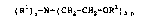Note: Descriptions are shown in the official language in which they were submitted.
2 ~
HOECHST AKTIENOE SELLSC~AFT ~OE 92/F 095 Dr. DA/b
De~cription
Polyurethane molding composition
The invention relate~ to a polyurethane molding
composition which, in order to achieve better mold
releace properties, contains a specific organic amide
having increa~ed reeistance to migration.
Both foamed polyurethane molding compositions and
polyurethane molding compo ition~ which can be proces~ed
thermoplastically have a very severe tendency to stick to
machine parts and to the molds. In order to prevent this
sticking, use i~ frequently made of externally applied
release agents, which are usually sprayed onto the
machine part~ or into the mold. In order to provide the
plastic components produced with mutual protection
against sticking together, the~e component~ are also
treated with external release agents.
Since, however, the application of an external release
agent signifies an additional and expensive operation,
attempts are i.ncreasingly being made to work with so-
called internal relea~e agents, which are incorporatedhomogeneously into the polymer matrix. However,~because
of incompatibilities, the adver~e effect of unde~ired
blooming from the polymer matrix at the surface of the
pla~tic component produced arises with thi~ type of
release agent. Con~equently, grea~y film~ can form on the
surfaces and enable sub equent proce~ing ~tep~, ~uch as,
for example, coating, to be carried out only with
additional expenditure on cleaning.
It i8 known that all of the relea~e ayent~ di~clo~ed
hitherto, for example including conventional montan waxes
and low molecular wei~ht fatty acid derivativeq, tend to
form depo~its (cf. US 4,889,908). Polyethers, which can
be obtained by reacting a sub~ituted 1,2-epoxyalk~ne
with a polyol~ have therefore been proposed as relea~e
~ 0 9 ~ 3
-- 2 --
agentY.
The object was now to find release agents which have a
good effect and a very low tendency to migration.
It has been found that the object can be achieved by
montan wax derivatives which are obtainable by reacting
amino alcohols with montanic acids.
The invention therefore relates to a polyurethane molding
composition comprising 90 to 99.99~ by weiqht, with
respect to the molding composition, of a polymer which
ha~ been obtained by reaction of a bifunctional higher
molecular weight polyhydroxy compound with a bifunctional
chain extender and an or~anic diisocyanate and 0.01 to
10% by weight, with respect to the molding composition,
of a compound of the formula
( Rl ~ n~N~ ( CH2-CH2-OR2 ) 3-n r
in which the R1s can be identical or different and are a
hydrogen atom, a C1 to C1a-alkyl group or the group R3-Co-,
in which R3 is an alkyl group having 23 - 35 carbon atoms,
the R2s can be identical or different and are a hydrogen
atom or the group R3-Co-, in which R3 has the
abovementioned meaning, n i~ 0, 1 or 2 and at least one
R3-Co- group is present in the moleculeO
The polyurethane molding composition according to the
invention comprises a polymer which is formed by reaction
of
A) essentially bifunctional, higher molecular weight
compounds essentially having two active hydrogen atoms
and a molecular mass of from 400 to 20,000, preferably
higher molecular weight polyhydroxy compounds,
~) es~entially bifunctional chain extenders, ~uch a~
diols or diamines having a molecular mass of from 32 to
399, and
C) organic dii~ocyanates, preferably aromatic
diiqocyanate~,
3 ~ ~
-- 3 --
D ) with the addition of, if appropri~te, ~tabilizer~ and
other additives known per se.
Starting components A), i.e. es~en$ially ~traight-chain
higher molecular weight compounds having predominantly
two active hydrogen atom~, ~uitable for the preparation
of the polyurethanes are virtually all essentially
straight-chain higher molecular weight compound~ known
per se which have about two reactive groups ~uch as
hydroxyl, primary and~or secondary amino, 5H, carboxyl
10 and/or other reactive groups, for example hydrazide
groups. Such compounds are, for instance, bifunctional
hydroxypolyesters, hydroxypolylactones,
hydroxypolyethers, hydroxypolythioether~,
hydroxypolye~ter amide~, hydroxypolycarbonate~, hydroxy
15 acetals or vinyl polymers containing hydroxyl groups or
other end groups or compounds which already contain
urethane and/or urea groups. These compound correspond
to the prior art and, for example, are de cribed in
detail in the literature.
20 These hydroxyl group-containing, higher molecular weight
compounds are preferably, for example, polyesterdiols
obtained from straight-chain or branched aliphatic and/or
cycloaliphatic diols and aliphatic dicarboxylic acids, in
particular adipic acid. Higher molecular weight polyamino
25 compounds, preferably having primary aromatic amino
groups, can optionally also be used. Particularly
prefexred polyols P.) are adipic acid polyester~ or
caprolactone diols or polycarbonate-diol~, optionally as
a mixture with polyether~.
30 The chain extenders B) to be u~ed are known per ~e and
widely described. The~e are in particular low molecular
weight polyalcohol~, preferably diols, and diamines, in
particular ( cyclo ) aromatic diamines . Diamines which may
be mention~d are 4, 4-diamino-dicyclohexylmethane,
35 isophoronediamine, ethylenediamine and 1, 3- or 1, 4
diaminocyclohexane. Preferred chain extender~ are diols
~93~ 8~
~uch as, for example, ethylene glycol, hexane-1,6-diol
and hydroquinone di-~-hydroxyethyl ether, as well as
butane-1,4-diol~ optionally in mixtures with other diols,
in p~rticular hexan~-1,6-diol~ The molecular mas~ of the
chain extender~ i~ from 32 to 399.
In addition, it is possible to u~e monofunctional
compounds as ~o-called chain terminator~, in amount~ of
from about 0.01 to 3% by weight, with re pect to PU
solid. Examples are monoalcohols, such as butanol, 2-
ethylenehexanol, isobutanol, l~octanol, and 3tearylalcohol, or monoamines, such as aniline, dibutylamine, N-
methylstearylamine or piperidine.
Dii~ocyanates C) to be used are the aliphatic,
cycloaliphatic, araliphatic, aromatic or heterocyclic
diisocyanate~ of the prior art known per se. Preferred
diisocyanates are hexamethylene diisocyanate, i60phorone
diisocyanate, naphthylene 1,5-diisocyanate, tetramethyl-
xylylene diisocyanate, 3,3-dimethyl-4,4-diisocyanato-
diphenyl (TODI~, 1,4-diisocyanatobenzene and the
corresponding hydrogenated product, toluylene
diisocyanates and, in particular, the diphenylmethyl
diisocyanate isomers and the corresponding hydrogenatsd
products. 4,4-diisocyanatodiphenylmethanP or the mixture
of it~ isomers may be mentioned. The said diisocyanate~
can optionally be used together with a polyisocyanate of
higher functionality; the amount of the polyi~ocyanate of
higher functionaliky must, however, ~e ~o restricted that
a polyurethane ela~tomer is obtained which iB ~till
meltable or thermoplastic. Example~ of i~ocyanates of
higher functionality and monofunctional compounds are
al~o described in the literature. Examples which may be
mentioned are monoamine~, such a~ butylamine or
dibutylamine, hydroxylamine, ~tearylamine,
N-methylstearylamine, pyrrolidone or tetrahexylamine or
butanone oxime, and al o monoalcohol~, such a~ l~butanol,
2-ethyl-1-hexanol, 1-dodecanol, i~obutanol or t-butanol,
- 5 -
cyclohexanol or ethylene glycol monomethyl ether.
Catalysts which can be used are, for example, tertiary
amines or organic metal compounds, in particular organic
tin, lead and titanium compounds, for example tin(II)
acetate, tin(II~ ethylhexoate, dibutyltin dilaurate or
lead acetate.
The amounts of reactants A~ to C) for the polyurethane3
are a3 a rule 80 cho~en that the NCO/OH equivalent ratio
of isocyanate to OH compounds is between 0.9 and l.2.
In addition to the polymer, the polyurethane molding
composition according to the invention contain~, as
release agent, a compound of the formula
( Rl ) n~N~ ( CEI2-C~12-OR2 ) 3-n ~
in which the Rls can be identical or different and are a
hydrogen atom, a C1 to Cl3-alkyl group, preferably a
hydrogen atom, or the group R3-Co-, in which R3 is an
alkyl group having 23-35, preferably 27-31 carbon atoms,
the R2s can be identical or different and are a hydrogen
atom or the group R3-Co-, in which R3 has the
abovementioned meaning, n is 0, 1 or 2, preferably 1 and
at least one R3~Co- group is present in the molecule. The
R3-Co- group i~ preferably the radical of a technical
grade montanic acid which has been obtained by oxidative
bleaching of crude montan wax and i8 a mixture of C24-C36-
carboxylic acids with the emphasis on C28-C32 and contains
about 15% by weight of dicarboxylic acids.
The compound~ to be used according to the invention are
prepared by reacting C24-C36-carboxylic acids, preferably
technical grade montanic acid, with amino alcohol~ of the
formula
( Rl ) n~N~ ( CEi[z-CHz-OH ) 3-n
in which Rl and n have the abovementioned meanings, in
accordance with conventional esterification and amidation
method~.
~3~
-- 6 --
Suitable amino alcohols are ethanolamine, diethanolamine,
triethanolamine and the alkyl derivatives thereof.
Suitable C24-C36-carboxylic acids are, for example,
tetracosanoic acid, cerotic acid, montanic acid and
melissic acid, preferably technical grade montanic acid
which has been obtained by oxidative bleaching of crude
montan wax and is a mixture of C24-C36-carboxylic acids
with the emphasis on C2~ ~o C3~ and contains about 15% by
weight of di~arboxylic acid~.
~he compounds ~re waxy bodie~ which have drop points of
from about 60 to 120C.
The polyurethane molding composition according to the
invention comprises 90 to 99.99, preferably 97 to 99.9%
by weight of the polymer and 0.01 to 10, preferably 0.1
to 3.0% by weight of the release agent to be used
according to the invention.
In addition, the polyurethane molding ~omposition can
contain antioxidants, light stabilizers, metal
deactivators, stabilizers, propellants, fillers,
reinforcing agents, lubricants, pigments, fluorescent
brighteners, flame retardants or antistatic agents.
Mixing of the release agents according to the invention
into the polyurethane moldin~ composition is effected in
the manner customary in pla~tics proces~ing, for example
by mixing the compounds and, optionally, further
additives into the melt prior to shaping. Incorporation
can also be effected by applying the dissolved or
dispersed compounds directly onto the polymer or by
mixing into a solution~ suspension or emulsion of the
~0 polymer, allowing the solvent to evaporate ~ubsequ0ntly,
if appropriate. The use of extruders or kneaders for
compounding or physical mixing, for example in slow-speed
mixexs, i~ also possible.
~ o ~
-- 7 --
The following examples are intended to illu~trate the
pre~ent invention.
Example 1
Reaction of technical grade montanic acid with
diethanolamine in a molar ratio of 2.25-1
The technical grade montanic acid was introduced into a
reactor and melted under an N2 atmosphere. The
diethanolamine was metered in at a temperature of 90 to
100C. The mixture was heated to 140C and ~tirred at
this temperature until the acid value had fallen below 15
mg KOH/g. The melt was then cooled and ground.
Drop point about 82C; acid value about 12 mg KOH/g;
saponifisation value about 135 mg KO~/g; drawing hardness
about 550 bar.
lS Example 2
Reaction of technical grade montanic acid with tallow fat
diethanolamine in a molar ratio of 1.6:1
The technical grade montanic acid was introduced into the
reactor and melted under an N2 atmosphere. The tallow fat
diethanolamine (Rl = radical of tallow fatty alcohol) was
addPd slowly to the hot melt~ which wa~ at a temperature
of 90 to 100C. The mixture was then heated to 140C and
stirred at thi~ temperature until the acid value had
fallen below 15 mg KOH/g. ~he melt wa~ then cooled and
ground.
Drop point about 75C; acid value about 12 mg KOH/g;
~aponification value about 100 mg KO~/g; drawing hardnefis
about 60 bar.
~ ~ 9 ~ 3
Example 3
1 mm shee~s having dimen~ions of 60 x 60 mm were prepared
for the migration teQt; plasticizing and homogenization
were carried out using a kneader and the subsequent
shaping was carried out u~ing a press. The ~pecimens thus
obtained were freely suspended in a circulating air oven
and conditioned for 24 h at 100C. After this 24 h, the
specimens were evaluated according to the criteria of
"visible" and "discernakle" deposit.
For characterization of the relea~e action, the release
characteri~tics of 1 mm sheets on ~teel sheet~ were
assessed. To this end, the specimen obtained after a
pressing operation (150C, 100 bar, 180 B) was manually
pulled off from the inserted steel ~heet. The evaluation
was subjectively determined and classified in accordance
with a compari~on ~cale.
The polymer used was a polyester-polyurethane having a
Shore A hardness of 93 and a density (23C) of 1.19 g/cm3
and a polyether-polyurethane having a Shore A hardness o
85 and a density (23~C) of 1.12 g/cm3.
The results are summarized in the following table.
Specimens:
1 mm pressed sheets, additive addition 1%
Migration tests after 24 h at 100C, criterium deposit
formation
~elea3e action: release characteri~tics o 1 mm sheets
from steel ~heet following a preceding pressing operation
Table
ExPmple Polyester-urethane Polyether-ureth~ne
Deposit Release Deposit R21ease
formation charact- formation charact-
eristics eristics
Comp. A none poor none poor
Comp. B slight good slight good
Comp. C moderate very good moderPte very goDd
Comp. D slight good slight moderate
10 Comp. E Rlight moderate slight moderate
Ex. 1 none good none good
Ex. 2 none good none good
Comparison A: Without additive
Comparison B: Ethanediol ester of montanic acid
Comparison C: Ethylenediamide of stearic acid
Comparison D: Trimethylolpropane ester of montanic acid
Comparison E: Pentaerythritol eseer of montanic acid
Assessment system: none - slight - moderate - severe deposit
formation
poor - moderate - good - very good release characteristics