and
MeOH and the solution is cooled to -78C~ Ozone i5
then introduced untilthe solution is coloured blue. Excess
ozone is driven off by means of oxygen gas. The
mixture is treated with S ml of dimethyl sulphide,
allowed to warm to room temperature and stirred
until peroxides .are no longer detectahle. It is
concentrated to dryness in vacuo, the residue is
recrystallised from ethyl acetate and 0.785 g (78%
of theory~ of the title compound are obtained.
Melting point: 181C.
Le A 28 861 - 32 -
: , : - ;., : :
.
,
: , :
, .
2~31~
0.748 g (1.94 mmol) of the compound from b) and
1.54 g (11.1 ~ol) of K2CO3 ar~ treated lnder argon
wlth 30 ml of DMF . After 1 h, 70 ml of water are
added and the suspension is stirred for 30 minutes.
S The solid is Eiltered of~ with suction, washed twice
with water and twice with ether and dried in high
vacuum. 0.62 g (88% of theory) o~ the title compound
is obtained (Example I).
Melting point: 245C.
Example II
Ethyl 6,7,8-trifluoro-4-oxo-1-l4-pherlyl-~ut-1-en-3-yl)-
1,4-dihydroquinoline-3-carboxylate
o O
~t
A solution of 2.10 g (14.3 mmol) of 3-amino-4-phPnyl-l-
butene is added dropwise at 0C to a solution of 4.57 g
(14.3 mmol) of ethyl 3-ethoxy-2-(2,3,4,5-tetrafluoro-
benæoyl)acrylate in 20 ml of ethanol. The mixture is
stirred at room temperature for 2 h and then concentrated
in vacuo and the residue is dried in high vacuum. The
residue is dissolved in 20 ml of anhydrou~ dimethoxy-
ethanç and the solution is added dropwise at 0C to a
LP A 28 861 - 33 -
,~ ,
20931~
susper1sior1 of fiO2 Ing (~0~ slr(~rlq~ 1oil1 ~0 Illlnol ) or so(liulll hydri(lc~ ir
50 ml of an~1ydrous dimethoxyethal-le. The mi~ture i5
stirred at 0C ~or l h, treated with 4.5 ml of ylacial
acetic acid and concentrated in vacuo. The residue is
partitioned between methylene chloride and water. The
organic phase is dried using sodium sulphate, concen-
trated and chromatographed on silica gel using tolu-
ene/ethyl acetate.
Yield: 3.63 g (63%).
Example III
Ethyl l~ benzyloxy-l-butene-3-yl)-6,7,8-trifluoro~4-
quinolone-3-carboxylate
o
F ~ CO2C2H5
A solution of l4.5 g (82 mmol) of 3-amino-4-benzyloxy-l-
butene is added at OC to a solution of 26.25 g (82 mmol)
of ethyl 3-ethoxy-2-(2,3,4,5-tetrafluorobenzoyl)acrylate
in 105 ml of ethanol. The mixture is stirred at room
temperature for ~ h, then concentrated in vacuo and dried
in a high vacuum. The residue i5 dissolved in 200 ml of
anhydrous dimethoxyethane and added dropwise at 0C to a
suspension of 2.93 g (97 mmol) of sodium hydride (80%
strength in oil) in 235 ml of anhydrous dimethoxyethane.
The mixture is stirred at 0C ~or l h, treated with 22 ml
of glacial acetic acid and concentrated in vacuo. The
residue is partitioned between methylene chloride and
water. The organic phase is dried with sodium sulphate,
concentrated and chromatographed on silica gel using
toluene/ethyl acetate.
Yield: lS g (42%)
Melting point: 61-63C.
Le A 28 861 - 34 -
.
:. : :
Example IV 2093~08
Ethyl 3-benzyl-9,10-dL~lLtoro-7-oxo-7ll-pyrido[l,2~3
de][],4~benzoxazine-6-carboxylate
o o
'~1
Ozone i5 passed at -78C into a solution of 3.6 g
(9.0 mmol) of the compound from Example II in 80 ml of
methanol and 20 ml of methylene chloride until the onset
of a blue co].oration. Excess ozone is driven off with
nitrogen. 7.1 ml of dimethyl sulphide are added at -78C
and the mixture is allowed to come to room temperature.
It is conaentrated in vacuo, the residue i8 taken up in
50 ml of anhydrous DMF and the mixture i8 treated with
1~25 y (9.0 mmol) of potassium carhonate. ~fter 1 h ~t
room temperature 5 ml of glacial acetic acid are added
and then 500 ml of water, and the precipitate which
deposits is filtered off with suction and dried in high
vacuum.
Yield: 3-.3 g (95%); melting point: >260C~
Example V
Ethyl 3-~enzyloxymethyl-9,10-difluoro-7-oxo-7H-
pyrido[1,2,3-de]rl,4]benzoxazine-6-carboxylate
Le A 28 861 - 35 -
. ~ .
.
` 20g31~
o o
F ~E~
The title compound was prepared by use of the compound
from Example III in analogy to the procedure of Example
IV.
Yield: 45%.
Example VI
3-Benzyl-9,10-difluoro-7-oxo-7H-pyridoL1,2,3-de]tl,4~ben-
~oxazine-6-carboxylic acid
o o
F` ~
3.3 g (8.6 mmol) of the compound from Examp~le IV, 47 ml
of glacial acetic acid, 41 ml of water and 1 ml of
concentrated sulphuric acid are heated together under
reflux for 4 h. After cooling, the mixture i9 treated
with water, and the precipitate which deposits is
filtered off with ~uction and dried in high vacuum.
Yield 2.5 g (81%); melting point:>260C.
Le A 28 861 - 36 -
: ' ,. :
., , :
'
209~8
Example VII
3-Benzyloxymethyl-9,10-dLfluoro-7-oxo~-7H-pyrido[1,2,3-
d~][l,4]benzoxazine-6-carboxyllc acid
o o
~o~
The title compound wa~ prepared by reaction of tho
compound from Example V in analogy to the procedure of
Example VI.
Yield: 77%; melting point: 168-170C.
Example VIII
Ethyl 2-(1,1-dimethyl-4-phenoxy-butyl)-9,10-difluoro-7-
oxo 7H-pyrido[1,2,3-de][1,4]-benzoxazine-6~carboxylate
F ~ CO2C2Hs
0~
H3C >L (CH2)~ 0 ~3
Le A 28 861 - 37 -
~......... : , . : :.:. . . .
,
' - .'' - ~' :,.
2~)93~
2 g (7.38 mmolj of ethyl 6,7,8-trifluoro-4-hydroxy-3--
quinolonecarboxylate are ~u-qpended in 20 ml of abs. DME
under argon. 2.55 g (18.44 mmol~ of K2CO3 and 3.76 g
(14.75 mmol) o~ 1-chloro-3,3-dimekhyl-6-phenoxy-2-
S hexanone in 20 ml of abs. DMF are added and -the mixture
is heated at 80C for 6 h. A further 1.8 g (7.38 mmol)
of chloroketone are added and the mixture i5 heated at
80C for a further 14 h. After cooling, the mixture is
added to 400 ml of water and acidified with lN HC1. It
is extracted several times with CH2Cl2, and the organic
phase is washed with water, dried over Na2SO4 and con-
centrated. The residue is dissolved ln a little
CH2Cl2:MeOH = 100:3 and purified on a silica gel column
(same eluent).
Yield: 1.15 g (34 % of theory); melting point: 96C.
Example IX
2-(1,1-Dimethyl-4-phenoxybutyl)-9,10-difluoro-7-oxo-7H-
pyridotl,2,3-de][1,4]benzoxazine-6-carboxylic acid
o o
F ~ OH
' O~ ~
CH3 - _H3 ~
1.1 g (2.34 mmol) of the compound from Example VIII are
suspended in a mixture of l.S ml of H2SO4, 12 ml of H2O
Le A 28 861 - 38 -
, .
.: ,,
209~ 8
and 18 ml of ace~ic acid and the mixt:~lre is heat~d at
120C for 4 h. It is allowed to cool, and the precipitate
is filtered off with suction, washed with water and dried
in high vacuum. 0.89 g (86~ of theory) of the title
compound ar~ obtained.
Melting point: 212C.
Example X
9,10-Difluoro~2-(2-am.i.no-3-chloro-5-pyridyl)~7-oxo-7H-
pyrido[1,2,3-de][1,4]benzoxazine-6 carboxylic acid
O n
o~,~
CIJ~N
NH2
1.55 g (18.44 mmol) of K2CO3 are added under argon to 2 g
(7.38 mmol) of ethyl 6,7,8~trifluoro-4-hydroxy-3-
quinolonecarboxylate in 20 ml of abs. DMF. A solution of
3.7 g (14.75 mmol) of 1-ibromo-2-(2-amino-3-chloro-5-
pyridyl)~2-ethanone in 20 ml of abs. DMF is added to this
mixture and it is heated ~t 90C for 8 h~ The solvent i5
removed in vacuo, and the solid is stirred with water and
filtered o~f with suction. 'rhe residue is taken up with
acetone and the solution is concentrated until crystals
form. 'rhe crude product is filtered off with suction and
Le A 28 861 - 39 -
''
.'. ' ' ' '
`', ~, ' ' .
2~31 ~
purified on ~ilic~ yel (eluent CH~Cl2:MeOH = 100:5).2.58 g (83~ of theory) of the title compound are
obtain~d.
Mel-ting pOillt: 200C (decomposi.tion~.
Example XI
9,10 Difluoro-2-(2-amino~3-chloro-5-pyridyl)-7-oxo 7H-
pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid
F ~ ~ OH
0~
~ ' '. '
CI~N
NH2
12 ml of acetic acid, 8 ml of water and 3 ml of ~2SO4 are
added to 2.5 g (5.96 mmol) of the compound from
Ex~nple X. The su~pension is heated at 120C for 2 h.
After cooling, it is diluted with water, and the solid is
filtered off with suction and washed with water until
neutral. The product is dried in high vacuum. 702 mg
(30~ of theory) of the title compound are obtained.
Melting point: >230C.
Le A 28 861 - 40 -
.
- ' ' . ' , , ':
'
E m~e_XII 2093108
Ethyl 3~ imidazolylmethyl)-9,lO-di~luc)ro-7-oxo-7H-
pyrido[l,2,3-de][l,~]benzoxazine 6-carboxy].ate
O O
,~J~OEt
1==1
O ~ N ~ N
1.35 g (19.89 mmol) of imidazo:Le are added to 3.23 g
(9.94 mmol) of the compound from ~xample XIII and 1.37
(9.94 mmol) of K2CO3 in 30 ml of DMF and the mixture is
stirred at room temperature for 2.5 h. It i~ treated with
water, and the product is iltered off with suction,
washed with water and petroleum ether and dried in high
vacuum. 3.~1 g (92% o theory) of the title compound are
obtained.
Le A 28 861 - 41 -
: : ~ . , ...... ' , :: .
,: . ,' . i , ~
. . ~ . , ,
` ' . ;", ' ' , ~ , ,.: , ' ~''
, ' ,' ''. ~ . ,, ' ,
~093~0~
Example XIII
Ethyl 6,7,8-trifluoro-1-(2-oxo-1-methyl~ne~ethyl)-4-
quinolone-3~carboxylate
o
F ~,C2C2H5
~CH2
Ozone i~ introduced at -78C into a ~olution of 30.85 g
(~1.5 mmol) of the compound from Example III in 640 ml of
methanol and 160 ml of methylene chloride until it is
coloured blue. Excess ozone is driven of~ with nitrogen
at -7~C. 57 ml of dimethyl sulphide are added, and the
mixture is allowed to come to room temperature and is
stirred overnight at this temperature. After concentra-
tion in vacuo, the residue is chromatographed on silica
gel using methylene chloride/methanol.
Yield: 22.1 g (97%)
Melting point: 185-187Co
Le A 28 861 - 42 -
2093~
Example XIV
2 ethoxycarbonyl-3-(9,10-difluoro-6-ethoxycarbonyl-7-oxo-
7H-pyrido[1,2,3-de][1,4]benzoxazin-3-yl)propionic acid
F ~ CO2C2H5
F ~ N ~ CO2C2Hs
C02C2H5
1.75 g (10.9 mmol) of diethyl malonate are added to a
suspension of 329 mg (10.9 mmol) of sodium hydride (80~
strength in oil) in 55 ml of anhydrous DMF. As soon as a
clear solution is fo~ed, 3.55 g (10.9 mmol) of the
compound from Example XIII are added and the mixture is
stirred at room temperature for 4 h.5,5 ml of ylacial
acetic acid and 500 ml of water are added, and the
resulting precipitate is stirred overnight~ filtered off
with suction and washed with w~ter. After chromatography
on silica gel using methylene chloride/methanol, 3.44 g
(68%) of the title compound are obtained.
Example XV
3-(6-Carboxy-9,10-difluoro 7-oxo-7H-pyrido[1,2,3-
de][l,4]benzoxazin-3-yl)-propionic acid
Le A 28 861 - 43 -
. , ;
,: .
: ,, ., ,: , ~
.
, ,. . , - :
, : "
2093~
~ CO2H
400 mg (0.86 mmol) of the compouIId ~rom Example XIV are
heated under reflux for 4 h in 12 ml of glacial acetic
acid, 10 ml of water and 1 ml of sulphuric acid. After
cooling, the mixture is treated wlth 100 ml of water, and
the resulting precipitate i~ filtered off with suction,
washed with water and dried in high vacuum.
Yield: 271 mg ~94%)
Melting point: 252 254C.
Example XVI
Ethyl 1~ cyclopropyl-2-propenyl)-6~7,8-trifluoro-4-oxo-
1,4-dihydro-quinoline-3-carboxylate
o
; ~Co2c2Hs
,~7
The title compound is prepared from l-amino-1-cyclo-
propyl-2-propene in analogy to the procedure of Example
III.
Le A 28 861 - 44 -
,, .,.~ , .
.. : ~' ' ~: :` ,
, .. , .: .
. ~ .
20931~
Example XVI:[
3-Cyclopropyl-9,10-difluoro-7-oxo-7H-pyrido[1,2,3- -
de][l,4]benzoxazine 6-carboxylate
X~ ~ J
o~7
The compound i.s prepared from the compound of Example XVI
in analogy to the procedure of Example IV.
Example XVIII
3-Cyclopropyl-9,10-difluoro-7-oxo-pyrido[1,2,3
de][1~4]benzoxazine-6-carboxylic acid
F ~ CO2H
0~
The title compound is prepared from the compound of
Example XVII in analogy to the procedure of Example VI.
Le A 28 861 - 45 -
.. . . ~ "
. , - . :
. .
.,
~0~3~0~
Example XIX
Bromomethyl cyclopropyl ketone
Br
10.5 g (0.125 mol) of cyclopropyl methyl ketone are
dissolved in 100 ml of methanol and cooled to 0C.
19.95 g (0.125 mol) of brom.ine are added in one portion
and the mixture is stirred further at +10C until the
solution has been completely decolorised~ It is warmed
to room temperature, stirred for a further 30 min. and
40 ml o~ H20 are added. 'rhe mixture is again stirred for
15 min and added to 120 ml of H20. It is extracted four
times with 100 ml of ether each ~i.me. ~he organic phases
are combined and washed twice with 10% strength K2CO3
solution. They are then washed with satd. NaCl solution,
dried over MgS04 and concentrated in vacuo.
The brownish crude product is distilled and 12.2 g (60%
of theory) of the title compound are obtained,
B.p.12~ ~ 80-90C.
Le A 28 861 - 46 -
. , :, . . :~ ~ ;
:
.
20931~
_xc~1l3 XX
Ethyl 2~cyclopropyl-9,10-diEluoro-7-oxo-7H-pyrido[1,2,3-
de][1,4]benzoxazine-6-carboxylate
O O
F ~ ~ ` OEt
0~
5 g (18.4 mmol) of ethyl 6,7,8-trifluoro-4-hydroxy-3-
~uinoline~carboxylate are suspended under argon in 100 ml
of ab~. DMF. 6.37 g (46.1 mmol) of K2CO3 and 6.0 g
(36.9 mmol) o~ bromomethyl cyclopropyl ketone are added
and the mixture is heated at 90C for 12 h. The DMF is
distilled off in racuo. The residue is taken up with
CH2C12 and the solution is washed three times using 150 ml
of lN HC1 each time. The organic phase i~. washed with H2O
until neutral, dried over MgSO~ and concentrated. The
crude product is purified by column chromatography on
silica gel (elue~t C~2Cl~MeOH:NH3(~)= 100:2:1. The product
is taken up with toluene and the ~olution i9 filtered.
After concentrating again, it is recrystallised rom
ethyl acetate ~nd 2.1 g (34% of theory) of the title
compound are obtained.
Melting point 212C.
Le A 28 861 - 47 -
.
. . .
20931~
Exa~Le XXI
2-Cyclopropyl-9-lO-difluoro--7-oxo 7EI-pyrido[1,2,3-
de][1,4~benzoxazine-6-carboxylic acid
O O
F ~/1 ` J\OH
F~N
0~
200 mg (0.6 mmol) of the compound from Example XX are
S treated with 72 mg (3 mmol) of LioH and a mixture of 3 ml
of THF and 1 ml of H2O and stirred at 40C for 90 min. The
mixture iæ adjusted to p~ 1 with HCl and cooled to 0C,
and the precipitate is filtered off with suctionO The
residue is washed with H2O until neutral and dried in
vacuo. 130 mg (71% of theory) of the title compound are
obtained.
Melting point:>230C.
Le A 28 861 - 48 -
. ~ , . .
20931l~
Exanll)le XXI I
3~ Imidazolylmethyl)-9,10~difluoro-7-oxo-7H-
pyrido[l,2,3-de~[1,4]benzoxazine-6-carboxylicacidhydro-
chloride
O O
W )IJ~ x HCI
O~,N~,N
340 ml of 2N EICl are added to 3.41 g (9.13 mmol~ of the
compound from Example XII and the ~ixture is heated
under reflux for 4 h. It i9 filtered hot, the solution is
allowed to cool, and the crystals formed are filtered off
with suction and dried in high vacuum.
2.92 g (84% of theory~ of the title compound are thus
obtained.
Le A ?8 861 - 49 -
. . . . . . .
. : . : , , , ~ -
:,
:
.. . - ,
20~3:t~
reparation Exam~le~
Example 1
9-Fluoro-3-~ethyl-10-(4-(2-chlorophenyl)-piperazin-1-yl)-7-
oxo-7H-pyrido~1,2,3-de][1,4]benzoxazine-6-c~rboxylic ac.id
o
F ~ , ~ CO2H
N J O ~ CH3
Cl
300 mg (1.075 mmol) of 9,10-difluoro-3-methyl-7-oxo-7~
pyrido[l,2,3-de][1,4~benzoxazine-6-carboxylic acid are
suspended in 8 ml of DMSO and treated with 520 mg
t3.23 mmol) of N-(2-chlorophenyl-piperazine and the
mixture is heated at 100C for 2 h. The solvent is
removed in vacuum in a Kugelrohr device (bath temperature 80C),
the residue is stirred with ether, and the solid is
filtered off with suction and then stirred with isopropa-
nol, filtered off with suction and dried.
Yield 322 mg,.(95% of theory); m.p. 7265C.
The compounds listed in Tables 1 and 2 are prepared in
analogy to the procedure of Example 1:
Le A 28 861 - 50 -
~ :; . :
',
2093~
able 1 :.
o
¦~ N ~N~
~ N ~J O ~ CH3
Ex. No. R' M.p~ C Yield (% of
t,heory )
2 m-OCH3 2 3 7 5 7
3 o-C2Hs 2 8 0 5 6
4 o-OC2Hs 2 6 8 7 4
p-F 260 85
6 H 224 72
Le A 28 861 - 51 -
.; ~ ~. ''' ' . ' .
,
- 2~931~8
rrablQ 2:
F ~,3~ CO2H
R1~ J ~CH3
Ex. No. Rl M.p.C Yield (~ of
theory)
7 ~ dec. 45
N
8 /¢N~ 273 55
Example 9
10-(4-Cyclopropyl-piperazin-l-yl)9-fluoro-3-methyl-7-oxo-
7H-pyrido-[1,2,3 de]~l,4~ben~oxazine~ arboxylia acid
o
F ~ CO~H
~ N J ~ CH3
300 mg (1.075 mmol) oliii 9,10-difluoro-3-methyl-7-oxo-7H-
pyrido[l,2,3-de~1,4]benzoxazine-6-carboxylic acid are
Le A 28 861 - 52 -
- . .. .
' ' ' ' , ''. ~',.. ' , ' ~' '' ' ; : '.',' '
209~8
suspended Ln 8 ml of DMSO, -treated wi~h ]39.8 mg
(1.075 mmol) of 1-cyclopropylpiperazine and 240.8 mg
(2.15 mmol) of DA~CO and heated at 100C for 1 h. After
reaction is coMplete, the solvent is distilled off in a
S Kugelrohr device under vacuurn, the resiclue is suspended with isopro-
panol and the solid is filtered off with suction and
dried.
Yield: 398 mg (78% of theory) as a solid; m.p.C:>265.
The compounds listed in Tables 3, 4 and 5 are obtained in
analogy to the procedure of Example 9:
Table 3:
F~ ,CO2H
a~ N J o ~J~ CH3
Ex. No. R10 M.p.C Yield (~ of
theory~
-CH2CF3 255 87
Le A 28 861 - 53 -
.
2~93~
Table 4:
`f ~
HN~ J ~CH3
R
Ex. No. Rll Rl2 ~I.p. C Yield (% of
theory )
l l H -CH2-OC6H5 dec . 5 0
12 H -~CH2)20H 183 95
13 H -H2C-N~ O dec . 2 2
.
Le A 28 861 - 54 -
.
. .
20931~
Table 5:
R ')~ CO2H
~CI-13
Ex. No. R4 M.p. C Yield (~6 of
theory )
~`N--\NH
14 ~J 210 91 ;
Ç~N~
515 N~J 195 93
~N
16 l I dec. 47
N~
17 ~P'~ >265 64
H
H
18 ~N ~ >265 95
~o~N~
9 l >265 78
e A 28 861 - 55 -
- . . . . .
2 ~
Examp:le 2 0
3-Benzyl-9-fluoro-7-oxo-10-( 1-piperazinyl ) -7H-
pyrido[1, 2, 3-de ] [ 1, 4 ] benzoxazine-6-carboxylic acid
O O
F ~ OH
HN ~ ~ ~
355 mg ~1.0 n~ol) of the compound from Example VI, 5 ml
of DMSO, 1.7 ml of diisopropylethylamine (10 mmol) and
172 mg (2.0 mmol) of piperazine are heated under reflux
for 4 h. After cooling, tha mixture is treated with ether
and the precipitate which deposits is filtered off with
suction.
Yield: 290 mg (6~%); meltLn~ point: 169C (dec.).
The compounds listed in Table 6 are prspared in analogy
to the procedure of Example 20.
Le A 28 861 - 56
: . ' ', ..... , ,. ~ '
' . . , '` ' ~' ' . ` '
2093t ~
Table 6:
o
F ~ COzH
HN ~,J ~ R2
R~
Ex. No. R2 Rll M.p. C Yield (% of
theoxy)
21 -CH2C6H5 -CH3 248 250 36
(dec.)
2 2 -CH2--O-CH2-c6H5 H 2 2 8 -2 3 0 6 3
(dec.)
23 -CH2-O-CH2-C6H5 -C~3 186(dec.) 44
Example 24
2-(1,1,-Dimethyl-4-phenoxy-butyl)-9-fluoro-10-pipera-
zinyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-car-
boxylic acid
O O
F ~ OH
N ~ N
HN J O~ ~
CH3 ~ O ~
CH3
Le A 28 861 - 57 -
,,
.
.
` . . . . .
.
21~9~1 ~8
0.2 g (0.453 mmol) of the compound from Example IX are
suspended under argon in 2 ml of abso].ute DMF. 0.11 g
( 0 906 mmol ) of diisopropylethylamine and 0.12 g
( 1. 36 mmol ) of piperazine are added and the mixture is
heated at 120C for 4 h.
The solvent i9 dist.illed of~, the residue is treated with
ether and the product is filtered o~f with suction. It is
washed with water and dried in a high vacuum.
Exam~le 25
9 Fluoro-2-(1,1-dimethyl-4-phenoxy-butyl)-10 (3-methyl-
l-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-
6-carboxylic acid
(~
F ~ ~ CO2H
f N ~ N
CH3 H3C ~ (CH~3- O
H3C
The title compound is prepared using 2-methylpiperazine
in analogy to Example 24.
The examples listed in Table 7 are prepared in analogy to
the compounds mentioned therein:
Le A_28 861 - 58 -
.,
: : ;, . : ,
~93~ ~8
Table 7:
~, COzH
~N ~,J O ~J
R~ IP,11 ~`N
CI~J
N~2
Ex. No. R10 Rll Rl2 M.p.C Yield % In analogy
of theory to Example
26 -CH3 H H >230 60 24
27 H -CH3 H >230 67 24
28 H H H >230 59 24
29 H -CH3 -CH3 >230 40 24
Examp_e 30
Ethyl 9 fluoro-10-(4-methyl-1-piperazinyl)-7-oxo-7H-
pyrido[1,2,3~de][1 t 4]benzoxazine-2,7-dicarboxylate
F ~ CO2Et
N ~ O
CH3
CO2Et
Le A 28 861 - 59 -
: ,
2~93~ ~
O.S g (1.37 mmol) of the compound from Example I, 0.41 g
(4.1 n~ol) of N-methy].pipera~Lne and 0.35 g (2.74 mmol)
of HUnig base are treated with 15 ml of DMSO and stirred
at 120C for 4 h under argon. The mixture .is concentrated
in vacuo and the crude product is puri.fied by chromato-
graphy on si.lica gel (CH2Cl2:MeOH:NH3(~q) = 100:2.5:1).
0.116 g (19~ of theory) of the desired compound are
obtained.
Melting point: 168-170C.
Example 31
9-Fluoro-10- ( 4-methyl-1-piperazinyl) -7-oxo-7H-
pyrido [ 1, 2, 3-de ] ~ 1, 4 ] benzoxazine-2, 7 -dicarboxylic acid .
o
~--N~
CH3 . CO2H
104 mg (0.233 mmol) of the compound from Example 30 are
heated at 120C for 12 hours in 5 ml of an ~OAc/H~O/H2SO4
mixture (12:8-1). The solid is filt~red off with suction
and dried in a high vacuum~ 52 mg (57% of theory) of the
title compound are thu~ obtained.
M~lting point: >230C.
1H-NMR(DMSO-d6): 8.95 (s lH); 7.97 (s 1~); 7.49 (d J =
11.5; lH); 3.7-3.0 (m 8H); 2.85 (s 3H).
Le A 28 861 - 60 -
:~ :
: , :
:, ~. :
, ~ .:,, ' ' , , '
'
- ~
2~3~8
The compounds ].i~ted in Table 8 are prepared in analogy
to -the procedure of Example 20:
Table 8:
o
O ~ R
Ex. No. R4 R2
, - - -
32 N ~ N-CH3 -C~z-c6H5
33 -N 3 -CH2-C6Hs
-N O
34 ~ -(C~z~z-COzH
The compounds li~ted in Table 9 are prepared from the
compound of Example XVIII in analogy to the procedure of
Example 20.
Le A 28 861 - 61 -
'
,' , ,:
.
2~.93~ ~J8
Table 9:
o
F ~ 2
" ~ V
Ex. No. R4
~N N-
3 6 HO-(CH2)2-N~N
O N-
37 \ J
H3C
HN N-
3 8 ~
r~
H3C-N N-
3g ~
HN N-
/
Le A 28 861 - 62 -
.. . . .
. .
. . . , : :
:
2~93~ ~
Exampl~ 41
Ethyl 2-cyclopropyl-9-fluoro-10-(3-methyl-1-piperazinyl)-
7-oxo-7H-pyrido[1,2,3-de][1,4]-berlzoxazine-6-carbox-ylate
~OC2Hs
HN ~ ~J
Me ~,
0.5 g (1.5 mmol) of ethyl 2-cyclopropyl-9,10-di~luoro-7-
oxo-7H~pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylate are
~uspended under argon in 10 ml o ahs. DMSO. 0.364 g
(3 m~lol) of diisopropylethylamine and 0.451 g (4.5 mmol)
o~ 2-methylpiperaxine are added and the mixture is heated
at 120C for 6 h.
It i~ cooled and DMSO i~ ~tripped of on a rotary evapo-
rator. The residue is taken up in CH2C12, and the solution
is shaken with saturated NaC1 solution, dried over MgSO4
and concentrated. The crude product is purified on silica
gel (eluent CH2Cl2:MeOH:N~3(2~) - 100:2.5:1~ and yields
300 mg (48 % of theory) of the title compound.
Melting point: amorphou
H-NMR(CDCl3): 8.06 (~; lH); 7.57 (d J - 12.3; lH); 6.05
(s; lH); 4.36 (q J = 7; 2H); 3.2-2.7 (m); 1.52 (m; lH)
1.39 (tr J = 7;3H); 1.07 (d J = 6; 3~); 0.90 (m; 4H).
Le A 28 861 - 63 -
' . '.' - ' ' ,:
.
2~9~10~
Example 42
2-Cyclopropyl-9-fluoro-10-(3-methyl-1-pi.perazinyl)-7-oxo-
7H-pyrido[1,2,3-de][1,4]-ben~oxazine-6-carboxylic a~id
hydrochloride O O
~ ~ N
HN~J O~
Me ~
180 mg (O.43 mmol) of -the compound from Example 41 are
dissolved in a mixture of 6 ml of THF and 2 ml of H20,
and khe solution is treated with 52 mg (2.17 I~mOl) 0~
LioH and stirred at 40C ~or 6 h. The p~ i~ adjusted to
1 while cooling in an ice bath and the precipitate is
filtered off with suction af~er 30 min. Ik is washed with
cold wa~er until neutral and dri~d in a high vacuum.
92 mg (55% o~ theory) of the yellow title compound are
obtained.
Melting point:>230C.
Example 43
Ethyl 2-cyclopropyl-9-fluoro-10-(4-methyl-1-piperazinyl)-
7-oxo-7H-pyrido[1,2,3-de~ 4~-benzoxazine-6-carboxylate
. O
~N `o--CH3
~N
CH3
L~
Le A 28_861 - 64 -
: : '
~3~L~8
The ti-tle compound is obtalned in 52% of theory analo-
gously to the preparation of Example 41.
Melting point: 130C.
Example 44
Ethyl 2-cyclopropyl-9 fluoro-10-(4-methyl-1-pipera2inyl)-
7-oxo-7H-pyrido~1,2,3-de][1,4]-benzoxazine~6-carboxylic
acid hydrochloride O O
f ~ xHCl
CH3
238 mg (0.58 ~ol) of the compound from Example 43 are
di~solved in 5 ml o a CH3CO~H/~2O¦HzSO4 mixture (12:8:13
and the solution is heated at 100C for 4 hours. The
solvent i5 largely removed, the mixture is treated with
1 ml of conc. HCl and S0 ml of ethanol are added. It is
stirred overnight and the solid is filtered off with
suction in the cold~ The 95% pure crude product is
purified on silica gel (eluent CH2Cl2:CH3OH:CH3CO2H =
100:25:4) and again converted into the hydrochloride (see
above~. It is dried in a hiqh vacuum and 76 mg (32~ of
theory) of the title compound are obtained~
Melting point:>230C.
Le A 28 861 - 65 -
. ,
2~931L~8
The examples listed in Table ].0 are prepared in analogy
to the procedure of Example 20:
Table 10:
,~CO2H
O ~ ~ R
Ex~ No. R4 R2
____
-N NH
/ -H2C-N~N
-NN-CH3 ~ I
46 ~ -CH2-N~N
-N NH 1=1
-CH2-N~ N
CH3
4 8 -NAO -CH2-N ~ N
49 -N N~ -CH2 N~N
~ I I
5 0 -NN-(C ~l2)2H -CH2-N ~ N
Le A 28 861 - 66 -
., . -
:
, ~
,, . ~ . ,
. . ' ~ ' ~ ' ~ '