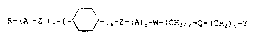Note: Descriptions are shown in the official language in which they were submitted.
~t~C~1~
Liquid-cryHtalline compounds
The invention relate~ to liquid-crystalline
compound~ of the formula I
R-(Al-Z1)~-[_ ~ ]~-Z-(A)o~W~(CH2):~Q~(CH2)t-y
in which
R is H, an alkyl or alkenyl radical having 1 to
15 carbon atoms which is unsubstituted,
monosubstituted by CN or CF3 or at least mono-
substituted by halogen, it also being
possible for one or more C82 groups in these
radicals each to be replaced, independently
of one another, by -O-, -S-, - O -, -CO-,
-CO-O-, -O-CO- or -O-CO-O- in such a way that
oxygen atom~ ~ro not lin~od directly to one
another,
Z1 and Z are each, independently of one another,
-C~zCB2-, -C~-C~-t -C.C- or a single bond, and
one of the radicals Z~ and Z i9 alternatively
-(C~2),- or -c~c8-ca2c82-,
A and A1 are each, independently of one another,
trans-1,4-cyclohexylene in which, in addi-
tion,-one or two non-adjacent C~2 groups may
bo replaced by -O-, or aro 1,4-phenylene
which i8 un~ubstituted or monosubstituted or
disubstituted by fluorine and/or Cl atoms and
in which, in addition, one or two C~ groups
may bo replaced by N,
m i8 O, 1, 2 or 3,
o and ~ is O, 1 or 2, where (8+0) is 2 2,
30 W is -O-, -COO- or a single bond,
Q is -O-, -C8-CH- or a single bond,
r is 1 to 7,
t i~ O to 7, and
Y is F, Cl, CF3, OCF3, CHFz, OC8F2 or OC~2F.
- 2 - ~ ~ ~3212
The invention furthermore relates to the use of
these compounds as components of liquid-crystalline
media, and to liquid-crystal and electro-optical display
elements which contain the liquid-crystalline media
S according to the invention.
The compounds of the formula I can be used a~
components of liquid-crystalline media, in particular for
displays based on the principle of the twistQd cell, the
guest-host ef~ect, tho offect of deformation of aligned
phase~ or the effect of tynamic scattering.
The sub~tances employed hitherto for this purpose
have certain disadvantagea, for example excessively high
melting points, excessively low clearing points,
inadequate stability toward~ the action of heat, light or
electric fields, inadequate electrlcal re~istance,
exces~ively hiqh temperaturo dependence of the thre~hold
voltage, or unfavorable ela~tic and/or dielectric
proporti~3.
In particular in displays of the supertwi~t type
(STN) having twist angles of significantly greater than
220 or in displays having an activo matrix, the
material~ employed hithorto have disadvantage3.
The invention had the obj~ct of finding novel
stable liquid-crystalline or m sogcnic compounds which
are suitablo a~ components of liquid-cry~talline media
and at the same time, have, in particular, relatively low
vi~co~ity and relatively high dielectric anisotropy ant
high nematogeneity.
It has been found that compounds of the formula
I are eminently suitable as component~ of liquid-
crystalline media. In particular, they have relatively
low vi~cosities and high nematogeneities. They can be
used to obtain stable liquid-crystalline media havinq a
broad mesophase range and advantageou~ values for the
optical and dielectric anisotropy. These media
furthermore have very qood low-temperature behaviour, ie.
excellent solubility in the conventional LC materials, at
the same time occurrence of smectic phases being
- 3
effectivel~ suppressed.
Liquid crystals of the formula
CsHl; ~~oc6Hl 3
have already been di~closed in WO 8902425.
JP 59/155485-A2 m ntions a compound of the formula
CqHg~~O~C4Hg
Finally, EP 27B665 describe3 compounds of the formula
Alkyl- ~ ~ O-CH2CH--Alkyl
F
DE 31 36 624, DE 32 09 178 and DE 34 10 734 disclose
compound~ of tho following formulao:
CsHI 1 -~O-C2Hs
CsHll~O~CnH2n~.
n - 1 or 6
~ F n - 5 or 7,
CnH2ntl~~C2Hq~O~C~H2m~1 m - 1, 2, 6, 10 or 11-
CsH~ }C2Hs
JP 59/01,684 discloses 3-fluoro-4-substltuted
4-bicyclohexylbenzenes of the formula
- 4 _ 2093~1~
R ~ ~ ~ (R, R' - CllO-alkyl).
WO 88/05 803 A relates to esters of the general
formula
Alkyl- ~ COO-(CH2)ls~(CF2)s-1o~H
EP 0 360 521 A describes compounds of the general
formula
Alkyl-O-~}OCO-<~ (CO) ~/l-o-cH2- (CF2) 3~s--F
Cl
WO 89/02 884 discloses trifluoromethyl ethers of
the formula
R- ~ ~ GCF3
Finally, DE-A 39 09 802 describë~ compounds of
the formula
~30 (CH2) ncHF2
EP-A 0 168 683 discloses liquid crystals of the
following formulae:
alkyl- ~ ~ O-(CH2)n-CH=CH-alkyl
alkyl- ~ ~ ~ O-(CH2)n-CH=CH-alkyl
The compounds of the formula I where Y = CF3
facilitate both STN display~ having a very steep electro-
optical characteristic line and displays having an activematrix and excellent long-term 3tability. Compared with
non-fluorinated-compounds, the compounds according to the
invention have both higher ~ and higher ~1. Due to their
- s - 20~
particularly favourable elastic propertie~, they are
particularly suitable as components for TF~ mixture~ A
suitable choice of r and s allows the threshold voltages
to be signif icantly reduced in di~plays of both types.
However, in view of the very wide variety of
areas of application of such compounds, it was desirable
to have available further compounds which have propertie~
precisely customised to the particular application~.
- In addition, the provision of compounds of the
formula I very generally considerably broAdens the range
of liquid-crystalline substance~ which ara suitable, from
various applicational points of view, for the preparation
of liquid-crystalline mixtures.
~he compound~ of the formula I havs a broad range
of applications. Depending on the choice of substituents,
~he~e compounds can be used as ba~e materials from which
liquid-cryst~llina media are predominantly composed;
however, it i~ also po~sible to ~dd co~poundr of th0
formula I to liquid-crystalline b~e materials from other
classes of compound, in order, for example, to influence
the dielectric and/or optical anlsotropy of a dielectric
of this type and/or to optimize it8 threshold voltage
and/or its viscosity.
In the pure state, the compounds of the formula
I are colourless and form liquid-cry~talline mesophasQ~
in a temperatura range which is favor~bly located for
electro-optical use. They re stable ch~ic~lly,
thermally and to light.
The invention thus relate~ to the compounds of
the formula I and to the use of thase compounds as
components of liquid-crystalline media. The invention
furthermore relates to liquid-crystalline media
containing at least one compound of the formula I and to
liquid-crystal display elements, in particular electro-
optical display elements, which contain media of thistype.
Above and below, R, Al, A, zl, z, W, Q, Y, m, o,
r and t are as defined above, unless expressly stated
- 6 - ~ ~93~
otherwise. If A1 and A are a substituted 1,4-phenylene
ring, the phenylene ring is preferably substituted in the
2-, 2,3- or 2,6-position by fluorine.
Z~ and Z are preferably a single bond or -C82CH2-.
If one of the radicalg Z1 and Z i9 - ( CH2 ) 2-
-(CH2)4- or -CH-CH-CHzCH2-, the other radical Zl or Z i~
preferably a sinqle bond.
PrefQrred compounds of thi~ typ~ conorm to the
sub-formula I~
F
~ 3Z-~W_ (CH2) r~Q~ (CH2) t~Y I ~
in which Z is -(C~2)~- or -c~-ca-c~2c~2-, and R, r, t, L,
W, Q and Y are a~ defined in for~ula I.
Preference i8 likewise given to co~pounds of the
~ub-formula I''
F
R_~Zl~~}Z~W~ (CH2) r~ (CH2) t--Y I ~ ~
in which L i8 B or F, and R, Z~, W, Q, Y, r and t are as
defined in Claim 1. If t - O, Q i~ preferably a single
bond.
Y may also be ~ if W ~ O or denotes a single
bond.
In the following, for reasons of ~implicity, A''
i8 a 1,4-cyclohexylene radiGal, B i~ a radical of the
formula ~
~' .
where L i8 preferably F. Y is preferably Fr Cl or CF3, and
r and s are preferably 1, 2, 3 or 4. Q i9 preferably a
single bond.
The compoundq of the formula I accordingly
include compound~ of the sub-formulae
~ 7 ~ 2~3~
R-A" -A' ' -a-~ (cH2 ) r- (cH2 ) -Y I ' ' a
R-A' ' -A' ' -3-W- (CH2 ) r~0~ (CH2 ): ~Y I ' ' b
R-A' ' -Z:-A' ' -B-W--(CH2) r~ (CHz) -Y I ' ' c
R-A' ~ -Z l -A' ' -B-W (CH2 ) r~0~ (CH2 ) -Y I ' ' d
R-A' ' -A' ' -Z2-B-W- (CH2 ) r~ (Ci{2 ) ~-Y I ' ' e
R-A' ' -A~ ' -Z2-B-W--(CH2 ) c~~ (CH2 ) ~-Y I ' ' f
R-A' ' -Z' -A' ' -Z2-B-W- (CH2 ) r~ (CH2 ) c-Y I ' ' g
R-A~ ~ -Zl-A~ ' -Z2-B-W- (CH2) r~O~ (CH2~ ~~Y I ~ ~ h
Of the~e, particular preference is given to those
of the sub-formulae I~a, I~b, I~e and I~f. In the
compounds of the sub-formulae I~c to I~h, Z1 and Z are
-CH2C~2-, -CH-CH- or -C.C-, preferably -CH2CH2-.
Particular preference i8 al80 given to the
compound~ of the formuln LA,
CnH2n~l~D~ (CH2) ~~ [~a] ~ - Z~ (A) 0~O~ (CH2) -(CH=CH)b-CF3 IA
in which n i8 0 to 7, D i8 -0~~ -C~=CH- or a single bond,
a i8 1 to 5, b i3 0 or 1, and Z, A, o, and r are as
defin~d in Claim 1.
Particularly preferred sub-formulae of compounds
IA are
- the compounds of the formula Ia
CH3O- (CH2 ) ~~ [ ~ 9-Z- (A) o~O~(CH2)r-(CH=CH)b-CF3 Ia
in which a Ls 1 to 5, b i8 0 or 1, ~ is 1 or 2 and
A, Z, o and r are 8 defined in Claim l;
- the compound~ of the formula Ib
cnH2~ - - [ - a Z ~A) 0~O~ (CH2) r~ ~CH=CH)~-CF3 Ib
Ln which n i8 1 to 7, 8 is 1 or 2, b i3 0 or 1 and
A, Z, o and r are as defined in Claim 1;
- the compound3 of the formula Ic,
~3~
L
R-(Al-Z;)~-[- ~ L Ic
in which Ll is a, F or Cl, L2 i~ F or Cl, and R, Al, zl, z,
m and g are a~ defined in Claim 1.
If R in the formula I i~ an alkyl radical and/or
an alkoxy radical, this may be str~ight-chain or
branched. It i~ preferably ~traight-chain, has 2, 3, 4,
5, 6 or 7 carbon atom~ and i8 accordingly preferably
ethyl, propyl, butyl, pentyl, haxyl, heptyl, ethoxy,
propoxy, butoxy, pentoxy, hexoxy or heptoxy, further~ore
methyl, octyl, nonyl, decyl, undecyl, d`odecyl, ~ridecyl,
tetradecyl, pentadecyl, mothoxy, octoxy, nonoxy, decoxy,
undecoxy, dodecoxy, tridecoxy or tetradecoxy.
Oxa~lkyl i~ profor~bly straight-chain 2-oxapropyl
(- methoxymethyl), 2- l~ ethoxymethyl) or 3-oxabutyl
(~ 2-methoxyethyl)~ 2-, 3- or 4-oxapentyl, 2-, 3-, 4- or
5-oxah~xyl, 2-, 3-, 4-, S- or 6-ox~heptyl, 2-, 3-, 4-,
5-, 6- or 7-oxaoctyl, 2-, 3-, 4-, 5-, 6-, 7- or
8-oxanonyl, or 2-, 3-, 4-, 5-, 6-, 7-, a- or 9-oxadecyl.
If R is an alkenyl radical, it may be ~traight-
chai~ or branched. It i preferably straiqht-chain and
ha3 2 to 10 carbon atoms. Accordingly, it is in par-
ticular ~inyl, prop-1-, or prop-2-enyl, but-l-, 2- or
but-3-onyl, pent-1-, 2-, 3- or pent-4-enyl, hex-1-, 2-,
3-, 4- or hex-5-enyl, hept-1-, 2-, 3-, 4-, S- or
hept-6-enyl, oct-1-, 2-, 3-, 4-, 5-, 6- or oct-7-enyl,
non-1-, 2-, 3-, 4-, 5-, 6-, 7- or non-8-enyl, or dec-1-,
2-, 3-, 4-, 5-, 6-, 7-, 8- or dec-9-enyl.
If R i~ an alkyl radical in which one ca2 group
has been replaced by -O- and one has been replaced by
-CO-, the~e are preferably adjacent. These thus contain
an acyloxy group -CO-O- or an oxycarbonyl group -O-CO-.
These are preferably straight-chain and have 2 to 6
carbon atoms.
9 2~g~
Accordingly, they are ln particular acetyloxy,
propionyloxy, butyryloxy, pentanoyloxy, hexanoyloxy,
acetyloxymethyl, propionyloxymethyl, butyryloxymethyl,
pentanoyloxymethyl, 2-acetyloxyethyl, 2-propionyloxy-
ethyl, 2-butyryloxyethyl, 3-acetyloxypropyl, 3-propionyl-
oxypropyl, 4-acetyloxybutyl, methoxycarbonyl, ethoxycar-
bonyl, propoxycarbonyl, butoxycarbonyl, pentoxycarbonyl,
methoxycarbonylmethyl, ethoxycarbonylmethyl, propoxy-
carbonylmethyl, butoxycarbonylmethyl, 2-(methoxycar-
bonyl)ethyl, 2-(ethoxycarbonyl)ethyl, 2-(propoxycar-
bonyl)ethyl, 3-(methoxycarbonyl)propyl, 3-(ethoxycar-
bonyl)propyl or 4-~methoxycarbonyl)butyl.
If R i8 an alkenyl radical in which one C~2 group
adjacent to the vinyl group has been replaced by C0 or
C0-0 or 0-C0-, thi~ may be straiqht-cha~in or branched. It
iff preferably straight-chain and ha~ 4 to 13 carbon
atom~. Accordingly, it is in particular acryloyloxy-
methyl, 2-acryloyloxyethyl, 3-acryloyloxypropyl,
4-acryloyloxybutyl, 5-acryloyloxypentyl, 6-acryloyloxy-
hexyl, 7-acryloyloxyheptyl, 8- cryloyloxyoctyl, 9-acrylo-
yloxynonyl, 10-acryloyloxydecyl, metb~cryloyloxymethyl,
2-mothacryloyloxyethyl, 3-meth~cryloyloxypropyl,
4-methacryloyloxy-butyl, 5-mothacryloyloxypentyl,
6-mothacryloyloxyhexyl, 7-mothacryloyloxyheptyl,
8-methacryloyloxyoctyl or 9-methacryloyloxynonyl.
If R i~ an alkyl or alkenyl radical which is
~oAorub~tituted by CN or CF3, thi~ radical is preferably
straight-chain and the substitution by CN or CF3 i8 in the
~-po~ition.
If R i~ an alkyl or alkenyl radical which is at
lea~t mono~ubstituted by halogen, this radical is prefer-
ably straight-chain and halogen i~ preferably F or Cl. In
the caRe of multiple substitution, halogen i~ preferably
F. The resultant radicals also include perfluorinated
radical~. In the case of monosubstitution, the fluorine
or chlorine ~ubstituent may be in any desired po~ition,
but is preferably in the ~-position.
- 10~
Compound-Q of the formulae I which contain wing
groups R which are suitable for polymerisation reaction~
are suitable for the formation of liquid-cry~talline
polymer~.
Compounds of the formula I containing branchsd
wing groups R may occa ionally be of importance due to
better solubility in the customary liquid-cry~talline
base materials, but in particular as chiral dopants if
they are optically acti~e. Smectlc compounds of this type
are Auitable a~ component~ of ferroelectric materials.
Compound~ of the formula I having SA phase~ are suitable,
for example, for thermally addre~ed display~.
Branched groups of this type generally contain
not more than one chain br~nch. Preferred branched
radical~ R are isopropyl, 2-butyl (~ methylpropyl),
isobutyl (= 2-methylpropyl), 2-methylbutyl, isopentyl
(= 3-methylbutyl), 2-methylpentyl, 3-methylpentyl,
2-ethylhexyl, 2-propylpentyl, isopropoxy, 2-methyl-
propoxy, 2-methylbutoxy, 3-methylbutoxy, 2-methylpentoxy,
3-methylpentoxy, 2-ethylhexoxy, l-methylhexoxy and
l-methylheptoxy.
If R is an alkyl radic~l in which two or more CH2
groups have been replaced by -0- and/or -C0-0-, this may
be stra~ght-chain or branched. It i8 preferably branched
and has 3 to 12 carbon atoms. Accordingly, it i~ in par-
ticular biscarboxymethyl, 2,2-biscarboxyethyl, 3,3-biQ-
carbo~ypropyl,4,4-biscarboxybutyl,5,5-biscarboxypantyl,
6,6-biscarboxyhexyl, 7,7-biscarboxyheptyl, 8,8-biscar-
boxyoctyl, 9,9-biscarboxynonyl, 10,10-biscarboxydecyl,
bi~(methoxycarbonyl)methyl, 2,2-bi~(methoxycarbonyl)-
ethyl, 3,3-bis(methoxycarbonyl)propyl, 4,4-bis(methoxy-
carbonyl)butyl, 5,5-bis(methoxycarbonyl)pentyl, 6,6-bis-
(methoxycarbonyl)hexyl~ 7,7-bis(methoxycarbonyl)heptyl,
8,8-bis(methoxycarbonyl)octyl, bis(ethoxycarbonyl)methyl,
2,2-bis(ethoxycarbonyl)ethyl, 3,3-bis(ethoxycarbonyl)-
propyl, 4,4-bis(ethoxycarbonyl)butyl or 5,5-bis(ethoxy-
carbonyl)hexyl.
~ 2 ~ ~ 3 2 1 h
Of these compounds of the formula I and the sub-
formulae, those are preferred in which at least one of
the radical~ present therein has one of the preferred
meanings indicated.
S Some very particularly preferred ~maller groups
of compounds are those of the sub-formulae Il to ISO, in
which L is ~ or F, n ~ 1-14 and r and t are each 1-7.
R~O--(CH2) n~F ' Il
F
R~O--(CH2) n--Cl I2
R~O--(cH2 ) n~CF3 I 3
R~CH2CH2~0--(CH2) _-F I4
R~CH2CH2~0--~CH2) n-Cl I5
L
R~CH2CH2~0--~CH2 ) n~CF3 I 6
R~COO--~ CH2 ) n--F I 7
B 9 ~3 s ~ ~
R~COO--(CH2 ) n-Cl I 8
R~COO--(CH2) n--CF3 I9
R~CH2CH2~COO- ~CH2) r--O~ (CH2) t-F I10
R~CH2CH2~COO- (CH2) r~O~ (CH2) t-Cl Ill
R~CH2CH2~COO--~CH2) r~O~ ~CH2) t-CF3 I12
R~CH2CH2~ ~CH2 ) n~F I 13
-
R~CH2CH2~ ~CH2) n--Cl I14
R~}CH2cH2~ (CH2) n~CF3 I15
~ 13 - 2~93~
F
R~CH2CH2~ (CH2 ) n-CÆ2 I 16
R~}CH2c~32~ (cH2) n~CF3 I17
R~CH2CH2~CH2O (cH2) n-l~F I18
R~CH2CH2~CH20 (CH2), l-Cl Il9
R~CH2CH2~CH20~CH2)n ~--CF3 I20
.
R~CH2O (CH2) n l--F I21
F
R~H2O (CH2) n l-Cl I22
R_~CH2O (CH2 ) n- -CF3 I 2 3
- 14 ~ 3~2
R~O- (cH2) r~CF3 I24
R~O- (CH2) r-CH=CH-CF3 I25
R~O- ( CH2 ) r -CF3 I 2 6
R~(CH2) r~CF3 I27
R~ ~CH2) r~CF3 I28
~(cH23 r~CF3 I29
R~ (cH2) r~CF3 I30
R~ (cH2) r~CF3 I31
R-(}(cH2) r~CF3 I32
R~CH2CH2~} (CH2) -CF3 I33
R~ ~CH2) 4~ (CH2) .-CF; I34
R~ (cH2) 4~ (cH2) r~CF3 I35
~3~ ~
R~O- tCH2) ~-CH=C;{-CF3 I36
R~ (CH2 ) -CH=CH-_F3 I 37
R~ tCH2) r-CH=CH-CF3 , I38
R~(CH2) r-CH=CH-CF3 I39
R~{~(CH2) r-CH=CH-CF3 I40
R~)~(CH2) r-CH=CH-CF3 I41
R~ (CH2 ) r-CH=CH-CF3 I 4 2
R_~(CH2) r-CH=CH-CF3 I43
R_~CH2CH2 O tCH2) r-CH=CH-CF. I44
R~CH2CH2~ (CH2) -CH=CH-CF; I45
- 16- 20~3212
R~ (CH2) 4~} (CH2) c-CH=CH-CF3 I46
R~(CH2) 4~ (CH2)r-CH=CH-CF3 I47
R~OCH2CF, (L', L2 = H oder F) ~ I48
L2 L
R~ ~OCH2 CF3 I 4 9
N
L
R{ ~OCH2CF; I50
L
- 17 - 2~3~
The compounds of the formula I are prepared by
methods known per se, as described in the literature (for
example in the standard works such as Houben-Weyl,
Methoden der Organischen Chemie, Georg-Thieme-Verlag,
Stuttgart, Vol. IX, pp. 867 ff), to be precise under
reaction conditions which are known and suitable for the
said reactions. U~e may also be made here of variants
which are known per se, but are not de~cribed here in
greater detail.
The compounds of the formula I according to ~he
invention where W = -O- and A = ~ can be prepared,
for example, by metalating a compound of the formula II
F
R ~ Zl ~ z ~ II
in which L is H or P, and R, Z1 and Z are a~ defined
above, in accordance with the reaction ~cheme below, and
sub~equently reacting the product with a suitable
electrophile:
Scheme 1
F 1. n-3uLi/KOBu'
R ~ Z- ~ Z ~
L 2. B(OMe)3
II 3. H2O2
F
Z ~ H
III
The compounds of the formula II where L = F are
novel and are thus also the sub~ect-matter of the inven-
tion. Furthermore, compounds of the formula III where
L = F are a sub~ect-matter of the invention.
- 18 - 2~ 12
The invention furthenmore relate~ to liquid-
cry3talline media containing at least one compound of the
formula II.
The compounds of the formula I (W - -O- and A =
F
~ ) can be obtai.ned from the re~ultant phenol of the
formula III by known etherification methods, for example
by reaction with ~al-(C~2)~-Q-(CH2)~-Y (~al = I, Br or Cl)
in acetone~R2CO3, optionally in the presence of catalytic
amounts of RI.
The compounds according to the invention can al80
be obtained from the bromine derivative IV
IV
~r~O ~W-(c~2)r-Q-(cH2)t-Y
L
Further synthesi~ methods for the phenyl ethers
according to the invention are obvious to a perCon
skilled in the art. For example, appropriately
5-substituted 1,3-difluorobenzene compounds or mono-
fluorinated analogues (L = H) can be converted to tha
2-O- ( C~2 ) r~Q~ (CH2)~-Y-1,3-difluoro com~ounds or mono-
fluorinated analogues (L - H) in accordance with the above
20 scheme, and the radical R ~ z~ ~ z can sub~equently
be introduced by reactions which are customary in liquid-
cry~tallina chemistry (for example etherification or
coupling, for example as described in the article
E. Poetsch, Rontakte (Darmstadt) 1988 (2), p. 15).
~he compounds of the formula II can be prepared
for example, by the following synthesis:
- 19 - ~93~
Scheme
R ~ z: ~ CH2P~Phj ~ + OHC ~
R ~ Zl ~ CH=CH ~ L
l, H2~Pd-C
R ~ Zl ~ CH2CH2 ~ L
The synthesis of qome particularly preferred com-
pounds is indicated in greater detail below:
- 20 - ~ ~ 9 ~
Sch me 3 (L = H or F~
F 1. -H20
R~}Z {~eO + Li~ -
L 2. H2/Pd-C
F 1. n-BuLi
R ~ Zl ~
L 2. 8(0Me)3
3. H202
R ~ Zl ~ oHL
Aceton ~ ClCHzCH2CH2F/K2CO3
R ~ z: ~ 0CH2CH2CH2F
- 21 - 2~93~2
Sche~e 4
(R~ZI~) 2-zn
ul t rasound Br~OBz
~Luche et al. I F
Tetrahedron Lett.
1984, 3463)
R~z; ~roBz
H2 / Pd-C
R~Z:~OH
Acetone J, ICH2CH2CH2Cl/K2CO3
R~z: ~OCH2CH2CH2Cl
F
Schel~ 5
CH3I
Br~OH ~ Br~OCH3
ultrasound ¦ (R~Z;~})2-Zn
?.~ Z ~
- 22 -
Phenyl ethers of the formula I are obtainable by
etherification of corre~ponding hydroxyl compounds,
preferably corresponding phenol~, the hydroxyl compound
expediently fir~t being converted to a corresponding
metal derivative, for example to the corre~ponding alkali
metal alkoxide or alkali metal phenoxide by treatment
with NaH, NaNH2, NaOH, XOH, Na2CO3 or R2CO3. This can then
be reacted with the appropriate alkyl halide, alkyl
sulfonate or dialkyl sulfate, expediently in an inert
solvent, such as, for example, acetone, 1,2-dimethoxy-
ethane, DMF or dimethyl sulfoxide, or altornatively with
an excess of aqueou~ or agueous-alcoholic NaOH or KOH at
temperature~ between about 20 and 100C.
Furthermore, the alkyl ethers may al~o be pre-
pared by the method of Mitsunobu from the correcponding
phenols a~d a hydroxyl compound in the presence of
triphenylphosphine and diethyl azodicarboxylate
(S. Bittner, Y. A~saf, Che~istry and Industry, 281
(1975); M.S. Manhas, W.H. Hoffman, B. Lal, A.K. ~ose,
J. Chem. Soc. Perkin I, 461 (1975):
Sç~
R ~ ZI ~ Z ~ H ~ HO--(CH2)r-Q-(CH2)t-Y
PPh3 F
~n~Zl~Z~ (CH2) ~--(CH2) ~-Y
HsC20CN-NCOC2Hs L
Il 11
O O
The starting materials for the preparation
processe~ are either known or can be prepared analogously
to known compound~.
The compounds of the formula I~ where
Z - -(CH2)~- can be prepared in accordance with the
- 23 - ~ ~ ~3~
following scheme (n = 1-8):
Scb~e Z (L - H or F)
R ~ C~Y,CH2-3r
1 chain extension by mean~ of malonic
1 ester~ via hydroly~is, decarboxyla-
I tion, LiAlH~ reduction and subsequent
1 reaction with R~r
A
R ~ ,~ H~ (CH2)~-Br
Zn~r2/Li ultrasound (Luche et al.,
Tetrahedron Lett. 1984, 3463)
[R ~ (CH2)4]2-Zn I
F ~ 1 Br ~ ~o-(C;~2) -y
L PdII-Catalyst . L
R ~ (CH2)~ ~ R ~ (CH2)4 ~ -(CH2)n-Y I'''
In the Pd(II)-catalysed coupling reaction, the
target product ~ is either formed directly or a
preCUr80r i8 formed into which the radical
-O-(CH2)~-Q-lCHz)c-Y i8 introduced entirely analogously to
the abovementioned methods for compounds of the
formula I.
lS The phenyl esters of the formula I according to
the invention ~Q - -COO-) are obtained from the compounds
of the formula II in accordance with the following
reaction scheme:
- 24 _ 2~932~'~
Scheme_ (L - H or F)
R~Z:~Z~¢ ~ R~}Zl~}z~co2H
L 3. H20 L
1. R1OH, DMAP
R: = (CH2) n~X; 2. DCC,
n = 1-8; if X = CF3, CHF2, CH2F 3 . (COOH) 2
n = 2-8; if X = F, Cl, OCF3, OCHF2 ~ .
R~ZI ~Z~CO2Rl
The benzene derivatives of the formula I accord-
ing to the invention, in which Q is a ~ingle bond, are
obtained in accordance with the following schemes:
Scheme 9 ~L - ~ or F)
F 1. BuLi, XOtBu
R ~ Z1 ~ z ~ THF, -100 C
2. X~(CH2)n~Y
r Y = Cl, F, CF3, CHF2
R~Z ~}Z~(CH2) r~Y X = I or Br
:l = 2-8
- 25 - ~fl~3~2
Sche~e 10 (L = H or F)
FLiAlHq F
R ~ Z ~ Z ~ Co2H ~ R ~ Z ~ z ~ CH20H
1. DMS~/NaH F
-- ~ R~Zl~z~cH2-o- (cH2) n-l~Y
2. X~(CH2)n-l~Y L
(x = I or ar)
The trifluoroalkyl and -alkanyl ethers of the
formula R-~A1-ZI)~-[ ~ ]~-Z-A-o-(cH2)r-(c~=cH)ol-cF3 can
be obtained by known etherification methods, for
example by reacting 4-substituted phenols with
Hal-(CHr)2-(CH=CH)Ol-CF3, where Hal is I, Br or Cl, in
acetone and potassium carbonate, optionally in the
presence of catalytic amounts of potassium iodide.
The etherification is advantageou~ly carried out
in the presence of an inert solvent. Highly ~uitable
solvents are, in particular, ethers, such as diethyl
ether, di-n-butyl ether, THF, dioxane or anisole,
ketones, such as acetone, butanone or cyclohexanone,
hydrocarbons, such as benzene, toluene or xylene, or
halogenated hydrocarbons, such as tetrachloromethane,
dichloromethane or tetrachloroethylene.
The synthesis of the trifluoroal~yl and -alkenyl
compounds according to the invention is shown in the
following-schemes:
20 ScheD~ ll
R-(Al-z~ [ ~ ]5-Z- (A) o~OH
NaOH PPh3/3r2
-NaBr Br-(cH2)r-cF3 ~ HO-(CH2)r-CF3
R-(A1-z1) _[ ~ ]s-z-(A)o-o-(cH2) -CF3
- 26
Sche~_12
R--tA~--Z' ) m~ t {~~ ] ~~Z--(A~ o~OH
Br-(Ch2)r-P~Ph3Bre
Base
Br-(C~2~-CH=C~-CF~ +
~ ~C-CF3
R-(A1-Zl) m~ 1~ ] ~~Z~ (A) o~O~ (CH2)p-CH=CH-CF3
Sche~e 13
R~(Al~ZI)m~[~ O ~]~ ~ L2(L1, L2: H or F)
DEAD
PPh3 HO-(CH2) -CF3
R- (Al--zl ) m~ [ ~ ] ~~ (CH2)r--CF3
The compound~ of the formula Ic
R-(A;-Z!)~-[ ~ ] Z ~ OCH2CF3 Ic
L2
can be obtained from the 4-bromo-2-fluorophenol-(2,2,2-
trifluoroethyl) eth r IB,
Br ~ OCH2CF3 IB (L = H or F)
- 27 _ 2~3~
by the rea~tion of 4-bromo-2-fluoropheno~ide with 2,2,2-
trifluoroethyl methylsulfonate in the pre~ence of 1,3-di-
methyl-2-imidazolidinone (DMæU), a~ shown in Scheme 14.
ScheD~ 14 (L - ~ or F)
F CH3SO2-O-CH2CF3 F
Br ~ Na ) Br ~ ~2CF3
L DMEU L
Li/ZnCl2¦R-~A1-Z~) ~ Br
ultrasound
R-(Al~Zl)m ~ CH2CF3
Sche~ 15
Br ~ H2CF3 + R-~A;-Z-)~-[ ~ ],-Z ~ B~OH)2
F
Na2C03 F
~ R-(A~-Zl)~-[ ~ ],-Z ~ OCH2CF3
Pd-(0~-Cat. L
The liquid-crystalline media according to the
$nvention prsferably contain 2 to 40, in particular 4 to
30, components as further constituents besides one or
more compounds according to the invention. These media
very particularly preferably contain 7 to 25 components
besides one or more compounds according to the invention.
These further constituents are preferably selected from
- 28 - ~ ~93~
nematic or nematogenic (monotropic or i~otropic) sub-
stances, in particular substances from the clas~es of the
azoxybenzene~, benzylideneaniline~, biphenyls, ter-
phenyls, phenyl or cyclohexyl benzoates, phenyl or
cyclohexyl esters of cyclohexanecarboxylic acid, phenyl
or cyclohexyl esters of cyclohexylbenzoic acid, phenyl or
cyclohexyl esters o cyclohexylcyclohexanecarboxylic
acid, cyclohexylphenyl esters of benzoic acid, of cyclo-
hexanecarboxylic acid and of cyclohexylcyclohexanecar-
boxylic acid, phenylcyclohexane~, cyclohexylbiphenyls,phenylcyclohexylcyclohexanes, cyclohexylcyclohexanes,
cyclohexylcyclohexylcyclohexenes, 1,4-bi~-cyclohexyl-
benzene3, 4,4'-bis-cyclohexylbiphenyls, phenyl- or
cyclohexylpyrimidines, phenyl- or cyclohexylpyridines,
phenyl- or cyclohexyldioxane~, phenyl- or cyclohexyl-1,3-
dithiane~, 1,2-diphenylethane~, 1,2-dicyclohexylethanes,
l-phenyl-2-cyclohexylethanes, 1-cyclohexyl-2-(4-phenyl-
cyclohexyl)ethane~, l-cyclohexyl-2-biphenylylethane~,
l-phenyl-2-cyclohexylphenylethanesoptionallyhalogenated
stilbene~, benzyl phenyl ethers, tolans and substituted
cinnamic acids. The 1,4-phenylene groups in these com-
pound~ may alJo be fluorinated.
The mo~t important compounds suitable a~ further
constituent~ of ~edia according to the invention can be
characteri~ed by the formulae l, 2, 3, 4 and 5:
R'-L-E-R~ 1
R'-L-~OO-~-R~ 2
R'-L-OOC-E-R~ 3
R'-L-C~2CH2-E-R~ 4
R'-L-C.C-~-R~ 5
In the formulae 1, 2, 3, 4 and S, L and ~, which
may be identical or different, are in each case, inde-
pendently of one another, a divalent radical from the
group formed by -Phe-, -Cyc-, -Phe-Phe-, -Phe-Cyc-,
-Cyc-Cyc-, -Pyr-, -Dio-, -G-Phe- and -G-Cyc- and their
mirror images, where Phe is unsubstituted or fluorine-
2~321~
- 29 -
~ubstituted 1,4-phenylene, Cyc i8 trans-1,4-cyclohexylone
or 1,4-cyclohexenylone, Pyr i9 pyrimldino-2,5-diyl or
pyridine-2,5-diyl, Dio i9 1,3-dioxane-2,5-diyl and G is
2-(trans-1,4-cyclohexyl)ethyl, pyrimidine-2,5-diyl,
pyridine-2,5-diyl or 1,3-dioxane-2,5-diyl
One of the radicals L and ~ i~ preferably Cyc,
Ph~ or Pyr E i9 preferably Cye, Ph- or Ph~-Cye The
media aeeording to the invention preferably contain on~
or more eomponent~ seleetod from tho eompounds of th~
formulae 1, 2, 3, 4 and 5 in whieh L and E are selectad
from the group comprising Cye, Phe and Pyr and ~imul-
taneously on0 or more eomponents s~l~et6d from the
compound~ of the formula~ 1, 2, 3, 4 and 5 in which one
of the radi~als L and E i8 selected from the group
comprising Cye, Phe and Pyr and the ~othQr radical i8
seleeted from the group compri~ing -Phe-Phe~, -Phe-Cye-,
-Cye-Cye-, -G-Phe- and -G-Cye-, and optionally one or
more eomponents sel~etod fro~ tho eo~pound- of tho
formul~e 1, 2, 3, 4 and 5 in whieh tho radieal~ L and E
ara seleeted from the group eomprising -Phe-Cyc-,
-Cye-Cye-, -G-Phe- and -G-Cye-
In a maller sub-group of the eo pound~ of th~
formulao 1, 2, 3, 4 and 5, R' and R" are in each case,
indepondently of one another, alkyl, alk~nyl, alkoxy,
alkoxyalkyl, zlkenyloxy or alkanoyloxy having up to 8
earbon atomJ Thi~ smaller sub-group is ealled group A
below, and tho eompounds are labelled with tho sub-
formulae la, 2a, 3a, 4a and 5~. In most of th~ eom-
pounda, R' and R~ are diff~rent from one anothor, one of
th-~o radieals usually being alkyl, alkenyl, alkoxy or
alkoxyalkyl
In another smaller sub-group of the eompounds of
the formulae 1, 2, 3, 4 and 5 whieh is known as group ~,
R" i~ -F, -Cl, -NCS or -(O)1C~3~ F~Cll, where i is 0 or
1, and k+l is 1, 2 or 3; the eompounds in which R" has
this meaning are labelled with the sub-formulae lb, 2b,
3b, 4b and 5b Particular preference is given to those
compounds of the sub-formulae lb, 2b, 3b, 4b and 5b in
_ 30 _ 20~32~ ~
whioh R" is -F, -Cl, -NCS, -CF3, -OC~F2 or -OCF3.
In the compound~ of the sub-formulae lb, 2b, 3b,
4b and 5b, R' i9 as defined for the compounds of the ~ub-
formulae la-5a and is preferably alkyl, alkenyl, alkoxy
or alkoxyalkyl.
In a further smaller sub-group of the compounds
of the formulae 1, 2, 3, 4 and 5, R" i8 -CN; this sub-
group i8 known as group C below, and the compound~ of
this sub-group are correspondingly de~cribed by ~ub-
formulae lc, 2c, 3c, 4c and Sc. In the compound~ of thesub-formulae lc, 2c, 3c, 4c and 5c, R~ i~ as defined for
the compounds of the ~ub-formulae la-5a and i8 preferably
alkyl, alkoxy or alkenyl.
In addition to the preferred compounds of groups
A, ~ and C, other compounds of the farmul~e 1, 2, 1, 4
and 5 having other variants of the proposed substituents
are al~o cuRtomary. All these sub~tances can be obtained
by method~ which aro known fro~ the literaturo or analo-
gou~ly thereto.
~esides compounds of the formula I according to
the invention, the media according to the invention
preferably contain one or more compound~ selectod from
group A and/or group B and/or group C. The proportions by
weight of the compound~ from the~e groups in the ~edia
according to the invention are prefer~bly
Group A: 0 to 90%, preferably 20 to 90%, in particular
30 to 90%
Group B: 0 to 80%, preferably 10 to 80%, in part.icular
10 to ~5%
Group C: 0 to 80%, preferably 5 to 80%, in particular
5 to 50%,
the sum of the proportions by weight of the group A
and/or B and/or C compounds present in the particular
media according to the invention preferably being ~ to
90% and in particular 10 to 90%.
- 31 - 2~93~12
Th~ media according to the invention preferably
contain 1 to ~0%, particularly preferably 5 to 30%, of
compounds according to the invention. Further preferred
media are those which contain more than 40%, in parti-
cular 45 to 90%, of compounds according to the invention.
The media preferably contain three, four or five com-
poundq according to the invention.
The media according to the invention are prepared
in a manner which is customary per se. In general, the
components are dissolved in one another, expediently at
elevated temperature. By means of suit~ble additives, the
liquid-cry~talline phase~ can be modified in accordance
with the invention in a manner such that they can be u ed
in all types of liquid-crystal di~play elements which
have hitherto been disclosed. Additives of this type are
known to tho~e skilled in the art and are described in
detail in the literature (H. KelkertR. Hatz, ~andbook of
Liguid Crystals, V~rlag Chemie, Weinheim, 1980). For
example, pleochroic dyes can be added for the production
of colored gue~t-host systems, or substance3 can be added
to modify the dielectric anisotropy, the viscosity and/or
the orientation of the nematic phases.
The examples below are intendet to illustrate the
invention without representing a l;m;ta~ion. "C~tomary
work-up" means that water i8 added, the mixture i3
extracted with methylene chloride, the organic phase i~
separated off, dried and evaporated, and the product is
purified by crystallization and/or chromatography. Above
and below, per cent data are per cent by weight. All
temperature3 are given in degrees Celsius. m.p. is
melting point and c.p. ~ clearing point. Furthermore,
C - cry~talline state, N - nematic pha~e, S - smectic
phase and I - i~otropic phase. The data between the~e
symbol~ indicate the tran~ition temperatures. ~n is
optical anisotropy (589 nm, 20C). The vi~cosity ~mm2/sec)
wa~ determined at 20C.
The following abbreviations are used:
~93~
- 32 -
BuLi butyllithium
DAST diethylaminosulfur trifluoride
DCC dicyclohexylcarbodiimide
DIBALH diisobutylaluminum hydride
5 DMAP 2-dimethylaminopyridine
DDQ dichlorodicyanobenzoquinone
POT potas~ium tertiary butoxide
NH4Cl ammonium chlorido
T~F tetrahydrofuran
10 TMæDA tetramothylethylenediamine
pTSOH p-toluenesulfonic acid
E~ple 1
F
a) H,C3 ~ -OH
26 mmol of n-BuLi are added dropwi~e nt -100C to
a mixture comprising 26 mmol of 1-trans-4-(trans-4-n-pro-
pylcyclohexyl)-cyclohexyl-3-fluorobenzene (prepared as
described in scheme 1), 4.1 g of pota~sium tert.-butoxide
and 60 ml of THF. After the ~ixture ha- been stirrod at
-100C for one hour, 36 m~ol of trimethyl borate are
added dropwiso at from -85 to -90C. The mixture is
stirred for a further O.5 hour, and 42 mmol of acatic
acid are thon added dropwis- at -20C. The mixture i9
sub-oquontly warmed to 30C, and 4.2 ml Of ~22 are added
dropwi~e at this temperature, and the mixture i~ stirred
at from 50 to 60C for 2 hour~. The mixture is allowed to
cool to room temperature, and a 5% ~odium dithione
solution is added. Phase separation and conventional
work-up give the phenol.
b) H7C3 ~--OCH3
- 33 - 2~3212
The phenol obtained is reacted with methyl iodide
in acetone under reflux in the pre~ence of pota~3~3ium
carbonate to give the methyl ether. Conventional work-up
and chromatography on ~ilica gel using hexane give the
ether in pure form.
The following ethers of the formula
R ~ Zl ~ Z ~ OCH3
are prepared analogously.
R ~ z~ ~ z_
CH3 ~
C2H5 ~_
n-C5HI ~ ~
CH30CH2 _~_
CH2~CHCH2CH2 ~
CH3 ~ H2CH2-
CzHs ~}CH2CH2-
n-C3H~ ~H2cH2_
n--CsHll ~CHzCH2-
CH~OCH2 ~ CH2CH2-
CH2--CHCH2CH2 ~ H2CH2-
CH3 ~ H2CH2 ~
C2E{5 -~-CH2CH2
n--C3H7 ~CH2CH
n-CsHll ~CH2CH2~
CH30CH2 ~ CH2CH2 ~ }
CH2~CHCH2CH2 ~ CH2CY.
20~321~
-- 34 --
R {~ Z ~} Z -
_ _
CH3 ~ (CH2)
C2 H5 ~ ( Cf~
n-C3H7 ~ (CH2)
n~sHIl ~ (CH2)
c~{3oCH2 ~ ~CH2)
CH2-CHCH2CH2 ~ tCH2) ,~
Bxa~ 2
F
a) C3H7 ~ -OC~2C~2CH~
The fluorophenol prepared in` Example 1 a) i8
reacted with l-chloro-3-fluoropropane in boiling acetone
S in the presenca of potas~ium carbonate and a catalytic
amount of pota~ium iodlde to give the phenol ether.
Conventional work-up and chromatography on ~ilica gel
u~ing hexane gives the ether in pure form.
F
b) C3H7 ~ H2CH2CH2Cl
The fluorophenol prepared in Exzmple 1 a) i8
converted to the phenol ether using l-chloro-3-iodopro-
pane ~nalogou~ly to ~xample 2 a).
The following compounds of the formula
F
R ~ Zl ~ Z ~ H2CH2cH2Y
according to the invention are obtained analogously from
the corresponding precur~ors:
_ 35 _ 2~
R ~Z: ~Z Y
CH3
CH3 ~1~ C:
C2H5 ~ F
C2Hs ~ C'
n-C~ H3 ~ F
n--C4H3 ~ Cl
n-C5HIl ~ F
n-CsHl ~ } Cl
CH30CH2 -(~-- F
CH30CH2 ~ C '
CH25CEICH2CH2 ~ F
CHz-CHC~2~1z ~ Cl
CH3 ~CH2CH2~ F
CH3 - ~CH2CH2~ Cl
C2Hs _~CH2CH2~ F
C2H5 - (~'CH2C~12~ Cl
n-C3H7 ~CH2CH2~ F
n-C3H7 {~CH2CH2~ Cl
n--C4H9 ~ {~ H2CH2~ F
n-C~H~ {~CH2cH2~ Cl
n--C:sHl 1 ~CH2CH2~ F
n-CsHIl ~CH2CH2~ Cl
CH30CH2 ~CH2CH2{~ F
CH3OCH2 ~CH~CY~-(~ ^l
CH2-CHCH2CH2 ~CEI2CH ~
CH~=CHCH2CH2 ~CH~CH~-~} C!
- 36 - ~0~12
Xx~
a) 8-C3~7 ~ ~
Two drop~ of bromine are added to a solution of
6.0 ~ of mAgnesium turning- in 60 ml of ether. A ~olution
S of 48.2 g of 3,5-difluorobromobsnsQne in 60 ml of ether
i9 sub~equently added dropwis~. The mixture i8 ~tirred
for a further 0.5 hour, and a ~olution of 4~.5 g of
4-trans-~4-propylcyclohexyl)cyclohexanone in 50 ml of
ether i8 then added dropwise to ths Grign~rd re~gent at
20-25C. The mixture i~ stirred for ~ further two hourY,
poured into 500 ml of water, acidified~by mean~ of 30 ml
of conc. hydrochloric acid and extract~d by sh~king with
ether. ~he organic phase i8 evaporated to give a residue
and ub~equ~ntly ref lux-d for 1 hour with 1,000 ~1 o~
toluene and 120 ml of 20% sulfuric ~cid.
Af ter phase ~ep~ration and neutralization by
mean~ of saturated sodium bicarbonate 801ut~on ~ the
product i8 hydrogenated at 1 bar and 609C u~ing 10 g of
Pd/C (5%). Tho mixture i8 ub~equently filtered and
evaporated. Flash chro~atography gives th~ purs product.
C 60 N 87.6 I, ~ - 3.3
F
b ~ C3 H, ~-OH
31 ml of n-BuLi (15% in hexane) are added drop-
wise at from -65C to -70C to a mixture of 47 mmol of
4-,trans-(4-n-propylcyclohexyl)cyclohexyl-1-trans-(3,5-di-
fluorobenzene), 50 mmol of TMEDA and 150 ml of T~F, and
the mixture is ~tirred at -70C for a further hour. Then,
57 mmol of trimethyl borate are added dropwise at from
-85 to -90C, followed by 65 mmol of acetic acid at
-20C.
- 37 -
2 0 9 ~ 2 ~ ~
c ) C~H,~-OCH2CH2CH2F'
The dif luorophenol is r~acted analogouQly to
Example 2 a) with 1-chloro-3-fluoropropane to give the
phenol ether.
F
d) C3H7 ~ ~2CH2CH2Cl
Tha difluorophenol prepared in ~xample 4 b~ i9
reacted analogously to Example 2 b) with 1-chloro-3-iodo-
propane. C 50 N 157.2 I, Q t ~ 5 . 7 -
1~D1~ ~
F
a) C3H7 ~ H2CHlF
1 mmol of diethyl azodicarboxyl~te i3 addeddropwi~e at 0-10C to a solution comprising 1 mmol of
4-tran~-(4-n-propylcyclohexyl)cyclohe~yl-1-tran~-(3,5-di-
fluorophenol), 1 mmol of triphenylpho2phine, 1 mmol of
2-fluoroothanol and 25 ml of T~F. The mixture i~ ~ubse-
quently stirred at room temperature for a further hour
and then 8ubjected to customary work-up. C 82 N 165.5 I,
ac - 2.07.
F
b) C3H7 ~ ~3
F
1 mmol of 4-trans-(4-n-propylcyclohexyl)cyclo-
hexyl-l-trans-(3,5-difluorophenol) i8 reacted with
- 38 - 2 ~ 321 2
methanol ~nalogously to Example 4 a). C 41 N 151.8 I,
0.038.
The following compounds of the formula
R ~ Z~- ~ Z ~ O-~CH2)~-Y:
according to tho invontion are obtained analogously from
the corre~ponding pr~cur-or-:
R ~ Z~ ~ Z- n Y
CH3 ~ 1 H
CH3 ~r~ 1 F
CH3 ~ 1 `Cl
CH3 ~ 2 F
CH3 ~ 2 Cl
CH3 ~ 3 F
CH3 ~ 3 Cl
CH3 .~ 4 F
CH3 ~ 4 Cl
CH3 ~ 5 F
CH3 ~ 5 Cl
C2H~ ~ 1 F
C2Hs ~ 1 Cl
C2H5 ~r 2 F
C2Hs ~} 2 Cl
C2Hs {~ 3 F
C2H5 ~ 3 Cl
C2H5 {~ 4 F
C2H5 ~} 4 Cl
C2Hs ~ 5 F
C2Hs ~ 5 Cl
C3H7 ~r~ l :~ c 41 ~ ;~ , 3
39- 7~i332~
R ~2~ {~Z- n Y
n-C3H7 ~ 1 F
n--C3H7 {~ 1 Cl
n-C3H~ ~ 2 Cl
n~:3H7 ~ 2 F C 82 N 165. 5 I
n-C3H? ~} Cl
n~C3H7 ~ 4 F
n-C3H7 ~ 4 Cl
n-C3H7 ~ 5 F
n-C3H7 ~ 5 Cl~
n-C4Hg ~ 2 F
n-C~H9 ~ 2 Cl
n-C~Hg ~ 3 F
n-(C~Hg ~ 3 Cl
n-CsHll ~ 1 H C 43 N 154, 3 I
n-CsHl~ ~ 1 F
n-C5Hl. ~ 1 Cl
n-C:5HIl ~ 2 F
n-C5Hll ~ 2 Cl
n-C5Hll ~ F
n-CsHll ~ Cl
n-CsHll {~ F
n-(C5Hll ~ Cl
n-CsHll ~ F
n-CsH~ ~ S Cl
~ 40 ~ 2093212
R ~ Z:- ~ z- n Y
. ~
CH30CH2 ~ 2 F
CH30C.~l2 ~ 2 Cl
CH30CH~ ~ 3 F
CH3 OCH2 ~ 3 C 1
CH2-CHCH2cH2 ~ 2 F
CH2=CHCH2CH2 ~} 2 Cl
CH2=CHCH2CH2 ~ 3 F
CH2=CHCH2CH2 ~ 3 Cl
e 5
a ) Cs Hm ~CH=CH~
F
78 m~ol of potassium tert.-butoxidQ, di~solved in
80 ml of THF, are added dropwise at -S-C to 78 mmol of
1-(4-(4-n-pentylcyclohexyl)cyclohe~yl)methylenetriphenyl-
phosphonium iodide and 78 mmol of 3,5-difluorobenz-
aldehyde, dissolved in 50 ml of THF. The mixture i8 sub-
gequently stirred at the same temperature for lS minutes
and at room temperature for a further 1.5 hour~. The
reaction mixture is hydrolysed, neutrali~ed by means of
hydrochloric acid and subsequently sub~ected to customary
work-up.
F
b) CsH~ ~ CH=CH- ~ OCH2CH2CH2F
The product prepared in Example S a) i8 reacted
with 1-chloro-3-fluoropropane analogou~ly to Example 3 c)
to give the phenol ether.
- 41 -
F 20932~2
n-CsHm {~)~CH=CH~OCH2CH2CH2Cl
The product prepared in Example S a) is reacted
with l-chloro-3-iodopropane analogously to Ex~mple 3 d)
to give the phenol ether.
The following compounds of the formula
R ~ Zl ~ Z ~ -oc82cH2cH2y
according to the invention are obtainsd analogously from
the corresponding precursors of the formula II (L - F):
R ~ Zl ~ z_ Y
CH3 ~ H=CH- F
CH3 ~ H=CH- Cl
C2H5 ~ H-CH- F
CzH5 ~ -CH- Cl
n-C3H~ ~ ~ CH=CH- F
n-C3H7 ~ ~ CH--CH- Cl
n-C~Hg ~ ~ CH=CH- F
n-C~Hg ~ ~ =CH- Cl
CR30CHz ~ H=CH- F
CH30CH2 ~ H=CH- Cl
CH2-CHCH2CH2 ~ CH=CH- F
CH2-CHCH2CH2 ~ CH=CH- Cl
~ aL~ 6
a) n-C5H~l ~ ~ CH2CH
- 42 - ~ ~ ~3212
9 mmol of the product from Example 5 a) are
dissolved in 30 ml of THF, 0.3 g of 4% Pd-C i8 added, and
the mixture i9 hydrogenated. The cataly~t is subsequently
filtered off, and the filtrate i~ evaporated in Yacuo to
give a residue. This i9 chromatographed on a silica gel
column using pentane.
An analogQus reaction gives
n-C3H7 ~ CH2C.~2- ~ , C 52 N 9S.1 I, a 6 ~ 4.7.
b) n--C5~ ~ ~ C~2CH2 ~ 2CH2Ci2F
The product obtained in ~xample 6 a) is reacted
with l-chloro-3-fluoropropane an~logously to gxaople
3 c).
C) r~-csE~ 2cH2~~ CH C~ Cl
~ ho product prepared in Example 6 al i~
reacted with 1-chloro-3-iodopropane analogously to
Example 3 d) to give the phenol ethcr.
The following compounds of tha formula
R ~ ~ -ocH2cH2cH2y
F
are obtained analogously from the corre~ponding precur-
~ors of the formula II (L ~ F):
43 ~93212
R ~ZI ~Z Y
.
CH3 ~ CU2C 2- F
C~3 ~~C~2C 2 C1
C2H5 ~ C12CH2- F
C2H5 _~-C~2CK2- C1
n C3H7 ~CH2CH2- F
n-C3H~ ~CH2CH2- C1
n-C4H9 -<~CH2CH2- F
n--C~H9 -~-<~CH2CH2- C1
CH30CH2 ~CH2CH2- F
CH30CH2 _~ - CH2CH2 - C1
CH2=~CHCH2CH2 {~CH2CH2- F
CH2 CHC~2CH2 {~GCH2CH2- C1
~1~ 7
F F
a) CsH~CH2) 4-8r + 3r-(~ ~CsHIl~(CH2) 4-<~
F F
ll.S g of anhydrou~ zinc bromide and th~n 1.4 g
of lithium granules are added to 0.1 mol of 4-(4-(n-pen-
tylcyclohexyl)cyclohexylbutyl bromide in 150 ml of a
~olvent mixture compri~ing T~F/toluene tl:4). The mixture
i8 treated with ultrasound for 4 hours at between 0C and
10C under argon and with stirring. The organozinc
compound produced i9 treated with 0.1 mol of 3,5-di-
fluoro-l-dibromobenzene and 2 mol~ of l,l'-bi~(diphenyl-
phosphino)ferrocenepalladium(II) dichloride, the ultra-
~ound bath and the cooling are removed, and the mixture
is ~tirred at room temperature for 24 hour~. 100 ml of
~ 44 ~ 22~321~
saturated N~,Cl solution are added with stirrin~, the
organic phase is separated off, and the aqueous phase i9
extracted with toluene. The combined organic extracts are
dried, evaporated and chromatographed on 8ilica gel.
b) C;~m ~ ~ (CH2)~ ~ -OH
The reaction of the difluorobenzen~ with ~uLi and
trimethyl borate to give the difluorophenol i8 carried
out analogou ly to Example 1.
c) CsHIl~ (cH2) 4~-OCHzCH2C~2F
Th~ difluorophonol from ~x3mpl~ 7 b~ is re~cted
with l-chloro-3-fluoropropane analogously to Example 3 c)
to give the phenol ether.
d) C~HIl ~ (CHz) ~-OCH2CH~CH2Cl
The product fro~ Exa~plo 7 ~ reacted with
l-chloro-3-iodopropane analogouJly to Exa~ple 3 d).
The following compound~ of the formula
R~zl:~z-~cHzcH2cH2y:
L
according to the invention are obtained analogously from
the corresponding precur~or~ of the formula II (L - ~ or
F)
~ 45 ~ 2~93212
R ~Zl~Z- L Y
.
CH3 ~(CH2)~ H F
CH3 ~ (CH2) 4~ H Cl
CH3 ~ (CH2) ~ F Cl
c2~s ~ (CH2~ 4~ H F
C2H5 ~--~CH2) 4~ F F
n~3H7 ~ (CH2) 4-(~ H F
n~3H7 ~ (CH2) ~ H Cl
n C3H7 ~ (CH2) 4~ F Cl
n~Hg ~ (CH2) q~ F F
n--CjHg ~ (CH2) ~ ~ F Cl
n--CsH 1 ~(CH2)q~ H F
n-CSH ' ~ (CH2 ) 4~ F F
n-CSH i ~--(cH2)4{~ F Cl
CH3OCH2 ~ (CH2) 4~ H F
CH30CH2 ~ (cli2) q~3~ F F
CH2=cHcH2cH2 ~ (CH2) q~ H F
CH2=CHCH2cH2 ~ (CH2) q~ F F
CH2=CHCH2CH2 ~ (CH2) 4-<~ F Cl
13~1~ 8
n-CsHI1 ~ < ~ COOCH2CH2F
26 ml of 2-fluoroethanol and 0.1 g of DMAP are
added to a suspension of 26 mmol of 4-trans-(4-pentyl-
cyclohexyl)cyclohexyl-l-trans-3,5-difluorobenzoic acid
S (prepared as described in scheme 7) in 160 ml of di-
chloromethane. A solution of 6.6 g of DCC in 40 ml of
dichloromethAno i~ then added dropwise with stirring. The
- 46 - ~093212
mixture i9 stirred overnight at room temperature. Oxalic
acid is added, the mixture is stirred for a further hour,
chromatographed using hexane and recrystallised. C 63 N
148.8 I
S The following compounds of the formula:
~ ~ -COOR'
are obtained an~logou31y:
R L R'
CH3 H CH2CH2F
C2H5 H CH2CHiF
n~3H7 ~ CH2CH2F
n-C~H~ H CH2CH2F
n-CsH~l H CH2CH2F
CH30CH2 H CH2CH2F
CH2=CHCH2CH2 H CH2CH2F
CHI F C~2CH2F
C2H5 F CH2CH2F
n-C3H~ F CH2CH2F
n-C4H9 F CH2CH2F
CH30CH2 F CH2CH2F
CH2~CHCH2CHz F CH2CH2F
CH3 F CH2CH2Cl
C2H5 F CH2CH2Cl
n.-C3H7 F CH2CH2Cl
n-CsH1~ F CH2CH2Cl
CH30CH2 F CH2CH2Cl
2(J932~2
R L R'
CH3 F CH2CH2CH2Cl
C2H5 F CH2CH2CH2Cl
n-C3H, F CH2CH2CH2Cl
n-C5H:i F CH2CH2CH2Cl C 58 N 102 I
CH3OCH2 F CH2CH2CH2Cl
CH2=CHCH2CH2 F CH2CH2CH2Cl
CH3 F CH2CH2CH2CF3
C2H5 F CH2cH2cH2cF3
n-C3H, F CH2CH2CH2CF3
n--CsH ~ F CH2CH2CI~2CF3 C 65 N 86. 9 I
CH3ocH2 F CH2CH2CH2CF3
CH2=CHCH2CH2 F CH2CH2CH2CF3
CH3 F CR2CH20CF3
C2~5 F CH2CH2OCF3
n-C3H~ F CH2CH20CF3
n-c5H-~ 1 F CH2CH2OCF3
CH3ocH2 F CHzCH2OCF3
CH2=CHCH2CH2 F CH2CH20CF3
CH3 - F CH2CH2CHF2
C2Hs F CH2CH2CHF2
n-C3H7 F CH2CH2CHF2
n-CsHll F CH2cH2cHF2
CH3ocH2 F CH2CH2CHF2
CH2-CHCH2CH2 F CH2CH2CHF2
~9~
- 48 -
~2
n~CsHm ~2 H ~ n-CsHIs ~ CHzOH
F A
n-C,HII m~CH20CH2CH2CH2Cl
- B F
A suspension of 50 mmol of 4-tranY-(4-pentyl-
cyclohexyl)cyclohexyl-l-tran~-3,5-~difluorobenzoic acid)
in 150 ml of THF i8 added dropwise to a suspension o
S S0 mmol of lithium aluminum hydrids in 50 ml of T~P. The
mixture is subsequently refluxed for a further 1 hour
with stirring. The mixture i~ cooled i~n an ice bath and
hydrolysed by addition of 6 ml of 10% NaHC03 solution.
12 ml of 20% sodium hydroxide solution are added, and the
organi~ ph~se i~ soparated off, dried and ~vaporated.
A ~olution of 28 mmol of the difluorobenzyl
alcohol A in 90 ml of ~HF is added to a 301ution of
30 mmol of sodium hydrid~ in 30 ml of DMS0. 31 mmol of
3-chloro-1-iodopropane are then added, and the mixture i8
stirred overnight at 50C. Hydroly~is and conventional
work-up give the ether B.
The following compounds of the formula
-
R~{ ~H20 (CH2 ) n~Y:
are obtained analogously:
_ 49 _ 2~93212
R L n Y
CH3 H 2 F
CH3 F 2 F
C2Hs H 2 Cl
C2Hs H 3 Cl
C2H5 F 2 Cl
C2H5 F 3 Cl
C2Hs H 2 F
CzHs H 3 F
C2Hj F 2 r
C2Hs F 3 F'
C2Hs H 2 CF3
C2H5 H 3 CF3
C2H5 F 2 CF3
C2Hs F 3 CF3
n-C3H7 H 2 F
n-C3H7 F 2 F
n~C3~ H 2 Cl
n-C3H~ F 2 Cl
n-C3H7 H 2 OCF3
n-C3H7 F 2 OCF3
n-c3H7 H 2 CHF2
n--C3H7 F 2 CHF2
n-C3H7 H 2 CF3
n-C3H7 F 2 CF3
- 50 ~ 3 '~ ~ 2
R L n Y
n~sHI I H 2 Cl
n~sHIl H 2 F
n-CsH~ ~ F 2 F
n~5Hl ~ H 2 CF3
n~sHm F 2 CF3
n~jH~l ~ 3 F
n-C5HI ~ F 3 Cl
n~,HI 1 F 3 CF;
~sapç~Le lQ
H7C3~0-C~2CH2CH2CF3
14.5 g (0.065 mol) of 4-(4-propylcyclohexyl)-
cyclohexanol are di~solved in 15 ml of T~F, and 12.; g
(0.065 mol) of trifluorobutyl bromide, 1.~ g of cetyl-
trLmethyl~mmonium bromlde, 5.2 g (0.13 mol) of ~odium
hydroxide solution and 0.3 ml of water are added ~ucce~-
~ively. The mixture i~ stirred overnight at 70C. The
mixture i~ sub~equently allowed to cool to room tempera-
ture and i~ taken up in diethyl ether. Conventionalextractive work-up qive~ the ~ther, which i~ purified by
chromatography on ~ilica gel (hexane:ethyl acetate -
9:1). C 10 S~ 47 I.
The following trifluoroalkyl ether~ of the
formula
R-[ ~ l.-Z ~ ~CH2)r-CF,
are prepared analogou~ly:
1 - 2~32 ~ 2
R ~ z ~ -o-~CH2),-CF~
C2H5 _~ ~ OCH2CH2CF~
n-C3H, ~ OCH2CHzCE'3
n-C5~l. ~} C)CH2CH2CF,
CH3C~2 _< ~ 3,, OCH2CH2CF,
CH2--CH CHaCH2 ~ OCH2CH2CF~
C2~5 ~ OCH2CH2C~aCF3
n-C~H~ ~ OC~zCH2CH2CF3
n-cs~ ~, OCH2CH2CR2CF~
CH30C~ ~ OCH2CHzC~2CF3
C~2 = CH CHaC~2 ~ OCH2C~2C~2CF3
C2H5 ~ cH2cR2c~2c~I2cF3
n-C3H7 ~ OCH2C~2C~2CR2CF3
n-C5Hll. ~ OCH2CH2CH2C~12CF~
CH30a~2 _< ~ , OCR2CH2CH2CH2CF3
CH2--CH CH2CH2 ~ OCX2CH2CH,CH2CF3
~a~yle 11
Br-~CH2)2-P-Ph38re + C-CF3 3r-CH2-CH-CH-C~3
11.5 g of potassium tert.-butoxide are added in
portions at 0-10C to a ~uspen~ion of 0.1 mol of Wittig
salt in 200 ml of THF. Trifluoroacetald~hyde gas is
subseguently pas~ed in at the 8amo temperature until the
orange ylide suspension has become colourless. The
mixture i8 subsequently stirred at room temperature for
24 hours, poured into water, neutralised and extracted a
nu~ber of times with toluene, and the toluene extract is
dried, evaporated and filtered on silica gel using
hexane.
52 - ~ ~93~
C;H~- ~ ~ ~CH2CH=CH~
0.065 mol of 4-(4-propylcyclohexyl)cyclohexanol~
15 ml of THF, 0.065 mol of 1,1,1-trifluoro-4-bromobut-
2-ene, 1.2 g of cetyltrimethylammonium bromide, 0.13 mol
of sodium hydroxide solution and 0~3 ml of water are
S reacted analoqously to ~x~mple 1.
The following trifluoroalkenyl ether~ of the
fonmula
R-t ~ -],-Z ~ -O-(CH2) -CH~H-CF3
are prepared analogou~ly:
R _~ ~ ] J_Z_~ - O - (CH2 ) r -CH=CH-CF3
_. _
CzH5 ~ OCH2CH = CHCF3
n-C~H9 ~ OCH2CH = CHCF3
n-CsHll ~ OCH2CH = CHCF3
CH30CH2 ~ ~ OCH2CH = CHCF3
CH2 = CHCH2CH2 ~ OCH2CH = CHCF3
CzHs ~ ~ OCH~C:-~CH = CHC-3
n-C~H7 ~ CCH~C:i~CH = CHC~ 3
n~CsHII - ~ OCH~CHaCH = CHCr 3
CH30CH2 ~-- CCH2--H~CH = CHCFl
CH2 ~ CHCH2CH2 ~ CCH~CH~CH = CHC~3
~ample 12
CsH~ 0-(CHz)3-CF3
0.102 mol of diethyl azodicarboxylate i~ added
dropwise with cooling to 0.1 mol of 4-(4-pentylcyclohex-
~ 53 ~ 2~93212
yl)phenol, 0.102 mol of triphenylphosphine and 0.1 mol oftrifluorohutan-4-ol in 250 ml of tetrahydrofuran at room
temperature. The mixture i~ subsequently stirred over-
night. The mixture is evaporated and the residue is
S filtered on silica gel using toluene.
The following trifluoroalkyl ethers of the
formula
- R-[ ~ ~-Z-(A)o-O-(CH2),-CF3
are prepared analogoUsly:
- 54 - 2~3212
R
- t O-) ,-2- (~) J- - (CH2) _r~ 3
C2H5 ~ ~
n-C5H,~ OCH2CF3 C 50 I
CH30CH2 ~ OC~2CF3
C~2 ~ CHcH2cH2 J~3_ OCH2CF,
C2H5 ~ OcH2cH2cF3
n-C3H~ ~ OCH2CH2CF3
n-CsH~ _ OCH2CH2CF3
CH30CH2 _~3_ OCH2CH2Cr 3
C~l2 ' C~IcH2cH2 ~} OcH2cH2cF3
--C3~ _~F OcH2cH2cF3
FF
n--C5H~ OcH2cH2cF3
C2H5 _~,F OCH2CH2CH~CF3 C 50 I
n-C3H7 ~ OCH2CH2C~12CF3
n-C~H9 ~ OCH2CH2CH2CF3
CH30(CH2 -~- 0cH2c~2c~l2cF3
C~2 ' CHCH2CH2 _<~_ OCH2CH2CH~CF3
n~H~ ~ OCH2CH2CH2CF3
n~CSHl 1 _~;_ OCH2CH2CH2Cr 3
- 55 - ~ 3212
R[ {} ~ ,-Z- (A): - -O- (CH2 ) -CF3
C2Hs ~ OCH2CH2CH2CH2CF3
n-C3H7 -~ OCH2CH2CH2CH~CF,
n-CsH~l (~ OCH2CH2CH2CH2CF3
CH3OCH2 ~<~ OCH2CH2CH2CH2CF3
CH2 = CHcH2cH2 ~ OCH2CH2CH2CH2CF3
n-C3H7 ~ OCH2CH2CH2CH2CF3
n-CsHll ~ F OCH2CH2CH2CH2CF3
C2Hs ~ } OCH2CF3
n-C3H7 ~ OCH2CF3
n-CsHll ~ OCH2CF3 C 10,7 I
CH3OCH2 ~ } OCH2CF3
CH2 = CHcH2cH2 ~ OCH2CF3
CH3CH20~}~} OCH2CF3
CH3CH2CH2CH20 ~ OCH2CF3
n-C3H7 -~ OCH2CF3
F
n-CsH~ OCH2CF3
- 56 - 2~932 ~ 2
R ~ -Z- (A) ~- -O- (CH2 ) r-CF7
_ _ _
C2H5 -@--<~ OCH2CH2CF3
n-C3H. ~} CCH2CH2CF3
n-CsHl ~ } OCH2CH2CF3
CH3OCH2 ~} OCH2CH2CF3
CH2 = CHcH2cH2 -~ } OCH2CH2CF,
CH3CH20 ~} OCH2CH2CF3
CH3CH2CH2CH20 ~ OCH2CH2CF3
n-c3H7 F OCH2CH2CF3
n-CsH~ , OCH2CH2CF3
C2H5 ~ OCH2CH2CH2CF3
n-C3H7 ~}<~ OCH2CH2CH2CFj
n-CsHll ~<~ OCH2CH2CH2CF3
CH3OCH2 ~<~ OCH2CH2CH2CF3
CH2 = CHcH2cH2 ~ OCH2CH2CH2CF3
CH3CH20 ~ OCH2CH2CH2CF3
CH3CH2CH2CH20 ~ OCEI2CH2CH2CF3
n-C3H7 -<~ OCH2CH2CH2CF3
FF
n-CsH~ OCH2CH2CH2CF3
~932~2
R_ _~] ,-Z- (A) ~- -O- (CH2) -CF~
C2H~ ~ OcH2cH2cH2c~2cF3
n-C3H, -/~@- CCHzCH2CH2C.~ F3
n-C5H~; -~ OCH2CH2CH2CH2CF3
CH3OCH2(~(~} OCH2CH2CH2CH2CF3
CH2 - CHCH2CH2 ~}~ OcH2cH2cH2cH2cF3
CH3CH20 ~ OCH2CH2CH2CH2CF3
CH3CH2CH2CH20 ~} OCH2CH2CH2CH2CF3
n-C3H7 ~ OCH2CH2CH2CH2CF3
F
n-CsHll ~ OCH2CH,CH2al2CF3
C2Hs ~}<~ OCH2CF3
n-C3H7 -<~(~ OCH2CF3 K ' 00 ~3170
SA: 3~;
n-CsHl i(~(~} OCH2CF3
CH3OCH2 3(~ OCH2CF3
CH2 S CHCH2CH2 ~<~} OCH2CF3
- 58 - ~ 3 ~ ~ 2
R - [-~] ,-Z- (A) ,- -O- ~CH2) ~-CF3
C2 H5 _~H2CH2CF,
.l-C3H~ OCH2CH2CF3
n-C5H~ } OCH2CH2CF3
CH3OCH2~} OCH2CH2CF3
CH2 = CHcH2cH2 ~}~ OCHzCH2CF3
n-C3H~ ~ OCH2CH2CF3
CH2 = CHCH2CH2 -~ OCH2CH2CF3
c2H5 G ~ ocH~cH2cH2cF3
n-C3H7~~<~ OCH2CH2CH2CF3
n-CsHl: <~ OCH2CH2CH2CF3
CH3OCH2~<~ } OCH2CH2CH2CF3
CH2 - CHCH2CH2 ~OE~ OCH2CH2CH2CF3
n-C3H~ -~ OCH2CH2CH2CF3
n-CsHll ~ OCH2CH2CH2CF3
2~93~
R - [ -~ ], -Z- (A) ,- -O- ~CHz ) ,-CF;
. .
C2Hs ~} OCH2CH2CH2CH2CF3
n-C3H, -~(~ OCH2CH2CH2CH2CF3
n-CsH:: ~}<~} OCH2CH2C~2CH2CF3
CH3OCH2 ~<~} OCH2CHzCH2CH2CF3
CH2 = CHCH2CH2 ~ OCH2CH2CH2CH2CF3
n-C3H, ~ OCH2CH2CH2CH2CF3
F
n-C3H7 ~ QCH2CH2CH2CH2CF3
n-C5H~ OCX2CH2CH2CH2CF3
n-CsHl 1 ~}~ OCH2CH2CH2CH2CF3
C2H5 <~}(~ OCH2CF3
n-C3H7 ~ OCH2CF3
n-CsHl 1 ~ OCH2CF3
CH3OCH2 ~}<~ OCH2CF3
CH2 ~ CHCH2CH2 ~}~} OCH2CF3
- 60 ~ 3212
- ~ -a I g-Z- (A) - -O- ~CH2) ,-CF3
OCH2 CH2 CF3
n-C ~ } OCH~CH2CF3
n-C,H; ~~ OCH2CH2CF3
CH30CH2 ~} OCH2CH2CF,
CH2 = CHcH2cH2 ~ OCH2CH2CF3
n-C3H~ {~ OCH2CH2CF3
n-C;Hl 1~~<~~ OCH2CH2CF3
C2Hs ~_ OCH2CH2CH2CF3
n-C3H7 ~ OCH2CH2CH2CF3
n-CsHIl ~ OCH2CH2CH2CF3
CH3OCH2 ~ OCH2CH2CH2CF3
CH2 S CHcH2cH2 ~ OCH2CH2CH2CF3
n-C3H~ ~ OCH2CH2CH2CF3
n-CsHll ~ F OCH2CH2CH2CF3
F K 61 S3~56) N 145,2 I,
~n - O, 096
CH3OCH2 ~ OCH2CH2CH2CF3
-- 61 --
2~3~
R ~ Z- (A) - -O- (CH2) -CFl
n-C3H, ~<~ OCH2CH,CH2CH,CF3
n-CsH ~ OCH2CHzCH2CH2CF3
CH3OCH2 ~ OCH2CH2CH2CH2CF3
CH2 = CHcH2cH2 ~ } OCH2CH2CH2CH2CF3
F
n-C3H7 ~ OCH2CH~CH2CH2CF3
C2Hs -<~CH2CH2~ OCH2CF3
n-C3H~ ~CH2CH2~ OCH2CF3
n-CsHIl -~CH2CH2~ OCH2CF3 x 66 Sg 166
CH3OCH2 ~H2CH2~ OCH2CF3
CH2 = CHcH2cH2 -~CH2CH2~ OCH2CF3
C2Hs ~CH2CH2~ OcH2cF3
FF
n-C3H7 ~H2CH2~ OCH2CF3
n-C3H7 ~}CH2CH2~ OCH2CF3
C2Hs ~}CH2CH2~ OCH2CH2CF3
n-C3H7 ~CH2CH2~ OCH2CH2CF3
n-CsHll ~}CH2CH2~ OCH2CH2CF3
CH3OCH2~CH2CH2-~ OCH2CH2CF3
- 62 - ~932
R - [-O-j ,-Z- (A) - -O- (CH2) -CF3
CH2 = CHcH2cH2 ~ cH2cH2 ~ OCH~CH2CF3
n-CsHIl ~CH2CH2~ OCH2CH2CF3
CH3OCH2CH2 ~CR2CH2~ OCH2CH2CF3
C2Hs ~ CH2CH2~ OCH2CH2CH2CF3
n-C3H7 ~CH2CH2-(~} OCH2CH2CH2CF3
n-CsHll ~CH2CH2-<~} OCH2CH2CH2CF3
CH3OCH2 ~CH2CH2-<~} OCH2CH2CH2CF3
CH2 = CHCH2CH2 ~ }CH2CH2-(~ OCH2CH2CH2CF3
CH2 ~ CHcH2cH2 ~CH2CH2~ OCH2CH2CH2CF3
FF
n ~3H7~ -CH2CH2 ~ OCH2CH2CH2CF3
F
C2Hs~-CH2CH2~} OCH2CH2CH2CH2CF3
n-C3H7~CH2CH2~} OCH2CH2CH2CH2CF3
n~Cs~ CH2CH2~ OCH2CH2CH2CH2CF3
CH3OCH2~CH2CH2-<~} OCHzCH2CH2CH2CF3
CH2 =CHcH2cH2 -<~CH2CH2~ OCHzCH2CH2CH2CF3
- 63 ~ 32~2
R -[{)-],-2-(A) - -O-(CH2)r-CF3
--
n-C3H~~CH2CH ~ )CH2CH2CH2CH2CF3
n-C5Hll~CH2CH2~ OCH2CH2CH2CH2CF3
n-CsHIl~CH2C~{2~ OCH2CH2CH2CH2CF3
C2Hs~}(CH2)4~ OCH2CF3
n-C3H7~ ~cH2) 4~} OCH2CF3
n-CsHll~ (CH2~ 4~} OCH2CF3
CH30CH2~ (CH2) 4~ OCH2CF3
CH2 = CHCH2CH2 ~} ~CH2) 4~ OCH2CF3
n-C3H7~} ~CH2) 4~- OCH2CF3
F
n-CsH~CH2) 4~ OCH2CF3
C2H5~ (CH2) 4~} OCH2CH2CF3
n-C3H7 - ~ (CH2) 4~} OCH2CH2CF3
n-CsHll ~ ~CH2) 4~} OCH2CH2CF3
CH3OCH2 {~ ~CH2) 4~} OCH2CH2CF3
CH2 = CHCH~CH2 ~ ~C~2 ) 4~} OCH2CH2CF3
CH3OCH2CH2 ~} (CH2) 4~ OCH2CH2CF3
F
- 64 ~ 93212
R - [ ~ ' ,-Z- (A) - -O- (CH2 ) .-CF3
C2Hs ~} (cH2) 4~ OCH~CH2CH2CFj
n-C3H, ~ (CH2) q~} OCH~C~12CH2CF3
n-C;H~L ~ ~CH2~ ~} OCH2CHzCH2CF3
CH3OCH2 ~ ~cH2) q~} OCH2CH2CH2CF3
CH2 = CHc~2cH2 ~ ~CH2) ~ OCH2CH2CH2CF3
n-C3H7 ~} (CH2) 4~ OCH2CH2CH2CF3
n-C5HLl ~ ~CH2) 4~ ` OCH2CH2CH2CF3
C2H5 ~} ~CH2) ~ OCH2CH2CH2CH2CF3
n C3H7 ~} ~CH2) 4~} OCH2CH2CH2CH2CF3
n-CsHL; ~ ~CH2) 4~ OCH2CH2CH2CH2CF3
CH3OCH2 ~ (CH2) 4~ OCH2CH2CH2CH2CF3
CH2 -- CHcH2cH2 ~ ~CH2) 4~} OcH2cH2cH2cH2cF3
C2Hs ~ ~CH2) 4~ OCH2CH2CH2CH2CF3
F
n-C3H7~ ~CH2) 4~ OCH2CH2CH2CH2CF3
F
n--CsH~CH2 ) 4~ OCH2CH2CH2CH2CF3
- 65 - 2~932~2
Exz~e 1~
CsH ~OCH2CH=CH-CF,
0.1 mol sf 4-(4-pentylcyclohexyl)phenol and
0.1 mol of 1,1,1-trifluoro-4-bromobut-2-ene are reacted
analogously to Example 3.
The following trifluoroalkenyl ether~ of the
formula
R- 1~] ,-Z- (A) 0-O- (CH2) ~-CH=CH-CF3
are prepared analogously.
R ~ -1 ,-Z- (A~ O~ O- (CH2) ~-CH=CH-CF3
C2Hs ~)~ OCH2CH=CHCF3
n-C3H7 ~ OCH2CH=CHCF3
n-C4Hg ~} OCH2CH=CHCF3
CH3OCH2 ~ OCH2CH=CHCF3
CH2 = CHCH2CH2 ~} OCH2CH=CHCF3
C2Hs ~ )'' OCH2CH2CH=CHCF3
n-C3H7 ~ OCH2CH2CH=CHCF3
n-CsH~ ocH2cH2cH=cHcFi
CH3OCH2 ~ OCH2CH2CH=CHCF3
CH2 = CHcH2cH2 ~} OCH2CH2CH=CHCF3
n-C3H7 ~ OCH2CH2CH=CHCF3
F
n-CsHl ~ GCH2CH2CH=CHCF3
66- 20~32~2
R - [ ~; ,-Z- (A) - -O- (CH2) -CH=CH-CF~
. . .. _ . ~_ . .
C2Hs ~(~3~ OCH~CH=CHCF3
n-C3H, ~ OCH2CH=CHCF3
n-CsHI ~ ~ OCH2CH=CHCF3
CH30CH2 ~ OCH2CH=CHCF3
CH2 - CHcH2cH2 ~ OCH2CH=CHCF3
CH3CH20 --~- OCH2CH~CHCF3
CH3CH2CH20 ~ OCH2CH=CHCF3
n-C3H7 ~ OCH2eH=CHCF3
F
n_C5Hll ~ OCH2CH=CHCF3
C2Hs _~_ OCH2CH2CH=CHCF3
n-C3H7 ~ OCH2CH2CH-CHCF3
n-C5Hll ~ OCH2CH2CH=CHCF3
CH3OCH2 ~ OCH2CH2CH-CHCF3
CH2 = CHcH2cH2 ~} OCH2CH2CH=CHCF3
CH3CH20 ~ OCH2CH2CH=CHCF3
CH3CH2CH2CH20 ~ OCH2CH2CH=CHCF3
C2Hs ~_ OCH2CH2CH=CHCF3
n-C3H~ ~ OCH2CH2CH=CHCF3
- 67 - ~93212
R - [ {>- ~ 5-Z- (A) ,- -O- (CH~ ) -CH=CH-CF3
n-C4Hg ~
~ OCH2CH2CH=CHCF3
CH3OCH2 ~ OCH2CH2CH=CHCF3
CH2 = CHcH2cH2 ~ OCH2CH2CH=CHCF3
CH2 = CHcH2cH2 ~ OCH2CH2CH=CHCF3
C2H5 ~} OCH2CH=CHCF3
n-C3H7 ~ OCH2CH=CKCF3
n-CsHI ~ ~ OCH2CH=CHCF3
CH3OCH2 ~ OCH2CH=CHCF3
CH2 = CHcH2cH2 ~ OCH2CH=CHCF3
n-C3H7 ~)~ OCH2CH=CHCF3
il-CsH~i ~ OCH2CH=CHCF3
C2H5 ~ OCH2CH2CH=CHCF3
n-C3H7 ~ OCH2CH2CH=CHCF3
n-CsHl 1 ~ OCH2CH2CH=CHCF3
CH3OCH2<} OCH2CH2CH=CHCF3
CH2 = CHCH2CH2 ~ OCH2CH2CH=CHCF3
- 68 - ~ J! ~ i3 21 ~
R ~ ;~~3] ~~Z~ (A) a~ ~O~ (CH2) r-CH=CH-CF3
n-CsH~: ~ OCH2CH2CH=CHCF3
F
CH30CH2CH~ ~ OCH2CH2CH=CHCF3
C2Hs ~ _ OCH2CH-CHCF3
n-C3H7 ~ OCHzCH=CHCF3
n-CsHll ~ OCH2CH=CHCF3
CH3OCH2 ~ OCH2CH=CHCF3
CH2 = CHcH2cH2 -< ~ OCH2CH=CHCF3
n-C3H7 ~ OCH2CHSCHCF3
F
n-CsH~ OCH2CH=CHCF3
C2Hs ~ OCH2CH2CHSCHCF3
n-C3H7 ~} OCH2C~l2CH-C~lCF3
n-C5HIl , ~ OCH2CH2CH=CHCF3
CH3OCH2 ~ OCH2CH2CHSCHCF3
CH2 = CHcH2cH2 ~ OCH2CH2CH=CHCF3
n-C3H, ~ OCH2CH2CH=CHCF3
- 69 - 21393212
R - [ ~ ~ ,-Z- (A) c~ -O- (CH2 ) ~-CH=CH-CF3
. _ _
n-CjH ~ OCH2CH2CH=ClICF3
F
CH3OCH2CH2 ~ OCH2CR2CH-CHCF3
C2H5~CH2CH2~ OCH2CH~CHCF3
n-C3H7~CH2CH2-~} OCH2CH=CHCF3
n-CsHIl~CH2CH2~ OCH2CH=CHCF3
CH3OCH2~CH2CH2~ OCH2CH=CHCF3
CH2 = CHCH2CH2 ~CH2CE~2~} OCH2CH=CHCF3
C2Hs~CH2CH2~ OCH2CHlCH=CHCF3
n-C3H7~H2CH2~ OC~2CH2CH~CHCF3
n-CsH~CH2CH2~ OCH2CH2CH~CF3
CH3OCH2-<~CH2CH2~} OCH2CH2CH-CHCF3
CHz = CHCH2CH2 ~CH2CHz~ OCH2CH2CH=CHCF3
C2Hs~ (CH2)~ ~ OCH2CH=CHCF3
n-C3H7~ ~CH2) 4~ OCH2CH=CHCF3
n~sHll~cH2) ~} OCH2CH=CHCF3
CH30CH2~ (CH2) ~-~ OCH2CH=CHCF3
CH2 = CHcH2cH2 ~} (CHz) ~ OCH2CH=CHCF3
_ 70 - 2!D~321`~
R ~ ],-Z- (A) ,- --O--(CH2) ~-CHaCH-CF3
-
C2Hs~} (CH2) 4~ OC~12CH2CH=CHCF3
n-C3H7~} (CH2 ) q~ OCH2CH2CH=CHCF3
n-CsHll~ (CH2) ~ OCH2CH2CH=CHCF3
CH3OCH2~} (CH2) 4~} OCH2CH2CH'CHCF3
CH2 - CHcH2cH2 ~ ~CH2~ ~} OCH2CH2CH=CHCF3
C2H5~ (CH2) 44~; OCH2CH2CH=CHCF3
F
n-C3H~(CH2) 4~ OCH2CH2CH=CHCF3
n{:sHl 1~ (CH2 ) 4 ~ OCH2CH2CH~CHCF3
F
CH3OCH2~ (CH2 ) 4 ~ OCH2CH2CH=CHCF3
CH2 =CHcH2cH2 ~ (CH2 ) 4~ OCH2CH2CH=CHCF3
~ 71 - ~09~212
~1~ v~
H7C3- ~ 0C~2CF3
a) 8r ~ H2CF3
0.085 mol of 4-bromo-2-fluorophenol are dissol~ed
in 100 ml of 1,3-dimethyl-2-imidazolidinone, the ~olution
S i~ heated to 140-C and 0.09 ml of 2,2,2-trifluoroethyl
methylsulfonste i~ added dropwi~e. The solution i8
stirred at 140-C for 24 hours. 500 ml of ice water are
~ubsequently added, and the product i8 ~3ub~ected to
conventional work-up.
F
b) H3C7 ~ H2CF3
O.05 ml of tran~-n-propylcyclohexylphenylboric
acid, 20 ml of toluene, 10 ml of ethanol, 0.030 mol of
sodium carbonate and 0.86 mmol of tetrakisttriphenyl-
pho~phine)p~ dium(0) are added to 0.015 mol of 4-bromo-
2-fluorophenol 2,2,2-trifluoroethyl ether, and the
mixture is refluxed for 2 hours. 100 ml of petroleum
ether (40-80-) are added, and the product is sub~ected to
conventional work-up. C 81 SB 101 S~ 133 N 139 . 6 I,
~n - 0.152.
The following compounds of the formula
L2 F
R~OCH2CF3
L3 Li
are prepared analo~ously:
- 72 - ~3321
R L: L2 L-
C2Hs H H H
C~H5 H F F K 86 I, ~ 0,111
C2Hs F H H K 7C N ~62, 4~ r,~ 0 137
C~Hs F F F K 96 I, Qn - ~0,105
n-ClH7 F H H K 8q N lOq, 6 t, l~n - ' 0,152
n~H7 F F F K sa I, ~ 0,111
n-C~H7 H F F xasr,~- ~0,120
n-C~H~ H H H
n--C5HI~ F H H K 65 N 108, ~ 0,142
n-C5H~I F F F K 90 I"~ 0,113
n-C5HIl H F F KilN ~78,2) I, ~-~0,119
CH,OCH2 H H H
CH,OCH2 F H H
CH,OCH2 F F F
CH~-CHCH~CH2 F F F
~uuqple 15
F
C3H7~H2CF3
n) ~r ~ H2CF3
F
2,2,2-Trifluoroethyl methylsulfonate i~ added
dropw$~e at 140-C to 0.085 mol of 4-bromo-2,6-difluoro-
phenol analogou~ly to Example 14 a).
C3H7~$~)CH2CF;
2~93212
- 73 -
1.2 g of zinc chloride and 0.14 g of lithium
granules are added to 0.01 mol of trans-4-n-propylcyclo-
hexyl bromide in 15 ml of a solvent mixture compri~ing
T~F and toluene (1:4). The mixture i8 treated with
ultrasound for 4 hours at between 0 and 10C under a
protective gas and with ~tirring. The organozinc compound
obtained i8 treated with 0.01 mol of 4-bromo-2,6-di-
fluorophenol 2,2,2-trifluoroethyl ~thor and 2 mol~ of
l,l'-bis~diphenylpho~phino)ferro~enepall~dium(II)
dichlorid~, the ultrasound bath and tho cooling are
removed, and the mixture is stirred at room temperature
for 24 hour~. 10 ml of ~aturated ammonium chloride
solution are added, and the organic phase i separated
off and subjected to customary work-up. C S I,
lS ~n ~ 0.038.
The following compounds of the formula
F
R-~ ~ ]' ~ H2CF3
ar- prepared analogously:
- 74 _ ~0~3~ ~ ~
R s L
.
n-C2Hs l H
n-C2Hs 1 F
n-C2Hs 2 H C 65 Sll 91 N l~9. 9 ~ 0. 093
n-C2Hs 2 F C 65 N 100.2 ~ 0.084
Il--C3H7 1 H
n-C3H7 1 F
n-C3H7 2 H
n--C3H~ 2 F C 69 N l~tO I; ~1 - ~O. 092
n-C5Hll 1 H
n-CsHll 1 F
n-CSHll 2 H C 62 59 92 N lSl.7 ~: ~ ~0,09
n-C5HIl 2 F C 60 N 137.~ 0.006
CH30CH2 1 H
CH3OCH2 1 F
CH3OCH2 2 H
CH3OCH2 2 F
CH2'CHCH2CH2 1 H
C~l2-CHCH2CH2 1 F
C~l2~CHCH2C~2 2 H
CH2~CHCH2C~2 2 F
~32~2
- 75 -
xample ~
O
H7C3 ~ ~ -OCH2CF,
o
F
a) H7c3{ )- ~
F
0.035 mol of 3,5-difluorobenzaldehyde, 0.35 mol
of propanediol and 3.5 g of p-toluenesulfonic ~cid are
dissolved in 500 ml of toluene and boiled on a water
separator.
F
b) H'C3 { ~ OH
F
O.044 mol of the dioxane derivativQ frsm Example
16 a) i8 dissolved in 130 ml of ab~olute THF, and the
solution i~ cooled to -70C. 51 mmol of n-3uLi are
subsequently added dropwi~e. The mixture is ~tirred at
-70C for 0.5 hour, and 51 mmol of trim~thyl borate are
thcn added dropwise to the reaction mixture. The mixture
i~ then allowed to warm to -20C, and 5 ml of acetic acid
are added in portions. Finally, 132 mmol of ~22 are added
dropwise to the reaction mixture at a temperature of
30-35C. The mixture i~ subsequently subjected to
cu~tomary work-up.
O
c ) H7C3~ OcH2cF3
F
23 mmol of sodium hydride are suspended in lO ml
of DMEU, and 23 mmol of the phenol derivative from
Example 16 b) in 20 ml of DMEU are ~ubsequently added
dropwi~e. The mixture i~ subsequently stirred at 45C for
1 hour. 25 mmol of 2,2,2-trifluoroethyl methylsulfonate
- 76 _ 2~ 2~2
in 5 ml of DMEU are added dropwise to the reaction
mixture at 140C, and the mixture is stirred at 140-C for
5 hours. After hydrolysi~3, the reaction mixture is
acidified and sub~ected to customary work-up.
C 27 I; a6 = 15.76; an = +0.038
The followinq compounds of the formula
R--(Al--Z: ) m~OCH2CF3
L2
are prepared analogously:
R (A ~Z ) ~ Ll ~ L2
.----
C2Hs { ~ H H
o
C2Hs { ~ H F
o
C2Hs { ~ F F
- 77 ~
~( ( A ~ r L-
C
n-C3H. { >_ H .
n-C3H, {~ H F
n~C4H3 {~ H H
n-C~ H9 { ~ H ~ F
n~CqHg {~ F F
n-CsHl { ~_ H H
n~CsHl: { >_ H F
n-CjHl: {~ F F
n-C6Hl 3 {~~ H H
n-C6H 3 {>_ H F
n-C6H: 3 {~_ F F
-- 78 --
~as32l2
R ( ~A - 7 ) ~
. ~
: s { ~
n-C,Hls { )~ H F
n-C7H~s {)~ F F
n--C3H7 { >~ H H
n-C3H7 ~X~ H ,.
n-c3H7 {~ F F
n-CsHl 1 {X~ H H
n-CsHl 1 {o~ H F
n-CsHll {X~ F F
_ 7~ 3~1~
~ample 17 F
H C~ { ~CC:~2 CF
F
13 mmol of 2-[2,6-difluoro-4-hydroxyphenyl]-5-
pentylpyrimidine are reacted analogously to Example 16 c)
with 13 mmol of sodium hydride in 300 ml of DMEU and
25 mmol of 2,2,2-trifluoroethyl methylsulfonate.
C 66 I; ~ = 18.49; ~n - 0.106
ThP following compounds of the formula
r<
R-(A'~Z )m ~ OCH2CF.
L2
are obtained analogously.
R ~Al-Z:) T L2
:
N
C2H4 ~ ~- .{ H
N
N
C2H4 ~ ~ H F
N
N
C2H4 {)- T F
N
N
n-C3H7 ~ ~ H H
N
N
n-C3H7 { ~ H F
N
N
n-C3H7 { ~ F
~J
- 80 _ ~ w~
R (A:-Z: ) ., L: L2
H~
N
n-C~Hg _,~N H F
N
n-C4H~ F F
N
N
n-C;H~ H H
n-CsH., _<~ N H
N
n-C6Hl 3_~ N H H
N
n-C6Hl 3_~ N H
N
n-C6Hl 3 ~ )~
N
n-C~Hls ~;~ H H
N
n-C7Hls ~N H
N
;\T
n-C~Hl s{
~93212
-- 81 --
R (A --Z! ) .r L: L2
_
~-C3`U~
n-C3H, -C )~ H F
n-C3H N F E`
n-C5H!: ~ ~ H H
n-CjH!: N H F
n-CsHll N F F